Hematopathology

On this page
🔬 The Hematopathology Command Center: Decoding Blood's Hidden Messages
You'll master the systematic approach to diagnosing blood disorders by learning how to decode peripheral smears, bone marrow findings, and flow cytometry patterns that reveal whether a patient has leukemia, lymphoma, or a benign mimic. This lesson builds your diagnostic framework from recognizing malignant transformation at the cellular level to applying quantitative discriminators that distinguish one hematologic disease from another. You'll integrate morphology, immunophenotyping, and cytogenetics into evidence-based treatment algorithms, then synthesize these tools across multi-system presentations to achieve clinical command in hematopathology.
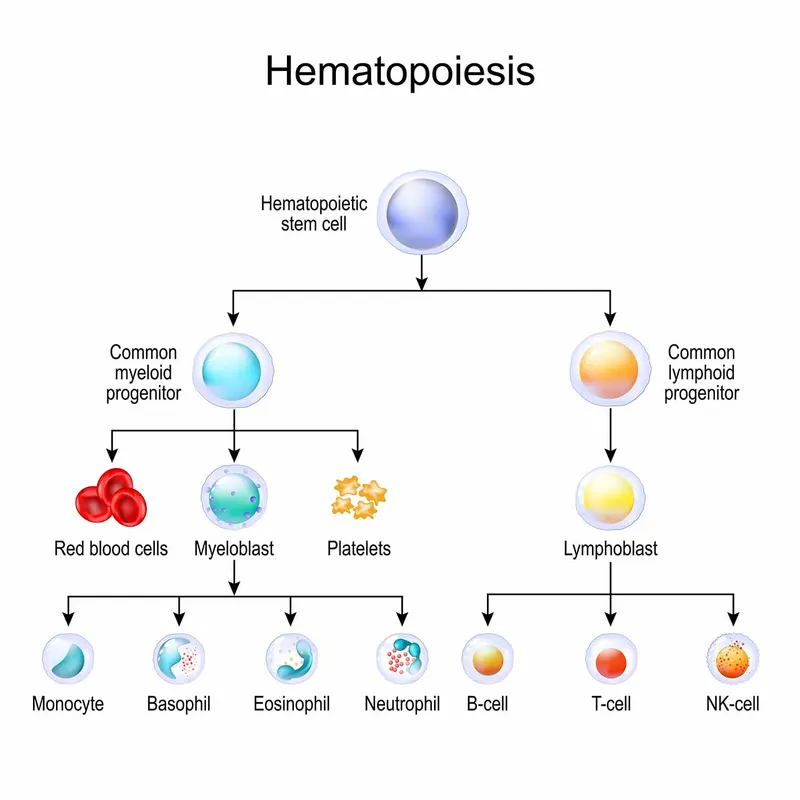
The Hematopoietic Hierarchy: Cellular Command Structure
Normal hematopoiesis follows a precisely orchestrated developmental cascade from pluripotent stem cells to mature effector cells. The bone marrow produces approximately 200 billion red blood cells, 10 billion white blood cells, and 400 billion platelets daily.
-
Hematopoietic Stem Cells (HSCs)
- Frequency: 1 in 10,000-15,000 bone marrow cells
- Self-renewal capacity: >50 population doublings
- Differentiation potential: All 8 major blood cell lineages
- Myeloid lineage: Granulocytes, monocytes, erythrocytes, megakaryocytes
- Lymphoid lineage: B-cells, T-cells, NK cells, dendritic cells
-
Progenitor Cell Compartments
- Common Myeloid Progenitor (CMP): 2-5% of bone marrow cells
- Common Lymphoid Progenitor (CLP): 0.02% of bone marrow cells
- Megakaryocyte-Erythroid Progenitor (MEP): 0.1% of bone marrow cells
- Maturation time: 7-10 days for erythropoiesis
- Platelet production: 1000-3000 platelets per megakaryocyte
📌 Remember: SLIM-CD for HSC surface markers - Sca-1, Lin-, IL-7R-, M-CSFR-, CD34+, Distinct from committed progenitors by maintaining CD34 expression while losing lineage-specific markers
Morphological Mastery: The Cellular Identification Matrix
| Cell Type | Size (μm) | Nuclear Features | Cytoplasm | Key Identifiers | Clinical Significance |
|---|---|---|---|---|---|
| Blast | 12-20 | Large, fine chromatin, 2-4 nucleoli | Basophilic, <20% granules | >20% in AML/ALL | Leukemia threshold |
| Promyelocyte | 16-25 | Oval, coarse chromatin | Heavy granulation | Auer rods possible | APL pathognomonic |
| Myelocyte | 12-18 | Round-oval, condensing | Specific granules | Last mitotic stage | Maturation arrest |
| Metamyelocyte | 10-15 | Kidney-shaped | Pink, few granules | Indented nucleus | Left shift indicator |
| Band | 10-15 | Horseshoe-shaped | Pink, specific granules | Parallel nuclear margins | >10% = left shift |
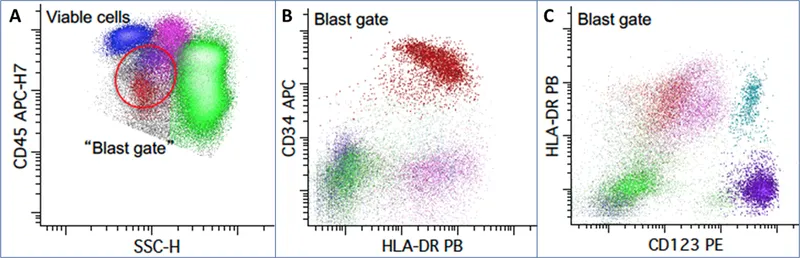
Immunophenotyping Arsenal: Surface Marker Signatures
Flow cytometry analysis identifies cellular lineages through characteristic surface antigen expression patterns. Modern flow cytometers analyze >10,000 cells per second across 8-12 fluorescent parameters simultaneously.
-
B-Cell Lineage Progression
- Early B-cell: CD19+, CD10+, TdT+, CD20-
- Pre-B cell: CD19+, CD10+, CD20+, surface Ig-
- Mature B-cell: CD19+, CD20+, CD10-, surface Ig+
- Memory B-cells: CD27+ expression
- Plasma cells: CD138+, CD38++, CD19-
-
T-Cell Development Markers
- Immature T-cell: CD7+, CD3+, TdT+, CD4+/CD8+ (double positive)
- Helper T-cell: CD3+, CD4+, CD8-, TCR αβ+
- Cytotoxic T-cell: CD3+, CD4-, CD8+, TCR αβ+
- Activation markers: CD25+, CD69+, HLA-DR+
💡 Master This: CD34 expression decreases as cells mature, while CD45 intensity varies by lineage - lymphoblasts show dim CD45, myeloblasts show moderate CD45, and mature lymphocytes show bright CD45. This pattern enables rapid blast identification with >90% accuracy.
Understanding these foundational principles establishes the framework for recognizing pathological deviations. The next section explores how normal hematopoietic mechanisms become disrupted in malignant transformation, creating the characteristic patterns that define specific hematologic disorders.
🔬 The Hematopathology Command Center: Decoding Blood's Hidden Messages
⚙️ The Malignant Transformation Engine: When Cellular Control Systems Fail
The Oncogenic Cascade: Multi-Hit Hypothesis in Action
Hematologic malignancies arise through sequential acquisition of genetic alterations, typically requiring 4-7 independent mutations for full malignant transformation. This process follows predictable patterns based on cellular context and environmental pressures.
-
Class I Mutations: Proliferative Advantage
- FLT3-ITD: Found in 30% of AML cases
- RAS mutations: Present in 25% of myeloid malignancies
- JAK2 V617F: Occurs in >95% of polycythemia vera cases
- Constitutive tyrosine kinase activation
- Cytokine-independent growth signaling
- 10-fold increased proliferation rates
-
Class II Mutations: Differentiation Blockade
- AML1-ETO: t(8;21) in 5-10% of AML
- PML-RARα: t(15;17) in >95% of APL cases
- RUNX1 mutations: 10-15% of AML patients
- Transcription factor disruption
- >90% reduction in lineage-specific gene expression
- Accumulation of immature blast cells
📌 Remember: CALM-AF10 for high-risk translocations - Core binding factor disruption, ALL1 gene rearrangements, Leukemia fusion proteins, Mixed lineage features, Aberrant immunophenotypes, Favorable vs unfavorable cytogenetics, 10% blast threshold variations
Epigenetic Dysregulation: The Silent Saboteur
Epigenetic modifications control gene expression without altering DNA sequence, representing >40% of pathogenic mechanisms in hematologic malignancies. These reversible changes offer therapeutic targets with >80% response rates in specific contexts.
| Epigenetic Mechanism | Target Genes | Clinical Impact | Therapeutic Agents | Response Rates |
|---|---|---|---|---|
| DNA Hypermethylation | p16, MLH1, CDKN2B | Tumor suppressor silencing | 5-azacytidine | 60-70% |
| Histone Deacetylation | p21, CDKN1A | Cell cycle arrest loss | Vorinostat | 30-40% |
| Chromatin Remodeling | SWI/SNF complex | Transcriptional dysregulation | BET inhibitors | 40-50% |
| MicroRNA Dysregulation | let-7, miR-15a | Post-transcriptional control | miRNA mimics | 20-30% |
| Histone Methylation | HOX genes, MEIS1 | Developmental program disruption | DOT1L inhibitors | 25-35% |
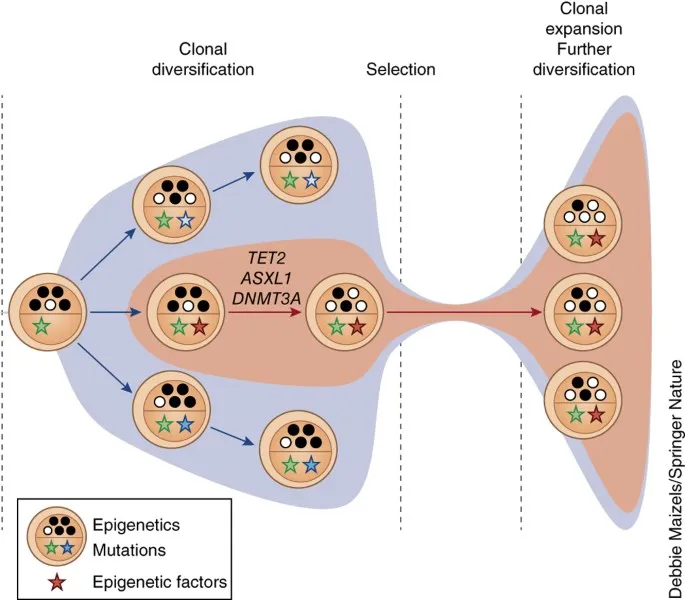
Clonal Evolution Dynamics: The Darwinian Battlefield
Hematologic malignancies evolve through clonal selection pressures, with dominant clones emerging based on growth advantages and treatment resistance. Single-cell sequencing reveals >50 distinct subclones within individual tumors.
-
Clonal Hematopoiesis of Indeterminate Potential (CHIP)
- Prevalence: 10-15% of individuals >70 years old
- Mutation frequency: >2% variant allele fraction
- Annual transformation risk: 0.5-1% to overt malignancy
- DNMT3A mutations: 40% of CHIP cases
- TET2 mutations: 20% of CHIP cases
- ASXL1 mutations: 15% of CHIP cases
-
Clonal Selection Mechanisms
- Chemotherapy pressure: >10-fold enrichment of resistant clones
- Immune surveillance escape: HLA-I loss in 30% of relapses
- Metabolic adaptation: >5-fold increased glycolysis rates
- Warburg effect activation
- LDHA overexpression in >80% of aggressive lymphomas
💡 Master This: Clonal evolution follows predictable patterns - early driver mutations (DNMT3A, TET2) provide clonal advantage, intermediate mutations (NPM1, FLT3) enhance proliferation, and late mutations (TP53, complex karyotype) confer treatment resistance and poor prognosis with <20% 5-year survival.
These molecular mechanisms create the pathological patterns that manifest as distinct clinical entities. The next section explores how to systematically recognize these patterns through morphological and immunophenotypic analysis, building the diagnostic framework essential for accurate classification.
⚙️ The Malignant Transformation Engine: When Cellular Control Systems Fail
🎯 The Pattern Recognition Matrix: Systematic Diagnostic Frameworks
The Blast Analysis Protocol: Systematic Cellular Evaluation
Blast identification and characterization forms the cornerstone of acute leukemia diagnosis. Standardized evaluation protocols ensure consistent interpretation across different observers and institutions.
- Morphological Blast Criteria
- Size: >12 μm diameter (larger than mature lymphocytes)
- Nuclear features: Fine, dispersed chromatin with 2-4 prominent nucleoli
- Cytoplasm: Moderate to abundant, basophilic staining
- Nuclear-cytoplasmic ratio: >3:1 in most cases
- Auer rods: Pathognomonic for myeloid lineage
- Hand-mirror cells: Characteristic of T-ALL
- Cytoplasmic vacuoles: Common in Burkitt lymphoma
📌 Remember: BLASTS criteria for acute leukemia - Basophilic cytoplasm, Large nucleoli (>2), Abundant cytoplasm, Size >12 μm, Twenty percent threshold, Specific lineage markers required for classification
Immunophenotypic Decision Trees: Lineage Assignment Logic
Flow cytometry provides definitive lineage assignment through systematic antigen expression analysis. Modern panels include >20 antibodies analyzed simultaneously across >10,000 cells.
-
B-Cell Acute Lymphoblastic Leukemia (B-ALL)
- Core markers: CD19+, CD22+, CD79a+, TdT+
- Maturation stages: Pro-B, Pre-B, Transitional B
- Prognostic markers: CD10+ (favorable), CD20+ (unfavorable)
- Philadelphia chromosome: t(9;22) in 25% of adult B-ALL
- Hyperdiploidy: >50 chromosomes in 30% of pediatric cases
- MLL rearrangements: 5-10% overall, >80% in infant ALL
-
T-Cell Acute Lymphoblastic Leukemia (T-ALL)
- Core markers: CD7+, CD3+, TdT+
- Maturation assessment: CD1a, CD4, CD8 expression patterns
- High-risk features: >100,000/μL WBC count, CNS involvement
- NOTCH1 mutations: >50% of T-ALL cases
- Early T-cell precursor: CD1a-, CD8-, weak CD5
⭐ Clinical Pearl: Aberrant antigen expression occurs in >80% of acute leukemias - myeloid antigens on lymphoblasts or lymphoid antigens on myeloblasts. This cross-lineage expression doesn't change lineage assignment but indicates higher relapse risk and may require intensified therapy.
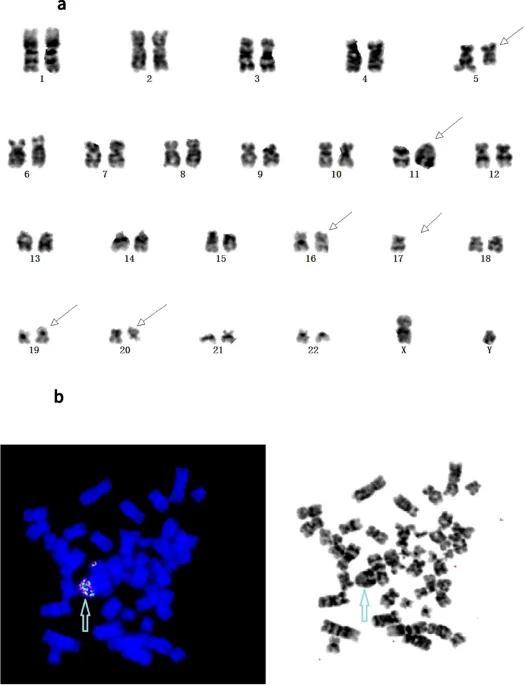
Cytogenetic Risk Stratification: Chromosomal Prognostic Mapping
Cytogenetic abnormalities provide the most powerful prognostic information in hematologic malignancies, with specific alterations determining treatment intensity and expected outcomes.
| Risk Category | Cytogenetic Abnormalities | Frequency | 5-Year Survival | Treatment Approach |
|---|---|---|---|---|
| Favorable | t(8;21), t(15;17), inv(16) | 15-20% | >80% | Standard therapy |
| Intermediate | Normal karyotype, +8, +21 | 50-60% | 40-60% | Risk-adapted |
| Unfavorable | Complex, -5, -7, 11q23 | 20-25% | <20% | Intensive/transplant |
| Very High Risk | Monosomal, chromothripsis | 5-10% | <10% | Experimental therapy |
- NPM1 mutations: 50-60% of normal karyotype AML
- FLT3-ITD: 30% of AML, allelic ratio >0.5 = poor prognosis
- CEBPA mutations: 10-15% of AML, biallelic = favorable
- IDH1/IDH2 mutations: 20% of AML, targetable with inhibitors
- TP53 mutations: 5-10% of de novo AML, >50% of therapy-related
💡 Master This: Complex karyotype (≥3 unrelated abnormalities) occurs in 10-15% of AML and confers <10% 5-year survival. When combined with TP53 mutations (>70% overlap), these patients require immediate allogeneic transplant in first remission for any chance of cure.
These systematic frameworks provide the foundation for accurate diagnosis and risk assessment. The next section examines how to differentiate between morphologically similar entities using quantitative discriminators and evidence-based criteria.
🎯 The Pattern Recognition Matrix: Systematic Diagnostic Frameworks
🔍 The Differential Diagnosis Decoder: Quantitative Discriminators
Acute Leukemia vs Myelodysplastic Syndrome: The Critical 20% Threshold
The distinction between acute myeloid leukemia and myelodysplastic syndrome hinges on precise blast enumeration and dysplastic feature assessment, with profound therapeutic implications.
| Parameter | AML | MDS | Discriminatory Value | Clinical Impact |
|---|---|---|---|---|
| Blast Percentage | ≥20% | <20% | Absolute threshold | Treatment intensity |
| Dysplastic Changes | <10% of cells | >10% in ≥2 lineages | Morphological assessment | Prognosis prediction |
| Cytogenetics | Balanced translocations | Unbalanced deletions | Chromosomal pattern | Risk stratification |
| Ring Sideroblasts | <15% | >15% (RS-MDS) | Iron stain quantification | Subtype classification |
| Median Survival | Variable by subtype | 2.5 years | Prognostic difference | Treatment goals |
- 500-cell differential count minimum for accuracy
- Exclude lymphocytes, plasma cells, mast cells from denominator
- Include promonocytes as blast equivalents in CMML
- Flow cytometry correlation required for borderline cases
- CD34+ cells: Should correlate with morphological blasts
- Immature myeloid cells: CD117+, HLA-DR+ population
- Dysplastic Feature Recognition
- Neutrophil dysplasia: Hypolobated nuclei, hypogranulation
- Erythroid dysplasia: Nuclear budding, multinucleation, ring sideroblasts
- Megakaryocytic dysplasia: Hypolobated nuclei, micromegakaryocytes
- Quantitative threshold: >10% dysplastic cells per lineage
- Inter-observer agreement: >85% for experienced hematopathologists
📌 Remember: RAEB-2 (Refractory Anemia with Excess Blasts-2) contains 10-19% blasts and represents high-risk MDS with median survival <2 years. These patients often benefit from intensive therapy similar to AML rather than supportive care alone.
Lymphoblastic vs Burkitt Lymphoma: Morphological Mimicry
Both entities present with sheets of immature-appearing lymphoid cells, but critical differences in morphology, immunophenotype, and genetics determine vastly different treatment approaches.
-
Morphological Discriminators
- Cell size: Lymphoblasts 12-15 μm, Burkitt cells 15-25 μm
- Nuclear features: Lymphoblasts have fine chromatin, Burkitt shows coarse chromatin
- Cytoplasm: Lymphoblasts scant, Burkitt moderate with vacuoles
- Mitotic rate: Lymphoblasts <10/hpf, Burkitt >40/hpf
- Starry-sky pattern: Pathognomonic for Burkitt lymphoma
- Apoptotic rate: >95% of Burkitt cells undergo apoptosis within 24 hours
-
Immunophenotypic Distinctions
- TdT expression: Positive in >95% of lymphoblastic lymphoma
- Ki-67 proliferation: >95% in Burkitt, <80% in lymphoblastic
- BCL-2 expression: Negative in Burkitt, variable in lymphoblastic
- MYC rearrangement: >95% of Burkitt cases
- Surface immunoglobulin: Positive in Burkitt, negative in precursor B-ALL
⭐ Clinical Pearl: Double-hit lymphomas contain both MYC and BCL-2 rearrangements, occurring in 5-10% of aggressive B-cell lymphomas. These require intensive chemotherapy regimens with >6 cycles rather than standard 3-4 cycle Burkitt protocols, as they show intermediate prognosis between the two entities.
Chronic Lymphocytic Leukemia vs Prolymphocytic Leukemia: Prognostic Precision
Both represent mature B-cell malignancies with circulating lymphocytes, but prolymphocyte percentage determines classification and dramatically affects prognosis.
-
Prolymphocyte Morphology
- Size: >15 μm (larger than small lymphocytes)
- Nuclear features: Prominent central nucleolus
- Chromatin: Less condensed than typical CLL cells
- Cytoplasm: Moderate amount, less basophilic
- Counting methodology: 200-cell differential minimum
- Flow cytometry correlation: Larger forward scatter population
-
Prognostic Implications
- CLL (<10% prolymphocytes): Median survival 10-15 years
- CLL/PL (10-55% prolymphocytes): Median survival 5-8 years
- B-PLL (>55% prolymphocytes): Median survival 2-3 years
- Treatment response: B-PLL shows <30% response to standard CLL therapy
- Transformation risk: CLL/PL has 2-fold higher Richter transformation rate
💡 Master This: Immunophenotypic scoring systems help distinguish CLL from other B-cell malignancies. Matutes score assigns points for CD5+ (1 point), CD23+ (1 point), weak CD79b (1 point), weak surface Ig (1 point), and CD22-/weak (1 point). Scores 4-5 indicate CLL, 0-2 suggest other B-cell lymphomas.
These quantitative discriminators enable precise classification that directly impacts treatment selection and prognostic counseling. The next section explores evidence-based treatment algorithms that translate diagnostic precision into optimal therapeutic outcomes.
🔍 The Differential Diagnosis Decoder: Quantitative Discriminators
⚖️ The Treatment Algorithm Engine: Evidence-Based Therapeutic Precision
Acute Myeloid Leukemia: Risk-Stratified Treatment Precision
AML treatment algorithms stratify patients based on cytogenetic and molecular risk factors, with treatment intensity adjusted to maximize cure probability while minimizing treatment-related mortality.
-
Induction Therapy Protocols
- 7+3 regimen: Cytarabine 100-200 mg/m² × 7 days + Daunorubicin 60-90 mg/m² × 3 days
- Complete remission rate: 60-80% in patients <60 years
- Treatment-related mortality: <5% in favorable risk, 10-15% in unfavorable risk
- FLAG-IDA: Fludarabine + Cytarabine + G-CSF + Idarubicin for high-risk disease
- CPX-351: Liposomal cytarabine/daunorubicin for therapy-related AML
-
Targeted Therapy Integration
- FLT3 inhibitors: Midostaurin with induction improves overall survival by 23%
- IDH inhibitors: Ivosidenib (IDH1), Enasidenib (IDH2) for relapsed/refractory
- Venetoclax combinations: >70% response rates in elderly patients
- BCL-2 inhibition: Particularly effective with hypomethylating agents
- Gemtuzumab ozogamicin: Anti-CD33 antibody-drug conjugate
📌 Remember: FLAMSA conditioning for high-risk AML - Fludarabine, L-PAM (melphalan), Ara-C (cytarabine), Melphalan, Stem cell rescue, Allogeneic transplant. This reduced-intensity conditioning enables transplant in patients up to age 70 with <20% treatment-related mortality.
Lymphoma Treatment Algorithms: Histology-Driven Precision
Lymphoma treatment varies dramatically by histologic subtype, with cure rates ranging from >90% in favorable Hodgkin lymphoma to <20% in certain T-cell lymphomas.
| Lymphoma Subtype | Standard Therapy | Response Rate | 5-Year Survival | Key Modifications |
|---|---|---|---|---|
| DLBCL | R-CHOP × 6 | 80-90% | 60-70% | CNS prophylaxis if high-risk |
| Follicular | R-CVP or BR | 90-95% | 80-90% | Maintenance rituximab |
| Mantle Cell | R-HyperCVAD | 70-80% | 50-60% | Autologous transplant |
| Burkitt | R-CODOX-M/IVAC | 85-95% | 80-90% | CNS prophylaxis mandatory |
| T-cell NHL | CHOP-based | 50-70% | 30-50% | Autologous transplant consideration |
- Mechanism: Anti-CD20 monoclonal antibody
- Dosing: 375 mg/m² every 3 weeks × 6-8 cycles
- Survival benefit: 10-15% improvement in overall survival
- Maintenance therapy: Every 2 months × 2 years in follicular lymphoma
- Biosimilar options: >95% efficacy compared to originator
- CAR-T Cell Therapy
- Indications: Relapsed/refractory large B-cell lymphoma after ≥2 prior therapies
- Response rates: >80% overall response, >50% complete response
- Toxicity management: Cytokine release syndrome in >70%, neurotoxicity in >30%
- Tocilizumab: First-line CRS management
- Corticosteroids: Second-line for severe CRS or neurotoxicity
⭐ Clinical Pearl: Double-expressor lymphomas (MYC >40% and BCL-2 >50% by immunohistochemistry) comprise 20-30% of DLBCL and show inferior outcomes with standard R-CHOP. These patients may benefit from dose-dense regimens like R-ACVBP or clinical trial enrollment.
Chronic Lymphocytic Leukemia: Era of Targeted Therapy
CLL treatment has been revolutionized by targeted agents that achieve superior outcomes compared to traditional chemotherapy, particularly in high-risk patients.
-
First-Line Treatment Selection
- Young, fit patients: FCR (Fludarabine, Cyclophosphamide, Rituximab) or BR (Bendamustine, Rituximab)
- Elderly or unfit: Ibrutinib or Acalabrutinib (BTK inhibitors)
- High-risk cytogenetics: Ibrutinib-based regimens preferred
- 17p deletion: <2-year median survival with chemotherapy vs >5 years with ibrutinib
- Unmutated IGHV: BTK inhibitors show superior progression-free survival
-
Novel Agent Mechanisms
- BTK inhibitors: Ibrutinib, Acalabrutinib - >90% overall response rates
- BCL-2 inhibitors: Venetoclax - >70% response in relapsed/refractory
- PI3K inhibitors: Idelalisib - >80% response but significant toxicity
- Combination strategies: Venetoclax + Rituximab achieves >80% undetectable MRD
- Fixed-duration therapy: 12-24 months treatment courses under investigation
💡 Master This: Tumor lysis syndrome risk with venetoclax requires careful dose escalation starting at 20 mg daily and increasing weekly to 400 mg daily. Patients with lymph nodes >5 cm or absolute lymphocyte count >25,000/μL require hospitalization for initial dosing due to >30% TLS risk.
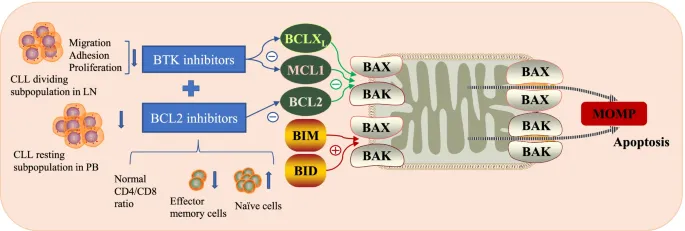
These evidence-based algorithms ensure optimal treatment selection based on individual patient and disease characteristics. The next section explores how multiple systems integrate to create comprehensive diagnostic and therapeutic frameworks for complex hematologic disorders.
⚖️ The Treatment Algorithm Engine: Evidence-Based Therapeutic Precision
🔗 The Multi-System Integration Hub: Advanced Hematopathology Synthesis
Hematolymphoid System-Solid Organ Interactions
Hematologic malignancies frequently involve solid organs, creating diagnostic challenges that require understanding of both hematopathology and organ-specific pathology patterns.
-
Bone Marrow Microenvironment Disruption
- Stromal cell dysfunction: >50% reduction in supportive cytokine production
- Angiogenesis dysregulation: 3-5 fold increased microvessel density in AML
- Immune microenvironment: >80% reduction in NK cell function
- Mesenchymal stem cell alterations: Abnormal differentiation in >70% of MDS cases
- Extracellular matrix changes: Increased fibrosis correlates with poor prognosis
-
Central Nervous System Involvement
- CNS leukemia: 5-10% of ALL, <5% of AML at diagnosis
- Sanctuary site protection: Blood-brain barrier limits chemotherapy penetration
- Intrathecal therapy: Methotrexate 12-15 mg based on age, cytarabine 30-70 mg
- High-risk features: >50,000/μL WBC, LDH >2× normal, certain cytogenetics
- Prophylactic approaches: >90% reduction in CNS relapse with appropriate therapy
Molecular Integration: Multi-Platform Diagnostics
Modern hematopathology integrates multiple molecular platforms to achieve comprehensive disease characterization, with each technique providing complementary information.
| Platform | Information Provided | Turnaround Time | Clinical Applications | Limitations |
|---|---|---|---|---|
| Flow Cytometry | Immunophenotype, MRD | Same day | Lineage assignment, monitoring | Limited antigen panel |
| Cytogenetics | Chromosomal abnormalities | 3-5 days | Risk stratification, prognosis | Resolution ~5-10 Mb |
| FISH | Specific translocations | 1-2 days | Targeted abnormality detection | Limited to known targets |
| NGS Panels | Mutations, CNVs | 7-14 days | Comprehensive mutation profiling | Interpretation complexity |
| Whole Genome | Complete genomic landscape | 14-21 days | Research, complex cases | Cost, data management |
- Myeloid panels: >40 genes commonly mutated in myeloid malignancies
- Lymphoid panels: >50 genes relevant to lymphomas and lymphoid leukemias
- Variant allele frequency: >5% for somatic mutations, >20% for germline
- Clonal evolution tracking: Serial monitoring of mutation burden
- Therapeutic targeting: >30 FDA-approved targeted agents based on mutations
- Minimal Residual Disease Monitoring
- Flow cytometry MRD: Sensitivity 10⁻⁴ (0.01%)
- Molecular MRD: Sensitivity 10⁻⁵-10⁻⁶ (0.001-0.0001%)
- Prognostic significance: MRD positivity predicts >70% relapse risk
- Treatment modification: MRD-guided therapy improves outcomes in >80% of studies
- Transplant decisions: MRD status influences conditioning intensity
📌 Remember: CHIP-CHOP for age-related clonal hematopoiesis - Clonal Hematopoiesis of Indeterminate Potential requires Careful History, Ongoing monitoring, Patient counseling about 0.5-1% annual transformation risk to overt malignancy
Therapeutic Resistance Mechanisms: Systems Biology Approach
Understanding resistance mechanisms requires integration of cellular, molecular, and microenvironmental factors that contribute to treatment failure and disease progression.
-
Primary Resistance Mechanisms
- Drug efflux pumps: MDR1 overexpression in >60% of relapsed AML
- Target mutations: BCR-ABL kinase domain mutations in >50% of imatinib resistance
- Apoptosis defects: p53 mutations confer >10-fold chemotherapy resistance
- Microenvironment protection: Stromal cell adhesion reduces drug sensitivity
- Metabolic reprogramming: Altered glucose metabolism supports survival
-
Acquired Resistance Patterns
- Clonal selection: Pre-existing resistant clones expand under treatment pressure
- New mutations: Secondary mutations develop in >30% of targeted therapy failures
- Pathway redundancy: Alternative signaling bypasses inhibited pathways
- Combination strategies: >2 mechanisms targeted simultaneously
- Sequential therapy: Rotation of non-cross-resistant agents
⭐ Clinical Pearl: Venetoclax resistance in CLL often involves BCL-2 mutations (G101V, D103Y) that reduce drug binding affinity by >100-fold. These mutations occur in <10% of patients but predict rapid disease progression and require alternative BCL-2 family targeting or combination approaches.
Precision Medicine Integration: Personalized Treatment Algorithms
The integration of genomic profiling, pharmacogenomics, and clinical factors enables truly personalized treatment approaches that optimize efficacy while minimizing toxicity.
-
Pharmacogenomic Considerations
- TPMT polymorphisms: 10% of population has reduced activity, requiring 6-MP dose reduction
- UGT1A1 variants: Gilbert syndrome patients need irinotecan dose modification
- CYP2D6 metabolism: Affects tamoxifen efficacy in hormone-positive malignancies
- DPYD deficiency: 5-fluorouracil toxicity risk in 3-5% of patients
- HLA typing: Abacavir hypersensitivity prediction with >95% accuracy
-
Germline Predisposition Recognition
- RUNX1 mutations: Familial platelet disorder with >40% leukemia risk
- CEBPA germline: Biallelic mutations in familial AML syndrome
- DDX41 mutations: Late-onset MDS/AML in >50% of carriers
- Genetic counseling: Family screening recommended for high-penetrance variants
- Surveillance protocols: Annual monitoring for at-risk family members
💡 Master This: Tumor-normal sequencing distinguishes somatic from germline mutations with >99% accuracy. Germline mutations in cancer predisposition genes occur in 5-10% of hematologic malignancy patients and require specialized counseling and family screening protocols to prevent secondary malignancies.
This multi-system integration approach enables recognition of complex disease patterns and optimal treatment selection. The final section synthesizes these concepts into practical mastery tools for immediate clinical application.
🔗 The Multi-System Integration Hub: Advanced Hematopathology Synthesis
🎯 The Hematopathology Mastery Arsenal: Clinical Command Tools
Essential Diagnostic Thresholds: The Numbers That Matter
Critical quantitative thresholds form the backbone of hematopathology diagnosis, with specific values determining classification, prognosis, and treatment approaches.
📌 Remember: 20-5-10-15 rule for acute leukemia - 20% blasts for AML diagnosis, 5% blasts normal bone marrow upper limit, 10% dysplastic cells for MDS lineage involvement, 15% ring sideroblasts for RARS diagnosis
-
Blast Enumeration Mastery
- Acute leukemia: ≥20% blasts (bone marrow or peripheral blood)
- MDS threshold: <20% blasts with >10% dysplasia in ≥2 lineages
- Normal variation: <5% blasts in healthy bone marrow
- Regenerating marrow: Up to 10% blasts acceptable post-chemotherapy
- Blast equivalent cells: Promonocytes in CMML, abnormal promyelocytes in APL
- Counting methodology: 500-cell differential minimum for accuracy
-
Cytogenetic Risk Stratification
- Favorable risk: t(8;21), t(15;17), inv(16) - >80% 5-year survival
- Intermediate risk: Normal karyotype, +8, +21 - 40-60% 5-year survival
- Unfavorable risk: Complex, -5, -7, 11q23 - <20% 5-year survival
- Very high risk: Monosomal karyotype - <10% 5-year survival
- Complex karyotype: ≥3 unrelated chromosomal abnormalities
- Monosomal karyotype: ≥2 autosomal monosomies or 1 monosomy + ≥1 structural abnormality
Rapid Pattern Recognition Matrix
| Clinical Presentation | Key Diagnostic Features | Critical Tests | Immediate Actions |
|---|---|---|---|
| Blast Crisis | >20% blasts, fever, bleeding | Flow cytometry, cytogenetics | Leukapheresis if WBC >100K |
| TLS Risk | High tumor burden, elevated LDH | Uric acid, phosphorus, calcium | Allopurinol, hydration |
| DIC Pattern | Schistocytes, low platelets | PT/PTT, fibrinogen, D-dimer | Platelet/plasma support |
| Hyperviscosity | >100K WBC or high paraprotein | Viscosity, protein electrophoresis | Plasmapheresis consideration |
| CNS Involvement | Neurologic symptoms | Lumbar puncture, flow cytometry | Intrathecal therapy |
Immunophenotypic Quick Reference
-
B-Cell Lineage Markers
- Pan-B markers: CD19 (earliest), CD20 (mature), CD22 (cytoplasmic early)
- Maturation sequence: CD34+ → CD10+ → CD20+ → surface Ig+
- Aberrant expression: Myeloid antigens in >80% of B-ALL cases
- Prognostic markers: CD10+ favorable in B-ALL, CD20+ unfavorable
-
T-Cell Development Pathway
- Early markers: CD7 (earliest), CD3 (cytoplasmic then surface)
- Maturation assessment: CD1a, CD4, CD8 double positive → single positive
- Aberrant patterns: CD4/CD8 double negative in >50% of T-ALL
- ETP-ALL: CD1a-, CD8-, weak CD5 = early T-cell precursor with poor prognosis
-
Myeloid Lineage Recognition
- Pan-myeloid: CD13, CD33 (early), CD15 (late)
- Monocytic: CD14, CD64, CD68 (cytoplasmic)
- Megakaryocytic: CD41, CD61 (specific for platelet lineage)
- Myeloperoxidase: >3% positive blasts confirms myeloid lineage
💡 Master This: Lineage infidelity occurs when >20% of blasts express antigens inappropriate for their assigned lineage. This doesn't change lineage assignment but indicates higher relapse risk and may require intensified therapy or alternative treatment approaches.
Treatment Decision Algorithms
-
Transplant Eligibility Criteria
- Age limits: Allogeneic <70-75 years, Autologous <75-80 years
- Performance status: ECOG 0-2 for intensive conditioning
- Organ function: Cardiac EF >45%, DLCO >50%, Creatinine <2.0
- HCT-CI score: Comorbidity index predicts transplant-related mortality
- Donor availability: >75% of patients have suitable donor through registries
-
Molecular Monitoring Protocols
- MRD assessment: Post-induction and pre-transplant time points
- Sensitivity requirements: 10⁻⁴ for flow cytometry, 10⁻⁵ for molecular
- Clinical significance: MRD positivity predicts >70% relapse probability
- Treatment modification: MRD-positive patients benefit from intensification
- Monitoring frequency: Every 3 months for 2 years post-treatment
Emergency Management Protocols
-
Tumor Lysis Syndrome Prevention
- Risk assessment: High tumor burden + rapid proliferation
- Prophylaxis: Allopurinol 300 mg daily or Rasburicase 0.2 mg/kg
- Monitoring: Uric acid, phosphorus, calcium, potassium every 6-8 hours
- Hydration: 2-3 L/day unless contraindicated
- Rasburicase contraindications: G6PD deficiency, pregnancy
-
Hypercalcemia Management
- Mild (10.5-12 mg/dL): Hydration and bisphosphonates
- Moderate (12-14 mg/dL): Calcitonin + bisphosphonates
- Severe (>14 mg/dL): Hemodialysis consideration
- Bisphosphonate choice: Zoledronic acid 4 mg IV most potent
- Response time: 24-48 hours for calcitonin, 2-4 days for bisphosphonates
📌 Remember: CAIRO-BISHOP criteria for tumor lysis syndrome - Calcium <7 mg/dL, Acute kidney injury, Increased uric acid >8 mg/dL, Rising potassium >6 mEq/L, Oliguria; Biomarkers, Increased phosphorus >4.5 mg/dL, Symptoms, Heart arrhythmias, Oliguria, Precipitation
These clinical command tools provide immediate access to critical diagnostic and therapeutic information, enabling confident management of complex hematopathology cases. Master these frameworks to transform comprehensive knowledge into precise clinical decision-making that optimizes patient outcomes.
🎯 The Hematopathology Mastery Arsenal: Clinical Command Tools
Practice Questions: Hematopathology
Test your understanding with these related questions
A 45-year-old woman comes to the physician because of a 1-week history of fatigue and bruises on her elbows. Examination shows a soft, nontender abdomen with no organomegaly. Laboratory studies show a hemoglobin concentration of 7 g/dL, a leukocyte count of 2,000/mm3, a platelet count of 40,000/mm3, and a reticulocyte count of 0.2%. Serum electrolyte concentrations are within normal limits. A bone marrow biopsy is most likely to show which of the following findings?













