Dermatopathology

On this page
🔬 The Microscopic Detective: Decoding Skin's Hidden Stories
Dermatopathology transforms you from observer to detective, teaching you to read skin's microscopic language where inflammation patterns, cellular architecture, and pigment behavior reveal diagnoses invisible to the naked eye. You'll master a systematic approach that moves from low-power pattern recognition through high-power cellular analysis, building a mental framework that integrates epidermal changes, dermal structures, and melanocyte behavior into confident diagnostic conclusions that directly guide patient care.
Understanding dermatopathological principles requires mastering the four-layer skin architecture, recognizing inflammatory patterns, and correlating microscopic findings with clinical presentations. This foundation enables rapid pattern recognition across the spectrum of skin diseases.
📌 Remember: EPIDERMIS - Epidermal thickness, Pigmentation patterns, Inflammatory infiltrates, Dermal changes, Epidermal-dermal junction, Rete ridge patterns, Melanocyte distribution, Immune cell types, Subcutaneous involvement
The epidermis consists of 5 distinct layers in thick skin, with the stratum corneum containing 15-20 cell layers and complete turnover occurring every 28 days. Keratinocytes comprise 95% of epidermal cells, while melanocytes represent only 3-5% but produce all skin pigmentation.
- Epidermal Architecture
- Stratum corneum: 10-15 μm thick in thin skin, 100-150 μm in palms/soles
- Stratum granulosum: 2-4 cell layers with keratohyalin granules
- Stratum spinosum: 5-10 cell layers with desmosomes
- Intercellular bridges visible at 400x magnification
- Tonofilaments concentrate around desmosomes
- Stratum basale: Single cell layer with 65% mitotic activity
⭐ Clinical Pearl: Normal epidermal thickness measures 50-100 μm, but increases to 200-300 μm in chronic dermatitis and 400-600 μm in psoriasis, providing quantitative diagnostic criteria.
| Layer | Thickness (μm) | Cell Types | Key Features | Clinical Significance |
|---|---|---|---|---|
| Stratum Corneum | 10-150 | Dead keratinocytes | Barrier function | Hyperkeratosis patterns |
| Stratum Granulosum | 15-25 | Granular cells | Keratohyalin granules | Granular layer changes |
| Stratum Spinosum | 75-150 | Spinous cells | Intercellular bridges | Acantholysis location |
| Stratum Basale | 10-15 | Basal keratinocytes | Mitotic activity | Dysplasia assessment |
| Basement Membrane | 0.1-0.3 | Specialized proteins | Dermal-epidermal junction | Blistering diseases |
💡 Master This: Rete ridge patterns distinguish inflammatory conditions - psoriasis shows regular elongated ridges, lichen planus displays saw-tooth patterns, while chronic dermatitis creates irregular, club-shaped ridges.

Connect epidermal architecture through dermal organization to understand complete tissue relationships and inflammatory response patterns.
🔬 The Microscopic Detective: Decoding Skin's Hidden Stories
🎯 Pattern Recognition Mastery: The Inflammatory Fingerprint System
📌 Remember: SPIV - Spongiotic (eczematous), Psoriasiform (hyperplastic), Interface (lichenoid), Vesiculobullous (blistering) - the four fundamental inflammatory patterns in dermatopathology
Spongiotic dermatitis demonstrates intercellular edema with widened intercellular spaces and inflammatory cell exocytosis. Acute spongiosis shows prominent fluid accumulation, while chronic forms develop acanthosis with irregular rete ridges and dermal fibrosis.
- Spongiotic Pattern Recognition
- Acute phase: Marked intercellular edema with lymphocyte exocytosis
- Subacute phase: Moderate spongiosis with parakeratosis and scale-crust
- Chronic phase: Minimal spongiosis with acanthosis and dermal fibrosis
- Rete ridges become irregularly elongated
- Dermal infiltrate shifts to deeper perivascular distribution
- Eosinophils suggest atopic dermatitis (15-25% of cases)
⭐ Clinical Pearl: Eosinophil count >5 per high-power field in spongiotic dermatitis suggests atopic dermatitis with 85% specificity, while neutrophil predominance indicates contact dermatitis or infection.
Psoriasiform dermatitis features regular acanthosis with uniform rete ridge elongation, confluent parakeratosis, and absent granular layer. The Munro microabscesses (neutrophils in stratum corneum) and Kogoj spongiform pustules (neutrophils in spinous layer) characterize psoriasis vulgaris.
| Pattern Type | Epidermal Changes | Dermal Infiltrate | Key Diagnostic Features | Clinical Correlation |
|---|---|---|---|---|
| Spongiotic | Intercellular edema | Superficial perivascular | Lymphocyte exocytosis | Eczematous dermatitis |
| Psoriasiform | Regular acanthosis | Deep perivascular | Munro microabscesses | Psoriasis spectrum |
| Interface | Basal vacuolization | Band-like infiltrate | Civatte bodies | Lichen planus group |
| Vesiculobullous | Cleavage planes | Variable patterns | Specific split levels | Blistering diseases |
| Granulomatous | Variable changes | Epithelioid cells | Giant cells present | Infectious/inflammatory |
💡 Master This: Band-like infiltrate density correlates with disease activity - dense infiltrates suggest active lichen planus, while sparse infiltrates indicate resolving or drug-induced interface dermatitis.
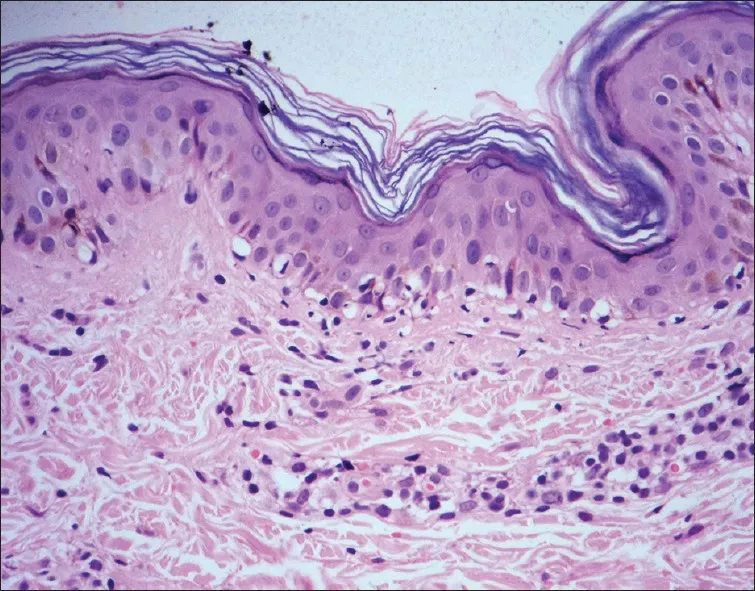
Vesiculobullous patterns depend on cleavage plane location: subcorneal (impetigo), intraepidermal (pemphigus), subepidermal (bullous pemphigoid), or dermal (porphyria cutanea tarda). Acantholysis (loss of intercellular adhesion) characterizes intraepidermal blistering.
- Vesiculobullous Cleavage Planes
- Subcorneal: Staphylococcal scalded skin syndrome, subcorneal pustular dermatosis
- Suprabasal: Pemphigus vulgaris (90% of cases), pemphigus foliaceus
- Subepidermal: Bullous pemphigoid, dermatitis herpetiformis, epidermolysis bullosa
- Eosinophil-rich: Bullous pemphigoid (80% specificity)
- Neutrophil-rich: Dermatitis herpetiformis (95% specificity)
- Pauci-inflammatory: Epidermolysis bullosa or porphyria
⭐ Clinical Pearl: Eosinophil count >20 per high-power field in subepidermal blisters indicates bullous pemphigoid with 90% positive predictive value, while neutrophil microabscesses suggest dermatitis herpetiformis.

Connect inflammatory patterns through cellular infiltrate analysis to understand immune-mediated disease mechanisms and therapeutic targets.
🎯 Pattern Recognition Mastery: The Inflammatory Fingerprint System
🧬 Cellular Architecture Decoded: The Keratinocyte Command Center
📌 Remember: ABCDE of keratinocyte assessment - Architecture, Basal layer integrity, Cellular atypia, Differentiation patterns, Epidermal thickness - systematic evaluation prevents diagnostic errors

Normal keratinocyte maturation demonstrates orderly progression with decreasing cell size, increasing eosinophilia, and nuclear condensation. Basal cells measure 8-12 μm with oval nuclei and prominent nucleoli, while spinous cells reach 15-20 μm with polygonal shapes and intercellular bridges.
- Keratinocyte Differentiation Markers
- Basal layer: Keratin 5/14, p63 positive, high proliferation index
- Spinous layer: Keratin 1/10, involucrin expression, decreased mitosis
- Granular layer: Keratohyalin granules, loricrin accumulation, filaggrin synthesis
- Profilaggrin converts to filaggrin during cornification
- Transglutaminase cross-links cornified envelope proteins
- Lipid lamellae organize barrier lipids between corneocytes
⭐ Clinical Pearl: p63 expression in >50% of suprabasal cells indicates squamous dysplasia, while loss of p63 in basal cells suggests invasive squamous cell carcinoma with 85% sensitivity.
Dysplastic keratinocytes show nuclear enlargement, hyperchromasia, irregular nuclear contours, and loss of polarity. Mild dysplasia affects lower third of epidermis, moderate dysplasia involves lower two-thirds, and severe dysplasia shows full-thickness atypia.
| Dysplasia Grade | Nuclear Features | Architecture | Mitotic Activity | Progression Risk |
|---|---|---|---|---|
| Mild | Slight enlargement | Lower 1/3 involved | Basal layer only | 5-10% over 10 years |
| Moderate | Moderate pleomorphism | Lower 2/3 involved | Parabasal mitoses | 15-25% over 10 years |
| Severe | Marked atypia | Full thickness | Throughout epidermis | 50-75% over 5 years |
| Carcinoma in situ | Severe pleomorphism | Full thickness | Atypical mitoses | 90% without treatment |
| Invasive SCC | Variable atypia | Dermal invasion | High mitotic rate | Metastasis potential |
💡 Master This: Mitotic figures above basal layer indicate dysplasia or malignancy - count mitoses per 10 high-power fields to grade squamous cell carcinoma aggressiveness and predict metastatic potential.
Squamous cell carcinoma demonstrates invasive growth with keratin pearl formation, individual cell keratinization, and desmoplastic stromal response. Well-differentiated SCC shows obvious keratinization, while poorly differentiated tumors lack squamous features and require immunohistochemistry.
- SCC Grading Criteria
- Well-differentiated: >75% keratinizing cells, keratin pearls, minimal atypia
- Moderately differentiated: 25-75% keratinizing cells, moderate atypia, some pearls
- Poorly differentiated: <25% keratinizing cells, marked atypia, rare pearls
- High-risk features: >2 mm depth, perineural invasion, poor differentiation
- Metastatic risk: Well-differentiated <5%, poorly differentiated 15-20%
- Immunostains: p40, p63, CK5/6 confirm squamous differentiation
⭐ Clinical Pearl: Perineural invasion in cutaneous SCC increases local recurrence to 25-30% and metastatic risk to 15-20%, requiring wider excision margins and adjuvant radiation.

Connect keratinocyte architecture through melanocyte interactions to understand pigmentary disorders and melanocytic neoplasia in comprehensive skin pathology.
🧬 Cellular Architecture Decoded: The Keratinocyte Command Center
🎨 Melanocyte Mysteries: Decoding Pigment Cell Behavior
📌 Remember: ABCDE of melanocytic assessment - Asymmetry, Border irregularity, Color variation, Diameter >6mm, Evolving characteristics - but histological ABCDE differs: Architecture, Basal proliferation, Cytological atypia, Dermal invasion, Epidermal consumption
Benign melanocytic nevi demonstrate orderly maturation from junctional to compound to intradermal patterns. Junctional nevi show melanocytes in nests at the dermal-epidermal junction, while compound nevi extend into superficial dermis with smaller, less pigmented cells at deeper levels.
- Nevus Maturation Patterns
- Junctional component: Large, pigmented cells in uniform nests
- Superficial dermal: Intermediate-sized cells with moderate pigment
- Deep dermal: Small, non-pigmented cells resembling lymphocytes
- Type A cells: Epithelioid, abundant cytoplasm, superficial dermis
- Type B cells: Lymphocytoid, scant cytoplasm, mid-dermis
- Type C cells: Spindle-shaped, neural features, deep dermis
⭐ Clinical Pearl: Loss of maturation in melanocytic lesions suggests dysplasia or melanoma - uniform cell size throughout dermis occurs in >90% of melanomas versus <5% of benign nevi.
Dysplastic nevi exhibit architectural disorder with bridging between nests, single-cell proliferation, and cytological atypia. Mild dysplasia shows slight architectural disorder, while severe dysplasia demonstrates marked atypia approaching melanoma in situ.
| Melanocytic Lesion | Architecture | Cytology | Maturation | Mitotic Activity |
|---|---|---|---|---|
| Benign nevus | Orderly nests | Uniform cells | Present | Rare, superficial |
| Mild dysplasia | Slight disorder | Mild atypia | Partial | Occasional |
| Moderate dysplasia | Moderate disorder | Moderate atypia | Impaired | Increased |
| Severe dysplasia | Marked disorder | Severe atypia | Absent | Frequent |
| Melanoma in situ | Pagetoid spread | Marked atypia | Absent | Variable |
💡 Master This: Pagetoid spread (melanocytes above basal layer) occurs in >95% of melanomas but <5% of benign nevi - this single feature provides excellent diagnostic discrimination.

Melanoma subtypes show distinct histological patterns: superficial spreading demonstrates pagetoid spread, nodular shows vertical growth without radial phase, lentigo maligna exhibits single-cell proliferation along dermal-epidermal junction, and acral lentiginous displays dendritic melanocytes in acral skin.
- Melanoma Prognostic Factors
- Breslow thickness: Most important prognostic factor
- Mitotic rate: >1 per mm² indicates poor prognosis
- Ulceration: Upstages tumor by one T category
- T1a: ≤1.0 mm, no ulceration, <1 mitosis/mm²
- T1b: ≤1.0 mm, ulcerated or ≥1 mitosis/mm²
- T2a: 1.01-2.0 mm, no ulceration
- T2b: 1.01-2.0 mm, ulcerated
⭐ Clinical Pearl: Breslow thickness predicts 5-year survival: <1.0 mm shows 95-99% survival, 1.0-2.0 mm demonstrates 85-95%, 2.0-4.0 mm indicates 70-85%, and >4.0 mm suggests 50-70% survival rates.
Immunohistochemistry aids melanocytic diagnosis: S-100 stains all melanocytes, Melan-A/MART-1 shows high sensitivity, SOX-10 demonstrates nuclear staining, and HMB-45 preferentially stains junctional and superficial dermal melanocytes.
Connect melanocytic pathology through dermal tumor recognition to understand complete skin neoplasia spectrum and differential diagnosis strategies.
🎨 Melanocyte Mysteries: Decoding Pigment Cell Behavior
🏗️ Dermal Architecture: The Structural Foundation Matrix
📌 Remember: DIVE into dermal assessment - Depth of involvement, Inflammatory pattern, Vascular changes, Elastosis degree - systematic evaluation reveals photoaging, inflammatory conditions, and neoplastic processes

Solar elastosis represents actinic damage with basophilic degeneration of elastic fibers in upper reticular dermis. Mild elastosis shows thin elastic fibers, moderate demonstrates thick, tangled fibers, and severe exhibits massive elastotic material replacing normal collagen.
- Dermal Inflammatory Patterns
- Superficial perivascular: Contact dermatitis, drug eruptions, viral exanthems
- Deep perivascular: Erythema nodosum, cellulitis, panniculitis
- Diffuse dermal: Urticaria, angioedema, systemic sclerosis
- Eosinophil-rich: Drug reactions, parasitic infections, hypereosinophilic syndrome
- Neutrophil-rich: Bacterial infections, Sweet syndrome, pyoderma gangrenosum
- Plasma cell-rich: Syphilis, chronic inflammation, plasmacytoma
⭐ Clinical Pearl: Plasma cell infiltrates in >20% of dermal inflammatory cells suggest secondary syphilis with 85% specificity, requiring serological testing and spirochete identification.
Granulomatous inflammation features epithelioid cells and multinucleated giant cells in organized collections. Sarcoidal granulomas show tight epithelioid clusters without necrosis, while infectious granulomas demonstrate central necrosis and neutrophilic infiltrates.
| Granuloma Type | Epithelioid Cells | Giant Cells | Necrosis | Associated Features |
|---|---|---|---|---|
| Sarcoidal | Tight clusters | Langhans type | Absent | Asteroid bodies |
| Infectious | Loose arrangement | Foreign body type | Present | Neutrophils |
| Foreign body | Scattered | Foreign body type | Variable | Polarizable material |
| Necrobiotic | Palisading | Langhans type | Central | Mucin deposition |
| Suppurative | Rare | Rare | Extensive | Neutrophil abscesses |
💡 Master This: Storiform pattern (cartwheel arrangement) characterizes dermatofibroma and dermatofibrosarcoma protuberans - distinguish using CD34 (positive in DFSP) and Factor XIIIa (positive in dermatofibroma).
Vascular proliferations range from reactive to neoplastic: pyogenic granuloma shows lobular capillary proliferation, cherry angioma demonstrates dilated capillaries, and angiosarcoma exhibits irregular vascular channels with endothelial atypia.
- Vascular Lesion Characteristics
- Hemangioma: Well-formed vessels, endothelial monolayer, no atypia
- Pyogenic granuloma: Lobular architecture, proliferating endothelium, surface ulceration
- Kaposi sarcoma: Spindle cells, slit-like vessels, HHV-8 positive
- Patch stage: Subtle vascular proliferation around existing vessels
- Plaque stage: Nodular proliferation with spindle cell bundles
- Nodular stage: Well-defined nodules with mitotic activity
⭐ Clinical Pearl: HHV-8 immunostaining shows nuclear positivity in >95% of Kaposi sarcoma cases, providing definitive diagnosis and distinguishing from other vascular proliferations.
Panniculitis affects subcutaneous fat with septal or lobular patterns. Septal panniculitis involves fibrous septa between fat lobules, while lobular panniculitis primarily affects fat lobules with secondary septal involvement.

Connect dermal pathology through adnexal tumor recognition to complete comprehensive skin pathology assessment and diagnostic mastery.
🏗️ Dermal Architecture: The Structural Foundation Matrix
🔧 Diagnostic Integration: The Pattern Recognition Engine
📌 Remember: MATCH diagnostic features - Morphology assessment, Architectural patterns, Tissue distribution, Cellular characteristics, Histochemical confirmation - comprehensive integration prevents diagnostic errors
Differential diagnosis frameworks organize morphologically similar conditions into systematic comparisons. Spindle cell lesions include dermatofibroma, neurofibroma, leiomyoma, dermatofibrosarcoma protuberans, and melanoma, each with distinctive immunohistochemical profiles.
- Spindle Cell Lesion Immunoprofile
- Dermatofibroma: Factor XIIIa+, CD68+, CD34-, S-100-
- Neurofibroma: S-100+, CD34+, Factor XIIIa-, CD68-
- Leiomyoma: Smooth muscle actin+, Desmin+, S-100-, CD34-
- DFSP: CD34+, Factor XIIIa-, S-100-, Smooth muscle actin-
- Melanoma: S-100+, Melan-A+, CD34-, Factor XIIIa-
- Fibrosarcoma: Vimentin+, specific markers negative
⭐ Clinical Pearl: CD34 positivity with storiform pattern indicates DFSP with 95% specificity, while Factor XIIIa positivity suggests dermatofibroma with 90% sensitivity.
Small round blue cell tumors require systematic immunohistochemical workup: Merkel cell carcinoma shows CK20+/TTF-1-, metastatic small cell carcinoma demonstrates CK20-/TTF-1+, and lymphoma exhibits leukocyte common antigen positivity.
| Tumor Type | CK20 | TTF-1 | LCA | Synaptophysin | Chromogranin | S-100 |
|---|---|---|---|---|---|---|
| Merkel cell carcinoma | + | - | - | + | + | - |
| Small cell lung cancer | - | + | - | + | + | - |
| Lymphoma | - | - | + | - | - | - |
| Melanoma | - | - | - | - | - | + |
| Ewing sarcoma | - | - | - | - | - | - |
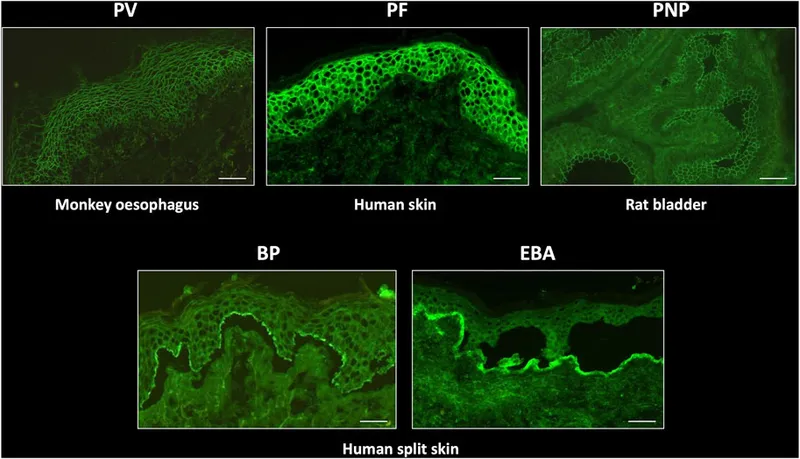
💡 Master This: Direct immunofluorescence patterns provide definitive diagnosis for autoimmune blistering diseases - intercellular staining indicates pemphigus, linear basement membrane suggests pemphigoid, and granular dermal-epidermal junction indicates dermatitis herpetiformis.
Molecular diagnostics enhance traditional morphology: BRAF mutations occur in 50-60% of melanomas, PIK3CA mutations characterize seborrheic keratoses, and PTCH1 mutations define basal cell carcinomas. Next-generation sequencing identifies targetable mutations for precision therapy.
- Molecular Diagnostic Applications
- Melanoma: BRAF, NRAS, KIT mutations guide targeted therapy
- Basal cell carcinoma: PTCH1, SMO mutations predict hedgehog inhibitor response
- Squamous cell carcinoma: TP53, CDKN2A mutations indicate aggressive behavior
- Spitz tumors: ALK, ROS1, NTRK fusions distinguish benign from malignant
- Dermatofibrosarcoma: COL1A1-PDGFB fusion confirms diagnosis
- Sebaceous tumors: Mismatch repair deficiency suggests Muir-Torre syndrome
⭐ Clinical Pearl: Mismatch repair protein loss in sebaceous tumors indicates Lynch syndrome in 25-30% of cases, requiring genetic counseling and cancer screening.
Quality assurance protocols ensure diagnostic accuracy: frozen section correlation, immunohistochemical controls, and molecular validation maintain >95% diagnostic concordance with expert consultation.
Connect diagnostic integration through clinical correlation to achieve complete dermatopathological mastery and optimal patient care.
🔧 Diagnostic Integration: The Pattern Recognition Engine
🎯 Clinical Mastery Arsenal: Your Diagnostic Command Center
Essential diagnostic thresholds provide quantitative anchors for pattern recognition: mitotic rate >1/mm² indicates aggressive melanoma, eosinophils >20/hpf suggest bullous pemphigoid, and plasma cells >20% of infiltrate indicate secondary syphilis.
📌 Remember: RAPID diagnostic mastery - Recognize patterns instantly, Assess key features systematically, Prioritize differential diagnoses, Integrate clinical correlation, Determine definitive diagnosis - your clinical command protocol
- High-Yield Diagnostic Numbers
- Melanoma thickness: <1mm (T1), 1-2mm (T2), 2-4mm (T3), >4mm (T4)
- SCC risk factors: >2mm depth, perineural invasion, poor differentiation
- Inflammatory thresholds: Eosinophils >5/hpf (atopic), >20/hpf (pemphigoid)
- Mitotic activity: >1/mm² (high-risk melanoma), >10/10hpf (malignancy)
- Breslow measurement: Nearest 0.1mm for T1 tumors, 0.2mm for others
- Immunostain interpretation: >50% positivity for diagnostic significance
⭐ Clinical Pearl: Emergency consultation criteria include melanoma >4mm, SCC with perineural invasion, atypical lymphoid infiltrates, and blistering diseases requiring immediate immunofluorescence.
| Clinical Scenario | Key Histological Features | Diagnostic Threshold | Immediate Action |
|---|---|---|---|
| Thick melanoma | Breslow >4mm, ulceration | T4 staging | Sentinel lymph node |
| High-risk SCC | Perineural invasion | Any degree | Wide excision |
| Bullous pemphigoid | Subepidermal blister, eosinophils | >20 eosinophils/hpf | Direct immunofluorescence |
| Cutaneous lymphoma | Atypical lymphoid infiltrate | Monoclonal population | Flow cytometry |
| Merkel cell carcinoma | Small blue cells, CK20+ | Any size | Staging workup |
💡 Master This: The 5-Second Rule - identify primary pattern within 5 seconds of low-power examination: inflammatory vs neoplastic vs infectious determines diagnostic pathway and workup strategy.
Immunohistochemical decision trees optimize stain selection: Spindle cell lesions require S-100, CD34, Factor XIIIa, and smooth muscle actin. Small blue cell tumors need CK20, TTF-1, LCA, and synaptophysin.
- Essential Immunostain Panels
- Melanocytic lesions: S-100, Melan-A, SOX-10, HMB-45
- Epithelial tumors: Pan-cytokeratin, p63, CK5/6, CK7/20
- Mesenchymal tumors: Vimentin, CD34, Factor XIIIa, SMA
- Lymphoid infiltrates: CD3, CD20, CD30, CD68
- Neuroendocrine tumors: Synaptophysin, chromogranin, CD56
- Vascular lesions: CD31, CD34, ERG, D2-40
⭐ Clinical Pearl: SOX-10 demonstrates superior sensitivity for melanoma compared to S-100 (95% vs 85%) and maintains specificity in poorly differentiated tumors.
Clinical correlation protocols ensure diagnostic accuracy: Age-appropriate diagnoses (melanoma rare <20 years), anatomical site considerations (acral melanoma patterns), and clinical presentation (rapid growth suggests malignancy).
Quality metrics maintain diagnostic excellence: Turnaround time <48 hours for routine cases, <24 hours for urgent consultations, >95% diagnostic accuracy on expert review, and <2% amendment rate for final reports.
🎯 Clinical Mastery Arsenal: Your Diagnostic Command Center
Practice Questions: Dermatopathology
Test your understanding with these related questions
A 52-year-old woman presents with erosions in her mouth that are persistent and painful. She says that symptoms appeared gradually 1 week ago and have progressively worsened. She also notes that, several days ago, flaccid blisters appeared on her skin, which almost immediately transformed to erosions as well. Which of the following is the most likely diagnosis?













