Cellular aging mechanisms US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Cellular aging mechanisms. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Cellular aging mechanisms US Medical PG Question 1: An investigator is comparing DNA replication in prokaryotes and eukaryotes. He finds that the entire genome of E. coli (4 × 106 base pairs) is replicated in approximately 30 minutes. A mammalian genome (3 × 109 base pairs) is usually replicated within 3 hours. Which of the following characteristics of eukaryotic DNA replication is the most accurate explanation for this finding?
- A. Replication inhibition at checkpoint
- B. Absence of telomerase enzyme activity
- C. DNA compaction in chromatin
- D. Simultaneous replication at multiple origins (Correct Answer)
- E. More efficient DNA polymerase activity
Cellular aging mechanisms Explanation: ***Simultaneous replication at multiple origins***
- Eukaryotic DNA replication initializes at **multiple origins of replication** along each chromosome, allowing synthesis to occur concurrently in many places.
- This strategy compensates for the much larger eukaryotic genome size, enabling its complete replication within a reasonable timeframe despite slower polymerase speed compared to prokaryotes.
*Replication inhibition at checkpoint*
- **Cell cycle checkpoints**, such as those in G1, S, and G2 phases, ensure the integrity of DNA replication and repair.
- While these checkpoints can *pause* or *inhibit* replication if errors occur, they do not fundamentally explain the *speed* or **efficiency** of replication across the entire genome.
*Absence of telomerase enzyme activity*
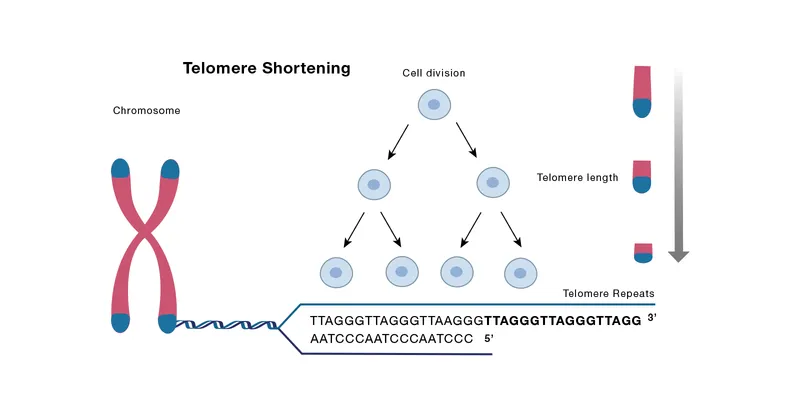
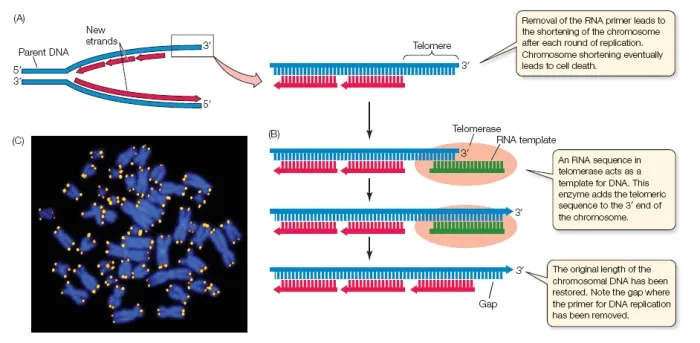
- **Telomerase** is an enzyme that maintains the ends of eukaryotic chromosomes (telomeres) by adding repetitive DNA sequences.
- Its presence or absence is related to telomere length regulation and cellular aging, not the overall speed of genome replication.
*DNA compaction in chromatin*
- Eukaryotic DNA is compact and organized into **chromatin** within the nucleus, which presents a challenge to replication by limiting access to the DNA.
- While enzymes must overcome this compaction, it is a *hindrance* rather than an enabler of replication speed. If anything, it would slow down replication.
*More efficient DNA polymerase activity*
- In actuality, **prokaryotic DNA polymerases** (e.g., DNA Pol III in *E. coli*) are generally more processive and faster than eukaryotic DNA polymerases.
- Therefore, more efficient polymerase activity is not a characteristic that would explain the relatively fast replication of a larger eukaryotic genome.
Cellular aging mechanisms US Medical PG Question 2: A 71-year-old man with colorectal cancer comes to the physician for follow-up examination after undergoing a sigmoid colectomy. The physician recommends adjuvant chemotherapy with an agent that results in single-stranded DNA breaks. This chemotherapeutic agent most likely has an effect on which of the following enzymes?
- A. DNA polymerase III
- B. Topoisomerase I (Correct Answer)
- C. Helicase
- D. Telomerase
- E. Topoisomerase II
Cellular aging mechanisms Explanation: ***Topoisomerase I***
- **Topoisomerase I** creates **single-stranded DNA (ssDNA) breaks** to relieve torsional stress during DNA replication and transcription.
- Many chemotherapeutic agents, such as camptothecin and its derivatives (e.g., irinotecan, topotecan), target topoisomerase I, leading to DNA damage and apoptosis in cancer cells.
*DNA polymerase III*
- **DNA polymerase III** is primarily involved in bacterial DNA replication, synthesizing new DNA strands in a 5' to 3' direction.
- While essential for bacterial survival, it is not the target of chemotherapeutic agents that induce single-stranded DNA breaks in human cells.
*Helicase*
- **Helicase** is responsible for unwinding the DNA double helix during replication and transcription, separating the two strands.
- While its function is critical for DNA processes, it does not directly create DNA breaks as its primary mechanism of action.
*Telomerase*
- **Telomerase** is an enzyme that maintains telomere length at the ends of chromosomes, particularly active in cancer cells.
- Inhibitors of telomerase aim to shorten telomeres, leading to cellular senescence or apoptosis, but they do not primarily cause single-stranded DNA breaks.
*Topoisomerase II*
- **Topoisomerase II** creates **double-stranded DNA (dsDNA) breaks** to untangle and decatenate DNA.
- Though also a target for chemotherapy (e.g., etoposide, doxorubicin), its mechanism involves double-stranded breaks, not single-stranded breaks as specified in the question.
Cellular aging mechanisms US Medical PG Question 3: A 3-year-old male child is found to have a disease involving DNA repair. Specifically, he is found to have a defect in the endonucleases involved in the nucleotide excision repair of pyrimidine dimers. Which of the following is a unique late-stage complication of this child's disease?
- A. Telangiectasia
- B. Colorectal cancer
- C. Malignant melanoma (Correct Answer)
- D. Lymphomas
- E. Endometrial cancer
Cellular aging mechanisms Explanation: **Malignant melanoma**
- The described condition is **xeroderma pigmentosum**, an autosomal recessive disorder characterized by a defect in **nucleotide excision repair (NER)**, specifically the inability to remove **pyrimidine dimers** caused by **UV radiation**.
- This severely impaired DNA repair leads to an extreme predisposition to **UV-induced skin cancers**, including basal cell carcinomas, squamous cell carcinomas, and, most aggressively, **malignant melanoma**, which is a unique and life-threatening late-stage complication.
*Telangiectasia*
- **Telangiectasias** are dilated small blood vessels that appear on the skin or mucous membranes and can be associated with various conditions.
- While skin abnormalities are prevalent in xeroderma pigmentosum due to sun damage, **melanoma** is a more specific and severe late-stage complication directly resulting from the DNA repair defect.
*Colorectal cancer*
- **Colorectal cancer** is typically associated with other DNA repair defects, such as those in the **mismatch repair system**, as seen in conditions like **Lynch syndrome**.
- It is not a primary or most significant late-stage complication of xeroderma pigmentosum, which is primarily characterized by skin cancers.
*Lymphomas*
- **Lymphomas** are cancers of the lymphatic system, often linked to immune deficiencies or specific genetic translocations.
- While individuals with genetic syndromes can have increased cancer risks, **lymphoma** is not the hallmark late-stage complication of xeroderma pigmentosum; skin cancers are the predominant concern.
*Endometrial cancer*
- **Endometrial cancer** is a gynecological cancer often associated with hormonal factors or genetic predispositions like Lynch syndrome, which involves mismatch repair defects.
- This type of cancer is not a characteristic or unique late-stage complication of xeroderma pigmentosum, whose pathology is centered on **UV-induced DNA damage** and subsequent skin malignancies.
Cellular aging mechanisms US Medical PG Question 4: An investigator is studying the biology of human sperm cells. She isolates spermatogonia obtained on a testicular biopsy from a group of healthy male volunteers. She finds that the DNA of spermatogonia obtained from these men show a large number of TTAGGG sequence repeats. This finding can best be explained by increased activity of an enzyme with which of the following functions?
- A. Ligation of Okazaki fragments
- B. Proofreading of synthesized daughter strands
- C. RNA-dependent synthesis of DNA (Correct Answer)
- D. Production of short RNA sequences
- E. Hemimethylation of DNA strand
Cellular aging mechanisms Explanation: ***RNA-dependent synthesis of DNA***
- The TTAGGG sequence repeats are **telomeric sequences**, which are maintained by **telomerase**, an enzyme that synthesizes DNA from an RNA template.
- **Spermatogonia** are germline stem cells that express high levels of telomerase to maintain telomere length across generations.
*Ligation of Okazaki fragments*
- This function is carried out by **DNA ligase**, which joins discontinuous DNA fragments during replication on the lagging strand.
- This process is essential for general DNA replication but is not specific to the formation or maintenance of telomeric repeats.
*Proofreading of synthesized daughter strands*
- This is a function of **DNA polymerase exonuclease activity**, which corrects errors during DNA replication.
- While important for genetic fidelity, it does not explain the presence or increase of specific TTAGGG repeat sequences at telomeres.
*Production of short RNA sequences*
- This function is performed by **primase**, which synthesizes RNA primers necessary to initiate DNA synthesis during replication.
- These RNA primers are later removed and replaced with DNA, and this process is not directly responsible for generating or extending telomeric repeats.
*Hemimethylation of DNA strand*
- Hemimethylation occurs during **DNA replication** when new DNA strands are unmethylated while parental strands are methylated.
- This phenomenon is involved in DNA repair and gene regulation but is unrelated to the synthesis or regulation of telomeric sequences.
Cellular aging mechanisms US Medical PG Question 5: A researcher is tracing the fate of C-peptide, a product of preproinsulin cleavage. Which of the following is a true statement regarding the fate of C-peptide?
- A. C-peptide exits the cells via a protein channel
- B. C-peptide is further cleaved into insulin
- C. C-peptide is packaged with insulin in secretory vesicles (Correct Answer)
- D. C-peptide is immediately degraded by the proteasome
- E. C-peptide activates an intracellular signaling cascade
Cellular aging mechanisms Explanation: ***C-peptide is packaged with insulin in secretory vesicles***
- Preproinsulin is cleaved in the **endoplasmic reticulum** to proinsulin (signal peptide removal), which is then transported to the **Golgi apparatus**.
- In the Golgi, proinsulin is cleaved by **prohormone convertases** into **insulin** and **C-peptide**, and both are stored together in **secretory vesicles** within the pancreatic beta cells.
- Upon stimulation, both insulin and C-peptide are **co-secreted** via exocytosis in equimolar amounts, making C-peptide a useful marker of endogenous insulin secretion.
*C-peptide exits the cells via a protein channel*
- C-peptide exits the beta cells via **exocytosis** of secretory granules, not through specific protein channels.
- It is **co-secreted with insulin** when secretory vesicles fuse with the plasma membrane.
- Its presence in the bloodstream in equimolar amounts with insulin makes it an indirect measure of **insulin secretion**.
*C-peptide is further cleaved into insulin*
- **C-peptide** is a product of proinsulin cleavage, alongside insulin; it is not further processed into insulin.
- Insulin itself is composed of two **peptide chains (A and B)** linked by disulfide bonds, formed after C-peptide is removed from proinsulin.
*C-peptide is immediately degraded by the proteasome*
- C-peptide is not immediately degraded by the **proteasome** upon synthesis.
- After secretion, it circulates in the blood with a **longer half-life** than insulin (approximately 30 minutes versus 4-6 minutes), allowing it to be a useful marker of endogenous insulin production.
- Its degradation occurs primarily in the **kidney**.
*C-peptide activates an intracellular signaling cascade*
- While there is some research suggesting C-peptide may have independent **biological activity** and activate certain signaling pathways extracellularly, its primary role in the context of the insulin synthesis pathway is as a **byproduct** of proinsulin processing.
- Its clinical utility is primarily as a **biomarker** of endogenous insulin secretion, particularly useful in distinguishing between endogenous and exogenous insulin in diabetic patients.
Cellular aging mechanisms US Medical PG Question 6: A 10-month-old boy is brought to the physician by his mother for evaluation of abnormal growth and skin abnormalities. His mother has also noticed that his eyes do not fully close when sleeping. He is at the 24th percentile for height, 17th percentile for weight, and 29th percentile for head circumference. Physical examination shows wrinkled skin, prominent veins on the scalp and extremities, and circumoral cyanosis. Genetic testing shows a point mutation in a gene that encodes for a scaffold protein of the inner nuclear membrane. The mutation causes a deformed and unstable nuclear membrane, which leads to premature aging. Which of the following is most likely to be the defective protein?
- A. Vimentin
- B. Lamin (Correct Answer)
- C. Plectin
- D. Nesprin
- E. Desmin
Cellular aging mechanisms Explanation: ***Lamin***
- The clinical presentation with **accelerated aging** symptoms (wrinkled skin, prominent veins, abnormal growth percentiles, lagophthalmos/difficulty closing eyes) combined with a defect in a **scaffold protein** of the **inner nuclear membrane** is diagnostic of **Hutchinson-Gilford Progeria Syndrome (HGPS)**.
- **Lamins** (specifically Lamin A/C) are intermediate filaments that form the **nuclear lamina**, the primary structural scaffold underlying the inner nuclear membrane, and mutations in the **LMNA gene** cause progeria and other laminopathies.
- The mutation typically produces progerin, an abnormal lamin protein that destabilizes the nuclear envelope leading to premature cellular senescence.
*Vimentin*
- **Vimentin** is an intermediate filament primarily found in **mesenchymal cells** and plays a role in cell shape, integrity, and motility within the **cytoplasm**.
- Defects in vimentin are not associated with disorders of the nuclear membrane or premature aging syndromes.
*Plectin*
- **Plectin** is a **cytoskeletal linker protein** that cross-links intermediate filaments to each other, to microtubules, and to actin filaments, reinforcing cellular stability.
- While important for cellular integrity, plectin is a **cytoplasmic protein**, not a component of the inner nuclear membrane scaffold.
*Nesprin*
- **Nesprins** (Nuclear Envelope Spectrin-repeat Proteins) are components of the **Linker of Nucleoskeleton and Cytoskeleton (LINC) complex**, bridging the nuclear lamina to the cytoskeleton at the **outer nuclear membrane**.
- While nesprins interact with the nuclear envelope, they are not the primary scaffold protein of the **inner nuclear membrane** itself (that role belongs to lamins), and mutations in nesprins are associated with muscular dystrophies, not progeria.
*Desmin*
- **Desmin** is an intermediate filament found predominantly in **muscle cells** (cardiac, skeletal, and smooth muscle), forming a scaffold that connects myofibrils to each other and to the sarcolemma.
- Mutations in desmin are associated with **myopathies** and **cardiomyopathies**, not with defects in the inner nuclear membrane or premature aging.
Cellular aging mechanisms US Medical PG Question 7: A 59-year-old man is brought to the physician by his wife for a psychiatric evaluation. Over the past 12 months, his behavior has become increasingly disruptive. His wife no longer brings him along shopping because he has attempted to grope a female cashier on 2 occasions. He has begun to address the mail carrier using a racial epithet. Three years later, the patient dies. Light microscopy of sections of the frontal and temporal lobes shows intracellular inclusions of transactive response DNA binding protein (TDP-43). These proteins are bound to a regulatory molecule that usually marks them for degradation. The regulatory molecule in question is most likely which of the following?
- A. Kinesin
- B. Chaperone
- C. Cyclin
- D. Ubiquitin (Correct Answer)
- E. Clathrin
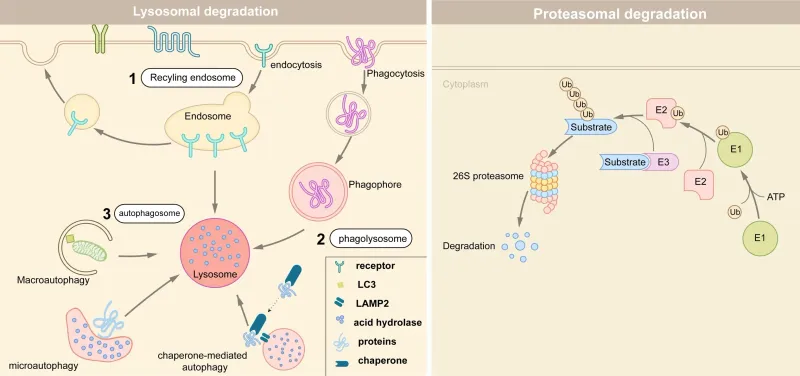
Cellular aging mechanisms Explanation: ***Ubiquitin***
- **Ubiquitin** is a small regulatory protein that marks other proteins for degradation, typically by the **proteasome**. In neurodegenerative diseases like **frontotemporal dementia (FTD)**, aggregates of misfolded proteins, such as **TDP-43**, can accumulate when the ubiquitin-proteasome system is overwhelmed or dysfunctional.
- The patient's clinical presentation of **behavioral changes** (disruptive, inappropriate, racial epithets) and the pathological finding of **TDP-43 inclusions** in the frontal and temporal lobes are highly characteristic of **FTD**. The accumulation of TDP-43, despite being marked for degradation, points to a failure of the normal ubiquitin-mediated protein disposal pathway.
*Kinesin*
- **Kinesin** is a motor protein that facilitates **anterograde axonal transport**, moving cargo away from the cell body along microtubules.
- While important for neuronal function, kinesin is not directly involved in marking proteins for degradation.
*Chaperone*
- **Chaperones** are proteins that assist in the proper **folding of other proteins** and can help refold misfolded proteins, preventing aggregation.
- While chaperones play a role in protein quality control, they do not directly mark proteins for degradation in the same way as ubiquitin.
*Cyclin*
- **Cyclins** are a family of proteins that regulate the progression of cells through the **cell cycle** by activating cyclin-dependent kinases (CDKs).
- They are primarily involved in cell division and growth, not protein degradation pathways.
*Clathrin*
- **Clathrin** is a protein that plays a key role in the formation of **coated vesicles** involved in endocytosis and intracellular trafficking.
- It is crucial for forming vesicles that transport cargo, but it is not directly involved in marking proteins for degradation.
Cellular aging mechanisms US Medical PG Question 8: A 67-year-old man comes to the physician for a follow-up examination after he was diagnosed with mantle cell lymphoma. The physician recommends a chemotherapeutic regimen containing bortezomib. Which of the following best describes the effect of this drug?
- A. Crosslinking of purine bases
- B. Preventing the relaxation of DNA supercoils
- C. Inhibition of tyrosine kinase receptors
- D. Accumulation of ubiquitinated proteins (Correct Answer)
- E. Stabilization of tubulin polymers
Cellular aging mechanisms Explanation: ***Accumulation of ubiquitinated proteins***
- **Bortezomib** is a **proteasome inhibitor**, specifically targeting the 26S proteasome, which is responsible for degrading ubiquitinated proteins.
- Its inhibition leads to the accumulation of various **ubiquitinated proteins**, including pro-apoptotic factors, ultimately inducing **apoptosis** in cancer cells.
*Crosslinking of purine bases*
- This mechanism is characteristic of **alkylating agents** such as cyclophosphamide or cisplatin, which form covalent bonds with DNA, preventing replication and transcription.
- **Bortezomib** does not directly crosslink DNA bases; its primary action is on protein degradation pathways.
*Preventing the relaxation of DNA supercoils*
- This describes the mechanism of **topoisomerase inhibitors**, such as etoposide (topoisomerase II) or irinotecan (topoisomerase I), which block DNA replication and repair.
- Bortezomib has a distinct mechanism involving proteasome inhibition, not direct interaction with DNA or topoisomerases.
*Inhibition of tyrosine kinase receptors*
- This is the action of **tyrosine kinase inhibitors**, a class of drugs like imatinib or gefitinib, that target specific signaling pathways involved in cell growth and proliferation.
- Bortezomib's anti-cancer effects are mediated through protein degradation pathways, not by inhibiting receptor tyrosine kinases.
*Stabilization of tubulin polymers*
- This mechanism is characteristic of **taxanes** (e.g., paclitaxel), which hyperstabilize microtubules, interfering with cell division.
- **Bortezomib** does not affect microtubule dynamics; its action is focused on the proteasomal degradation system.
Cellular aging mechanisms US Medical PG Question 9: An investigator is studying DNA repair processes in an experimental animal. The investigator inactivates a gene encoding a protein that physiologically excises nucleotides from damaged, bulky, helix-distorting DNA strands. A patient with a similar defect in this gene is most likely to present with which of the following findings?
- A. Ataxic gait and facial telangiectasias
- B. Malignant breast and ovarian growths
- C. Leukocoria and a painful bone mass
- D. Colorectal and endometrial cancers
- E. Dry skin and increased photosensitivity (Correct Answer)
Cellular aging mechanisms Explanation: ***Dry skin and increased photosensitivity***
- The description of excising **nucleotides from damaged, bulky, helix-distorting DNA strands** points to a defect in **Nucleotide Excision Repair (NER)**.
- Patients with defects in NER, such as those with **xeroderma pigmentosum**, are highly susceptible to UV-induced DNA damage, leading to **dry skin, increased photosensitivity**, and a high risk of skin cancers.
*Ataxic gait and facial telangiectasias*
- This constellation of symptoms is characteristic of **ataxia-telangiectasia**, a disorder caused by mutations in the **ATM gene**, which is involved in **DNA double-strand break repair**.
- While a DNA repair defect, it's not primarily linked to the excision of bulky, helix-distorting DNA strands.
*Malignant breast and ovarian growths*
- These cancers are commonly associated with mutations in the **BRCA1 and BRCA2 genes**, which play crucial roles in **homologous recombination repair of DNA double-strand breaks**.
- This type of repair is distinct from the excision of bulky, helix-distorting DNA strands described in the question.
*Leukocoria and a painful bone mass*
- **Leukocoria** can indicate **retinoblastoma**, linked to mutations in the **RB1 tumor suppressor gene**, which regulates the cell cycle but isn't primarily a DNA repair gene.
- A painful bone mass could suggest **osteosarcoma**, which is sometimes seen in retinoblastoma patients but not directly related to the specific DNA repair defect described.
*Colorectal and endometrial cancers*
- These cancers are hallmarks of **Lynch syndrome (hereditary nonpolyposis colorectal cancer - HNPCC)**, which is caused by defects in **Mismatch Repair (MMR)** genes (e.g., MLH1, MSH2, MSH6, PMS2).
- Mismatch repair corrects errors that arise during DNA replication, which is different from excising bulky, helix-distorting DNA damage.
Cellular aging mechanisms US Medical PG Question 10: A 52-year-old female was found upon mammography to have branching calcifications in the right lower breast. Physical exam revealed a palpable nodularity in the same location. A tissue biopsy was taken from the lesion, and the pathology report diagnosed the lesion as comedocarcinoma. Which of the following histological findings is most likely present in the lesion?
- A. Disordered glandular cells invading the ductal basement membrane
- B. Pleomorphic cells surrounding areas of comedonecrosis (Correct Answer)
- C. Extensive lymphocytic infiltrate
- D. Halo cells in epidermal tissue
- E. Orderly rows of cells surrounding lobules
Cellular aging mechanisms Explanation: ***Pleomorphic cells surrounding areas of comedonecrosis***
- **Comedocarcinoma** specifically refers to a high-grade subtype of **ductal carcinoma in situ (DCIS)** characterized by **central necrosis (comedonecrosis)** surrounded by **pleomorphic epithelial cells**.
- The presence of branching calcifications on mammography is also a classic sign often associated with **comedonecrosis** within the ducts.
*Disordered glandular cells invading the ductal basement membrane*
- This description is characteristic of **invasive ductal carcinoma**, where malignant cells breach the basement membrane and infiltrate surrounding tissues, which is not stated in the diagnosis of comedocarcinoma.
- Comedocarcinoma is a form of **carcinoma in situ**, meaning the cancerous cells are confined within the ductal system and have not yet invaded the basement membrane.
*Extensive lymphocytic infiltrate*
- While immune cell infiltrates can be seen in various cancers, an **extensive lymphocytic infiltrate** is more characteristic of conditions like **medullary carcinoma** of the breast or specific immune responses, not a defining feature of comedocarcinoma.
- It does not directly relate to the characteristic histological appearance of **comedonecrosis** and **pleomorphic cells** seen in comedocarcinoma.
*Halo cells in epidermal tissue*
- **Halo cells** (koilocytes) are characteristic of **human papillomavirus (HPV) infection** and are found in **cervical or anal squamous lesions**, not typically in breast tissue.
- This finding is completely unrelated to breast pathology and specifically to comedocarcinoma.
*Orderly rows of cells surrounding lobules*
- This description is more indicative of **lobular carcinoma in situ (LCIS)** or some benign proliferative lesions, where cellular architecture tends to maintain some order.
- **Comedocarcinoma** involves disordered, pleomorphic cells within ducts, often with central necrosis, and does not form orderly rows surrounding lobules.
More Cellular aging mechanisms US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.