Endocrine pathology

On this page
🎯 Endocrine Pathology: The Hormone Detective's Arsenal
Endocrine disorders are master mimics that can present as fatigue, weight changes, mood disturbances, or life-threatening crises, making diagnosis both challenging and essential. You'll learn to decode hormonal imbalances by understanding gland architecture, feedback loops, and cellular signaling, then apply systematic pattern recognition to distinguish hyperthyroidism from Cushing syndrome, primary from secondary failures, and functional from anatomic lesions. This lesson builds your diagnostic framework from molecular mechanisms through multi-system integration, equipping you with the precision tools to identify, differentiate, and manage the full spectrum of endocrine pathology.
🎯 Endocrine Pathology: The Hormone Detective's Arsenal
🏗️ The Endocrine Foundation: Architectural Blueprints
📌 Remember: FLAT PiG for major endocrine glands - Follicles (thyroid), Langerhans (pancreas), Adrenal, Testes/ovaries, Pituitary, islets, Gonads. Each gland has 3-5 distinct hormone products with different half-lives ranging from 3 minutes (insulin) to 7 days (T4).
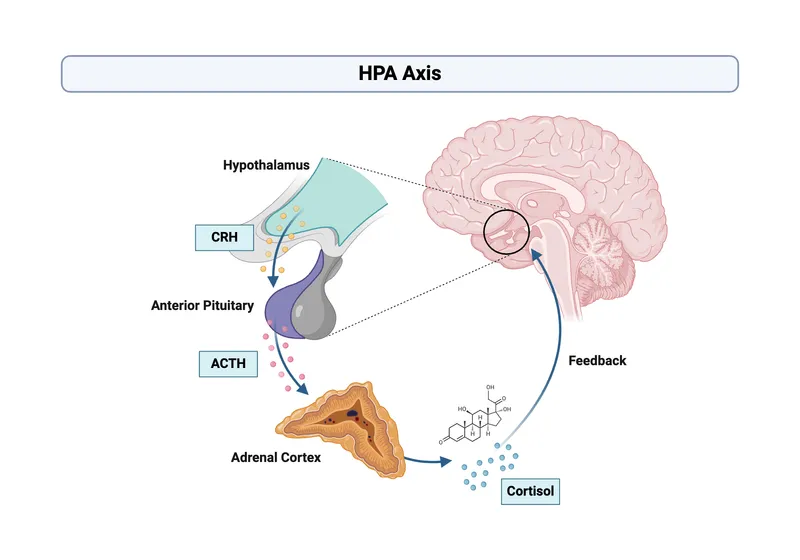
Hormonal Communication Hierarchy
-
Hypothalamic Level (Command Center)
- Releasing hormones: 6 major types with 2-20 minute half-lives
- Inhibiting factors: 3 primary with circadian variation of ±40%
- Portal circulation: 10-fold concentration advantage over systemic
- TRH: 2-4 minute half-life, 200-400 pg/mL portal levels
- CRH: 60 minute half-life, 10-50 pg/mL systemic levels
- GHRH: 7 minute half-life, pulsatile release every 90-120 minutes
-
Pituitary Level (Signal Amplifier)
- Anterior: 6 major hormones with 10-100x amplification
- Posterior: 2 hormones with direct neural control
- Feedback sensitivity: ±5% change triggers ±50% response
- TSH: 60 minute half-life, normal 0.4-4.0 mIU/L
- ACTH: 8 minute half-life, normal 10-60 pg/mL
- GH: 20 minute half-life, pulsatile with 8-10 peaks/day
| Gland System | Primary Hormones | Half-Life Range | Normal Levels | Clinical Threshold |
|---|---|---|---|---|
| Thyroid | T4, T3, Calcitonin | 7d, 1d, 5min | 4.5-12 μg/dL, 80-200 ng/dL | ±20% from baseline |
| Adrenal Cortex | Cortisol, Aldosterone | 90min, 20min | 5-25 μg/dL, 5-30 ng/dL | ±30% diurnal variation |
| Pancreas | Insulin, Glucagon | 5min, 3min | 5-25 μU/mL, 50-200 pg/mL | 2x baseline response |
| Parathyroid | PTH, Vitamin D | 4min, 15h | 10-65 pg/mL, 30-100 ng/mL | ±10% calcium change |
| Gonads | Testosterone, Estradiol | 10min, 36h | 300-1000 ng/dL, 30-400 pg/mL | Age-dependent decline |
💡 Master This: Feedback loop sensitivity explains why small primary gland changes cause large secondary effects. A 20% decrease in thyroid function triggers 300-500% increase in TSH, making TSH the most sensitive thyroid function marker.
The endocrine foundation operates through negative feedback loops with logarithmic sensitivity, where small percentage changes in target hormones trigger exponential responses in regulatory hormones. This amplification system enables precise homeostasis but creates diagnostic challenges when multiple loops interact.
Understanding hormone kinetics and feedback dynamics provides the framework for interpreting complex endocrine presentations and predicting treatment responses across all gland systems.
🏗️ The Endocrine Foundation: Architectural Blueprints
⚙️ Hormonal Machinery: The Cellular Command Network
📌 Remember: CAMP RAGE for rapid hormone mechanisms - CAMP, Adenylyl cyclase, Membrane receptors, Protein kinase A, Rapid response, Activation cascade, G-protein coupled, Effects in seconds to minutes. Nuclear receptors take 30-120 minutes for initial gene transcription.
Hormone Receptor Classifications
-
Membrane Receptors (Rapid Response)
- G-protein coupled: 85% of hormone receptors, millisecond binding
- Tyrosine kinase: insulin family, autophosphorylation in seconds
- Ion channels: direct electrical effects, microsecond responses
- cAMP pathway: TSH, ACTH, glucagon - 10-fold amplification per step
- IP3/DAG pathway: TRH, GnRH - calcium mobilization in <5 seconds
- Insulin receptor: tyrosine kinase with >100 substrate proteins
-
Nuclear Receptors (Genomic Response)
- Steroid family: cortisol, aldosterone, sex hormones - 2-6 hour onset
- Thyroid family: T3, T4 - 12-48 hour peak effects
- Vitamin D: calcitriol - 24-72 hour calcium responses
- DNA binding: zinc finger domains with nanomolar affinity
- Transcription: 100-1000x change in target gene expression
- Protein synthesis: new enzyme production in 4-24 hours
| Hormone Class | Receptor Type | Response Time | Mechanism | Clinical Onset |
|---|---|---|---|---|
| Catecholamines | GPCR/Adrenergic | Seconds | cAMP/IP3 | Immediate |
| Peptide Hormones | GPCR/Tyrosine Kinase | Minutes | Second messengers | 5-30 minutes |
| Steroid Hormones | Nuclear | Hours | Gene transcription | 2-6 hours |
| Thyroid Hormones | Nuclear | Days | Protein synthesis | 12-48 hours |
| Vitamin D | Nuclear | Days | Calcium transport | 24-72 hours |
Pathological Mechanism Patterns
-
Hormone Excess Syndromes
- Autonomous production: adenomas bypass feedback control
- Ectopic secretion: non-endocrine tumors produce inappropriate hormones
- Receptor hypersensitivity: antibody-mediated stimulation (Graves')
- Cushing's: cortisol >50 μg/dL (normal 5-25 μg/dL)
- Thyrotoxicosis: T3 >400 ng/dL (normal 80-200 ng/dL)
- Hyperaldosteronism: aldosterone >30 ng/dL with suppressed renin
-
Hormone Deficiency Syndromes
- Gland destruction: autoimmune, infectious, infiltrative
- Enzyme defects: congenital biosynthesis errors
- Receptor resistance: genetic or acquired insensitivity
- Addison's: cortisol <5 μg/dL with ACTH >100 pg/mL
- Hypothyroidism: T4 <4.5 μg/dL with TSH >10 mIU/L
- Diabetes insipidus: urine osmolality <300 mOsm/kg despite dehydration
💡 Master This: Hormone resistance syndromes show elevated hormone levels with clinical deficiency symptoms-the receptor defect prevents cellular response despite adequate hormone production. Type 2 diabetes exemplifies this with high insulin but poor glucose control.
The cellular machinery operates through amplification cascades where single hormone molecules trigger thousands of enzymatic reactions. Understanding these mechanisms explains both therapeutic drug targets and why small hormonal changes produce dramatic clinical effects.

⚙️ Hormonal Machinery: The Cellular Command Network
🔧 Pattern Recognition: The Diagnostic Framework
📌 Remember: TIRED SWEATY for hyperthyroid recognition - Tachycardia (>100 bpm), Insomnia, Restlessness, Exophthalmos, Diarrhea, Sweight loss, Warm skin, Enlarged thyroid, Anxiety, Tremor, Young onset. >3 symptoms = 85% sensitivity for hyperthyroidism.
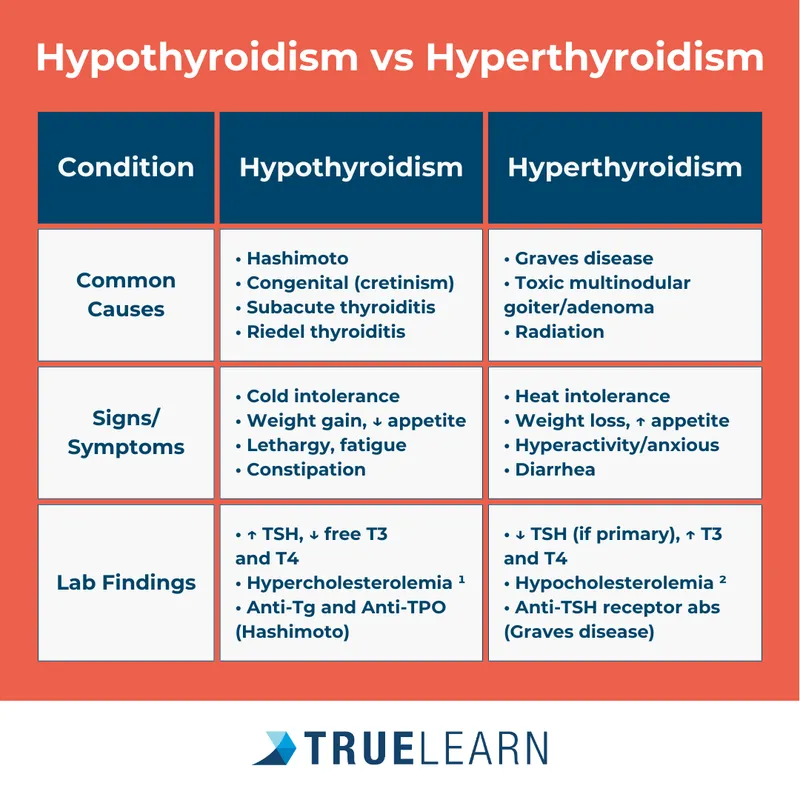
Metabolic Pattern Recognition
-
Hypermetabolic States (Accelerated Physiology)
- Hyperthyroidism: weight loss despite increased appetite
- Pheochromocytoma: paroxysmal hypertension with diaphoresis
- Hypercortisolism: central obesity with muscle wasting
- Heart rate: >100 bpm resting (normal 60-80 bpm)
- Weight loss: >10% in 3 months despite normal intake
- Heat intolerance: sweating at <70°F ambient temperature
- Tremor: fine at 8-12 Hz frequency (normal 4-6 Hz)
-
Hypometabolic States (Slowed Physiology)
- Hypothyroidism: weight gain with cold intolerance
- Adrenal insufficiency: fatigue with hyperpigmentation
- Hypogonadism: decreased libido with muscle loss
- Heart rate: <60 bpm resting (bradycardia)
- Weight gain: >5% in 6 months with decreased appetite
- Cold intolerance: shivering at >75°F ambient temperature
- Reflexes: delayed relaxation phase (>350 ms normal <300 ms)
| Pattern Type | Key Symptoms | Vital Sign Changes | Lab Clues | Time Course |
|---|---|---|---|---|
| Hypermetabolic | Weight loss, heat intolerance | HR >100, BP elevated | Low TSH, high T3/T4 | Weeks to months |
| Hypometabolic | Weight gain, cold intolerance | HR <60, BP low | High TSH, low T3/T4 | Months to years |
| Hypercortisol | Central obesity, striae | HTN, hyperglycemia | High cortisol, low K+ | Months to years |
| Hypocortisol | Fatigue, hyperpigmentation | Hypotension, hyponatremia | Low cortisol, high ACTH | Acute or chronic |
| Hyperparathyroid | Kidney stones, bone pain | HTN, arrhythmias | High Ca++, high PTH | Years |
Diagnostic Testing Strategies
-
Screening Tests (High Sensitivity)
- TSH: 99% sensitivity for thyroid dysfunction
- Morning cortisol: 95% sensitivity for adrenal insufficiency
- HbA1c: >6.5% diagnostic for diabetes mellitus
- TSH range: 0.4-4.0 mIU/L (varies by age and pregnancy)
- Cortisol: >18 μg/dL at 8 AM rules out insufficiency
- Random glucose: >200 mg/dL with symptoms = diabetes
-
Confirmatory Tests (High Specificity)
- Dexamethasone suppression: <1.8 μg/dL rules out Cushing's
- Cosyntropin stimulation: <18 μg/dL at 60 minutes = insufficiency
- Oral glucose tolerance: >200 mg/dL at 2 hours = diabetes
- Overnight dexamethasone: 1 mg at 11 PM, check 8 AM cortisol
- ACTH stimulation: 250 μg IV, measure cortisol at 30 and 60 minutes
- 75g glucose load: measure glucose at 0, 30, 60, 90, 120 minutes
💡 Master This: Dynamic testing reveals functional reserve better than static measurements. Stimulation tests detect early insufficiency, while suppression tests identify autonomous hypersecretion. Normal baseline values don't exclude functional disorders.
Pattern recognition transforms scattered symptoms into focused differential diagnoses. Understanding hormonal physiology enables prediction of which symptoms cluster together and which laboratory abnormalities accompany specific clinical presentations.
The diagnostic framework operates through Bayesian reasoning-pre-test probability based on clinical patterns determines post-test probability after laboratory confirmation. Master this approach, and you achieve diagnostic efficiency with minimal testing.
🔧 Pattern Recognition: The Diagnostic Framework
🔍 Differential Mastery: The Systematic Discriminator
📌 Remember: CUSHINGS vs PSEUDO - Cortisol >50 μg/dL, Urine free cortisol >300 μg/24h, Suppression failure with dexamethasone, High-dose suppression distinguishes pituitary vs adrenal, Imaging shows adenoma vs hyperplasia, No suppression = ectopic ACTH, Glucose intolerance, Striae vs Psychiatric medications, Stress response, Ethanol excess, Uncontrolled diabetes, Depression, Obesity alone.
Hypercortisolism Discrimination Matrix
-
Cushing's Disease (Pituitary Adenoma - 80% of cases)
- High-dose dexamethasone: >50% suppression of cortisol
- ACTH levels: elevated (>20 pg/mL)
- Imaging: pituitary adenoma on MRI (>4 mm)
- 24-hour urine cortisol: 300-1000 μg (normal <50 μg)
- Midnight salivary cortisol: >550 ng/dL (normal <100 ng/dL)
- CRH stimulation: >35% ACTH increase from baseline
-
Adrenal Adenoma (Primary Adrenal - 15% of cases)
- High-dose dexamethasone: no suppression (<10%)
- ACTH levels: suppressed (<10 pg/mL)
- Imaging: unilateral adrenal mass (>2 cm)
- Cortisol: >50 μg/dL with circadian rhythm loss
- Aldosterone: often co-secreted (>30 ng/dL)
- Contralateral adrenal: atrophic on CT/MRI
-
Ectopic ACTH (Non-pituitary tumors - 5% of cases)
- High-dose dexamethasone: no suppression
- ACTH levels: markedly elevated (>100 pg/mL)
- Imaging: lung, pancreas, or carcinoid tumor
- Rapid onset: weeks to months (vs years for pituitary)
- Severe hypokalemia: <2.5 mEq/L (vs mild in pituitary)
- Glucose intolerance: >90% of cases (vs 60% in pituitary)
| Cushing's Type | ACTH Level | Dex Suppression | Imaging Finding | Onset Pattern | K+ Level |
|---|---|---|---|---|---|
| Pituitary | 20-100 pg/mL | High-dose: >50% | Pituitary adenoma | Gradual (years) | Normal-low |
| Adrenal | <10 pg/mL | None | Adrenal mass | Moderate (months) | Low-normal |
| Ectopic | >100 pg/mL | None | Lung/pancreas tumor | Rapid (weeks) | Very low |
| Pseudo-Cushing | Normal | Variable | Normal glands | Variable | Normal |
| Cyclic | Variable | Variable | May be normal | Episodic | Variable |
Thyrotoxicosis Discrimination Framework
-
Graves' Disease (Autoimmune - 70% of hyperthyroidism)
- TSI/TRAb: positive in >95% of cases
- Radioiodine uptake: elevated (>35% at 24 hours)
- Ophthalmopathy: present in 50% of cases
- Diffuse goiter: smooth enlargement 2-4x normal size
- Pretibial myxedema: 5-10% of cases
- Thyroid bruit: audible in >80% of cases
-
Toxic Multinodular Goiter (Autonomous - 20% of hyperthyroidism)
- TSI/TRAb: negative
- Radioiodine uptake: heterogeneous with hot and cold areas
- Ophthalmopathy: absent
- Age: typically >50 years (vs <40 for Graves')
- Goiter: nodular with irregular surface
- Cardiac symptoms: more prominent (atrial fibrillation in >60%)
-
Thyroiditis (Inflammatory - 10% of hyperthyroidism)
- TSI/TRAb: negative
- Radioiodine uptake: suppressed (<5% at 24 hours)
- ESR: elevated (>50 mm/hr in subacute)
- Thyroid pain: present in subacute (absent in silent)
- Triphasic course: hyperthyroid → hypothyroid → recovery
- Duration: self-limited over 3-6 months
💡 Master This: Radioiodine uptake is the key discriminator for thyrotoxicosis-high uptake indicates overproduction (Graves', toxic nodules), while low uptake indicates hormone release from damaged tissue (thyroiditis, exogenous thyroid hormone).
Systematic discrimination prevents therapeutic errors-antithyroid drugs are ineffective for thyroiditis but essential for Graves' disease. Surgical planning requires distinguishing between benign adenomas and malignant masses using size thresholds and imaging characteristics.
The discrimination framework operates through hierarchical decision trees where initial tests determine broad categories, followed by specific tests that pinpoint exact diagnoses. This approach minimizes costs while maximizing diagnostic accuracy.
🔍 Differential Mastery: The Systematic Discriminator
⚖️ Treatment Algorithms: Evidence-Based Precision
📌 Remember: START LOW GO SLOW for hormone replacement - Start at 25-50% target dose, Titrate every 4-6 weeks, Assess clinical response, Reach steady state (5 half-lives), Target physiological levels, Low risk of overcorrection, Optimize timing with circadian rhythms, Watch for drug interactions.
Thyroid Hormone Replacement Precision
-
Levothyroxine Dosing Algorithm
- Initial dose: 1.6 μg/kg/day for healthy adults (<65 years)
- Elderly/cardiac: 25-50 μg/day with 25 μg increments every 6-8 weeks
- Target TSH: 0.4-2.5 mIU/L for most patients
- Absorption: 60-80% on empty stomach, 30-40% with food
- Steady state: 6-8 weeks after dose changes
- Drug interactions: calcium, iron, PPI reduce absorption by 30-50%
-
Monitoring Strategy
- Check TSH: 6-8 weeks after dose changes
- Stable patients: annual TSH monitoring
- Pregnancy: monthly monitoring with 30-50% dose increase
- Over-replacement signs: TSH <0.1 mIU/L, tachycardia, tremor
- Under-replacement: TSH >10 mIU/L, persistent symptoms
- Cardiac patients: avoid TSH <0.1 (increases atrial fibrillation risk 3-fold)
-
Special Populations
- Pregnancy: increase dose by 30-50% in first trimester
- Elderly: start 25 μg/day, slower titration (8-12 weeks)
- Post-thyroidectomy: higher doses (2.2-2.5 μg/kg/day)
- TSH targets: <2.5 mIU/L in pregnancy, <0.1 mIU/L for thyroid cancer
- Malabsorption: liquid or IV formulations for consistent absorption
| Patient Category | Starting Dose | Titration Interval | Target TSH | Special Considerations |
|---|---|---|---|---|
| Healthy Adult | 1.6 μg/kg/day | 6-8 weeks | 0.4-2.5 mIU/L | Full replacement dose |
| Elderly/Cardiac | 25-50 μg/day | 8-12 weeks | 0.4-4.0 mIU/L | Slower titration |
| Pregnancy | Pre-pregnancy + 30% | 4 weeks | <2.5 mIU/L | Monthly monitoring |
| Post-surgical | 2.2 μg/kg/day | 6-8 weeks | Variable by indication | Higher doses needed |
| Malabsorption | Liquid/IV form | 4-6 weeks | 0.4-2.5 mIU/L | Alternative formulations |
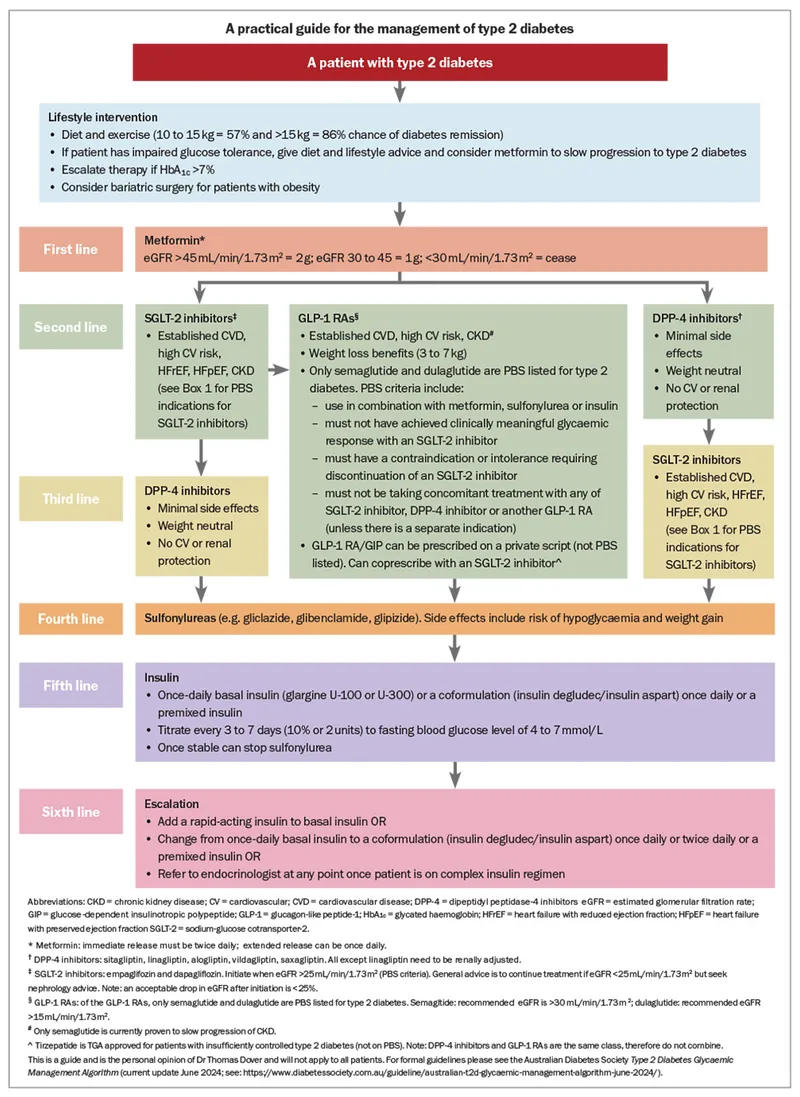
Diabetes Management Algorithms
-
Type 2 Diabetes Treatment Escalation
- First-line: Metformin 500-1000 mg twice daily
- HbA1c >7% at 3 months: add second agent
- HbA1c >8% at 6 months: triple therapy or insulin
- Metformin: weight neutral, 1-2% HbA1c reduction
- SGLT2 inhibitors: 3-7 kg weight loss, cardiovascular benefits
- GLP-1 agonists: 5-10 kg weight loss, 0.5-1.5% HbA1c reduction
-
Insulin Initiation Strategy
- Basal insulin: 10 units or 0.1-0.2 units/kg at bedtime
- Titrate: 2-4 units every 3 days to fasting glucose 80-130 mg/dL
- Prandial insulin: add if HbA1c >7% despite optimal basal
- Basal-bolus ratio: 50:50 for most patients
- Correction factor: 1800 rule (1800 ÷ total daily insulin)
- Carbohydrate ratio: 500 rule (500 ÷ total daily insulin)
💡 Master This: Individualized HbA1c targets prevent hypoglycemia while optimizing outcomes-<7% for healthy adults, <8% for elderly or multiple comorbidities, <6.5% for newly diagnosed without hypoglycemia risk.
Treatment algorithms operate through iterative optimization-initial therapy based on disease severity, followed by systematic adjustments based on response monitoring and adverse effect profiles. This approach achieves target goals in >80% of patients within 6-12 months.
Understanding pharmacokinetic principles enables prediction of dose requirements and timing optimization for maximum therapeutic benefit with minimal side effects.
⚖️ Treatment Algorithms: Evidence-Based Precision
🔗 Multi-System Integration: The Endocrine Network
📌 Remember: METABOLIC SYMPHONY for hormone interactions - Metabolism (thyroid-insulin), Electrolytes (aldosterone-PTH), Timing (cortisol-GH), Appetite (leptin-ghrelin), Blood pressure (renin-aldosterone), Ovulation (LH-FSH), Libido (testosterone-estrogen), Immunity (cortisol-thyroid), Calcium (PTH-vitamin D).
Hypothalamic-Pituitary Integration Networks
-
Stress Response Coordination
- HPA axis: cortisol suppresses TSH, GH, gonadotropins
- Sympathetic activation: catecholamines stimulate glucagon, inhibit insulin
- Metabolic shifts: cortisol promotes gluconeogenesis, protein catabolism
- Cortisol peak: 8 AM (15-25 μg/dL) suppresses other hormones by 30-50%
- Chronic stress: sustained cortisol causes secondary hypogonadism
- Recovery phase: cortisol normalization allows hormone axis recovery
-
Growth-Metabolism Integration
- GH-IGF-1 axis: anabolic effects require adequate thyroid and insulin
- Thyroid hormones: permissive for GH action and IGF-1 production
- Insulin: required for protein synthesis and growth responses
- GH deficiency: reduces IGF-1 by >50%, impairs growth velocity
- Hypothyroidism: blunts GH response to stimulation by 40-60%
- Diabetes: poor control reduces IGF-1 despite normal GH
-
Reproductive-Metabolic Coupling
- Energy balance: leptin signals nutritional status to reproductive axis
- Thyroid function: required for normal ovulation and spermatogenesis
- Insulin resistance: disrupts LH pulsatility and sex hormone binding
- PCOS: insulin resistance increases androgen production by 2-3 fold
- Hypothyroidism: prolactin elevation suppresses gonadotropins
- Weight loss: >10% body weight restores reproductive function
| Integration Network | Primary Hormones | Key Interactions | Clinical Consequences | Restoration Time |
|---|---|---|---|---|
| HPA-Thyroid | Cortisol, TSH, T3/T4 | Cortisol suppresses TSH | Secondary hypothyroidism | 3-6 months |
| HPA-Gonadal | Cortisol, LH/FSH | Stress suppresses reproduction | Amenorrhea, low testosterone | 6-12 months |
| Thyroid-Growth | T3/T4, GH, IGF-1 | Thyroid enables GH action | Growth retardation | 6-18 months |
| Insulin-Reproductive | Insulin, LH, Androgens | Insulin resistance increases androgens | PCOS, infertility | 3-9 months |
| Calcium-Bone | PTH, Vitamin D, Calcitonin | Coordinated calcium homeostasis | Bone disease | 6-24 months |
Metabolic Integration Cascades
-
Glucose Homeostasis Network
- Insulin: primary glucose lowering with anabolic effects
- Glucagon: counter-regulatory with hepatic glucose production
- Cortisol: stress response with insulin resistance induction
- Normal glucose: 80-100 mg/dL fasting, <140 mg/dL postprandial
- Hypoglycemia: <70 mg/dL triggers counter-regulatory response
- Stress hyperglycemia: cortisol can double glucose levels
-
Calcium-Phosphate Integration
- PTH: primary regulator with bone, kidney, intestinal effects
- Vitamin D: intestinal absorption and bone mineralization
- Calcitonin: bone protection during calcium excess
- Normal calcium: 8.5-10.5 mg/dL (ionized 4.5-5.5 mg/dL)
- PTH response: inverse relationship with calcium levels
- Vitamin D deficiency: secondary hyperparathyroidism in >60%
-
Fluid-Electrolyte Coordination
- ADH: water retention with osmolality regulation
- Aldosterone: sodium retention with potassium excretion
- ANP/BNP: natriuresis and vasodilation during volume excess
- Normal osmolality: 280-295 mOsm/kg with tight regulation
- Volume depletion: ADH and aldosterone work synergistically
- Heart failure: elevated BNP (>400 pg/mL) indicates volume overload
💡 Master This: Endocrine network failure creates cascading dysfunction-primary adrenal insufficiency leads to secondary hypothyroidism through reduced cortisol, which impairs thyroid hormone action and TSH response. Replacement therapy must address cortisol first to restore network function.
Multi-system integration operates through hierarchical control where hypothalamic-pituitary coordination orchestrates peripheral gland function. Understanding these network relationships enables prediction of complex presentations and optimization of multi-hormone replacement strategies.
The integration framework reveals why endocrine disorders rarely occur in isolation and why comprehensive evaluation often identifies multiple hormonal abnormalities requiring coordinated treatment approaches.
🔗 Multi-System Integration: The Endocrine Network
🎯 Clinical Mastery Arsenal: Rapid Reference Framework
📌 Remember: ENDOCRINE EMERGENCY recognition - Extreme vital signs, Neurological changes, Dehydration severe, Osmolality abnormal, Cardiac arrhythmias, Respiratory distress, Infection trigger, Nausea/vomiting, Electrolyte chaos. Any 3+ signs = immediate intervention required.
Critical Threshold Arsenal
-
Life-Threatening Values (Immediate Action Required)
- Glucose: <40 mg/dL or >600 mg/dL with altered mental status
- Sodium: <120 mEq/L or >160 mEq/L with neurological symptoms
- Calcium: <7.0 mg/dL or >14 mg/dL with cardiac effects
- Hypoglycemic coma: IV dextrose 25-50 grams immediately
- DKA: glucose >250 mg/dL, pH <7.3, ketones positive
- Thyroid storm: T3 >400 ng/dL, HR >140, fever >102°F
-
Diagnostic Thresholds (High-Yield Cutoffs)
- TSH: >10 mIU/L = overt hypothyroidism requiring treatment
- Cortisol: <5 μg/dL at 8 AM = adrenal insufficiency likely
- HbA1c: >6.5% = diabetes mellitus diagnosis
- Random glucose: >200 mg/dL + symptoms = diabetes
- Fasting glucose: >126 mg/dL on 2 occasions = diabetes
- OGTT: >200 mg/dL at 2 hours = diabetes
-
Treatment Targets (Optimal Ranges)
- Blood pressure: <130/80 mmHg for diabetes/CKD
- LDL cholesterol: <70 mg/dL for high cardiovascular risk
- HbA1c: <7% for most adults, <8% for elderly/comorbid
- Individualized targets: <6.5% for newly diagnosed, healthy
- Pregnancy: <6% pre-conception, <6.5% during pregnancy
- Avoid: <5.5% due to hypoglycemia risk and mortality increase
| Emergency Condition | Key Values | Immediate Treatment | Monitoring Frequency | Outcome Goals |
|---|---|---|---|---|
| DKA | Glucose >250, pH <7.3 | IV insulin, fluids | Hourly glucose/electrolytes | pH >7.3, gap <12 |
| Thyroid Storm | T3 >400, HR >140 | Beta-blockers, PTU | Q4h vitals | HR <100, temp normal |
| Adrenal Crisis | Cortisol <5, hypotension | IV hydrocortisone 100mg | Q2h vitals | BP stable, Na+ normal |
| Severe Hypoglycemia | Glucose <40, altered MS | IV dextrose 25-50g | Q15min glucose | Glucose >100, MS clear |
| Hypercalcemic Crisis | Ca++ >14, confusion | IV saline, calcitonin | Q6h electrolytes | Ca++ <12, symptoms resolve |
Rapid Diagnostic Sequences
-
Thyroid Function Assessment (3-Step Approach)
- Step 1: TSH (screening test with 99% sensitivity)
- Step 2: Free T4 if TSH abnormal (confirms thyroid dysfunction)
- Step 3: Free T3 if T4 normal but TSH low (T3 toxicosis)
- Normal TSH: 0.4-4.0 mIU/L (rules out thyroid disease in >95%)
- Subclinical hypo: TSH 4.5-10 mIU/L with normal T4
- Subclinical hyper: TSH <0.4 mIU/L with normal T4/T3
-
Adrenal Function Evaluation (Stress-Based Protocol)
- Morning cortisol: >18 μg/dL rules out insufficiency
- Cosyntropin test: if cortisol 5-18 μg/dL or clinical suspicion
- Dexamethasone suppression: if Cushing's suspected
- Normal response: cortisol >18 μg/dL at 60 minutes post-ACTH
- Insufficient response: <18 μg/dL indicates adrenal insufficiency
- Cushing's screening: 1 mg dexamethasone at 11 PM, cortisol <1.8 μg/dL at 8 AM
-
Diabetes Screening Strategy (Risk-Stratified Approach)
- High risk: annual screening with HbA1c or fasting glucose
- Average risk: screening every 3 years starting age 45
- Pregnancy: OGTT at 24-28 weeks gestation
- Risk factors: BMI >25, family history, previous GDM
- Prediabetes: HbA1c 5.7-6.4% or fasting glucose 100-125 mg/dL
- Follow-up: annual screening for prediabetes, lifestyle intervention
💡 Master This: Dynamic testing reveals functional capacity better than static measurements. Stimulation tests detect early insufficiency before baseline abnormalities appear, while suppression tests identify autonomous hypersecretion that escapes normal feedback.
The clinical mastery arsenal operates through pattern automation-repeated application of diagnostic sequences and treatment algorithms creates expert-level performance with reduced cognitive load and improved accuracy under clinical pressure.
Master these rapid-access tools, and you transform complex endocrine presentations into systematic evaluations that consistently achieve optimal diagnostic and therapeutic outcomes.
🎯 Clinical Mastery Arsenal: Rapid Reference Framework
Practice Questions: Endocrine pathology
Test your understanding with these related questions
A 21-year-old woman presents with irregular menses, acne, and increased body hair growth. She says her average menstrual cycle lasts 36 days and states that she has heavy menstrual bleeding. She had her menarche at the age of 13 years. Her blood pressure is 125/80 mm Hg, heart rate is 79/min, respiratory rate is 14/min, and temperature is 36.7°C (98.1°F). Her body weight is 101.0 kg (222.7 lb) and height is 170 cm (5 ft 7 in). Physical examination shows papular acne on her forehead and cheeks. There are dark hairs present on her upper lip, periareolar region, linea alba, and hips, as well as darkening of the skin on the axilla and posterior neck. Which of the following endocrine abnormalities would also most likely be found in this patient?













