Apoptosis pathways US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Apoptosis pathways. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Apoptosis pathways US Medical PG Question 1: A 78-year-old man dies suddenly from complications of acute kidney failure. An autopsy is performed and microscopic evaluation of the kidneys shows pale, swollen cells in the proximal convoluted tubules. Microscopic evaluation of the liver shows similar findings. Which of the following is the most likely underlying mechanism of these findings?
- A. Double-stranded DNA breakage
- B. Impaired Na+/K+-ATPase pump activity (Correct Answer)
- C. Free radical formation
- D. Cytochrome C release
- E. Cytoplasmic triglyceride accumulation
Apoptosis pathways Explanation: ***Impaired Na+/K+-ATPase pump activity***
- **Acute kidney failure** leads to **hypoxia** and ATP depletion, which impairs the function of the **Na+/K+-ATPase pump** on the cell membrane.
- Failure of this pump results in **intracellular accumulation of sodium** and water, causing **cellular swelling** and pallor as seen in the kidneys and liver.
*Double-stranded DNA breakage*
- This is primarily associated with **apoptosis** or **radiation injury**, which would lead to nuclear fragmentation and cellular death rather than simple cellular swelling.
- While cell death can occur in acute kidney failure, the initial changes described (pale, swollen cells) are characteristic of **reversible cell injury** before extensive DNA damage.
*Free radical formation*
- **Free radical formation** (oxidative stress) can cause cellular injury, but it primarily leads to **lipid peroxidation of membranes** and damage to proteins and DNA, not directly to the widespread intracellular water accumulation described.
- While part of the injury cascade, it's not the most direct mechanism for the initial gross and microscopic findings of swelling.
*Cytochrome C release*
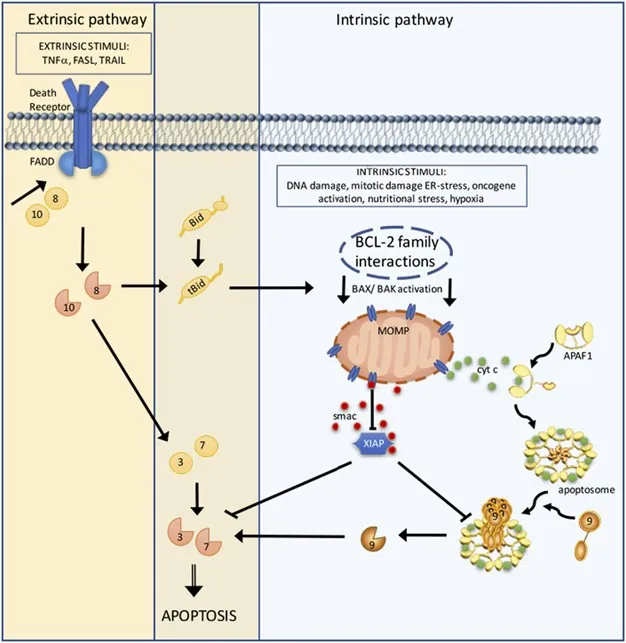
- **Cytochrome C release** from mitochondria is a critical step in the **intrinsic pathway of apoptosis**, leading to programmed cell death.
- The findings described (pale, swollen cells) are more indicative of **reversible cellular injury** or early necrosis, prior to the widespread activation of apoptosis.
*Cytoplasmic triglyceride accumulation*
- **Cytoplasmic triglyceride accumulation** (steatosis or fatty change) is often seen in conditions like **alcoholic liver disease** or **metabolic syndrome**.
- While it can be a sign of cellular injury, it does not directly explain the generalized "pale, swollen cells" observed in both the kidneys and liver following acute kidney failure, which points to water influx.
Apoptosis pathways US Medical PG Question 2: A 44-year-old Caucasian male presents with a fever, recent weight loss, and a cough productive of bloody sputum. A chest X-ray and CT scan were performed, revealing cavities near the apex of his lungs. The patient is started on rifampin, isoniazid, ethambutol and pyrazinamide. Formation of the cavities in the patient's lungs is mainly mediated by:
- A. NK cells
- B. Apoptosis
- C. B-cells
- D. Toxin secretion by the bacterium
- E. TH1 cells (Correct Answer)
Apoptosis pathways Explanation: ***TH1 cells***
- **Mycobacterium tuberculosis** infection primarily involves a **TH1 cell-mediated immune response**, which includes macrophages, epithelioid cells, and giant cells forming **granulomas**.
- The formation of **cavities** in tuberculosis is a result of **caseous necrosis** within these granulomas, driven by the intense destructive effects of TH1-driven inflammation attempting to contain the infection.
*NK cells*
- **Natural killer (NK) cells** play a role in early host defense against intracellular pathogens, including *Mycobacterium tuberculosis*, by producing **interferon-gamma** and directly killing infected cells.
- However, they are not the primary mediators of the extensive tissue destruction and cavitation seen in advanced pulmonary tuberculosis.
*Apoptosis*
- **Apoptosis**, or programmed cell death, plays a complex role in tuberculosis, both in host defense (killing infected cells) and potentially in M. tuberculosis survival mechanisms.
- While apoptosis contributes to cell death within granulomas and necrotic lesions, it is part of a broader immune response, not the main driving force for large-scale tissue cavitation.
*B-cells*
- **B-cells** are involved in the **humoral immune response**, producing antibodies that target extracellular pathogens or toxins.
- While antibodies can be detected in tuberculosis, they do not play a significant role in the cell-mediated immunity required to contain intracellular *M. tuberculosis* infection or in the formation of lung cavities.
*Toxin secretion by the bacterium*
- Unlike many bacterial infections that cause tissue damage through **exotoxins** or **endotoxins**, *Mycobacterium tuberculosis* does not secrete potent toxins that directly cause the extensive cavitary lesions.
- The destruction and cavitation are primarily due to the host's vigorous, but often dysregulated, **cell-mediated immune response**.
Apoptosis pathways US Medical PG Question 3: A researcher is tracing the fate of C-peptide, a product of preproinsulin cleavage. Which of the following is a true statement regarding the fate of C-peptide?
- A. C-peptide exits the cells via a protein channel
- B. C-peptide is further cleaved into insulin
- C. C-peptide is packaged with insulin in secretory vesicles (Correct Answer)
- D. C-peptide is immediately degraded by the proteasome
- E. C-peptide activates an intracellular signaling cascade
Apoptosis pathways Explanation: ***C-peptide is packaged with insulin in secretory vesicles***
- Preproinsulin is cleaved in the **endoplasmic reticulum** to proinsulin (signal peptide removal), which is then transported to the **Golgi apparatus**.
- In the Golgi, proinsulin is cleaved by **prohormone convertases** into **insulin** and **C-peptide**, and both are stored together in **secretory vesicles** within the pancreatic beta cells.
- Upon stimulation, both insulin and C-peptide are **co-secreted** via exocytosis in equimolar amounts, making C-peptide a useful marker of endogenous insulin secretion.
*C-peptide exits the cells via a protein channel*
- C-peptide exits the beta cells via **exocytosis** of secretory granules, not through specific protein channels.
- It is **co-secreted with insulin** when secretory vesicles fuse with the plasma membrane.
- Its presence in the bloodstream in equimolar amounts with insulin makes it an indirect measure of **insulin secretion**.
*C-peptide is further cleaved into insulin*
- **C-peptide** is a product of proinsulin cleavage, alongside insulin; it is not further processed into insulin.
- Insulin itself is composed of two **peptide chains (A and B)** linked by disulfide bonds, formed after C-peptide is removed from proinsulin.
*C-peptide is immediately degraded by the proteasome*
- C-peptide is not immediately degraded by the **proteasome** upon synthesis.
- After secretion, it circulates in the blood with a **longer half-life** than insulin (approximately 30 minutes versus 4-6 minutes), allowing it to be a useful marker of endogenous insulin production.
- Its degradation occurs primarily in the **kidney**.
*C-peptide activates an intracellular signaling cascade*
- While there is some research suggesting C-peptide may have independent **biological activity** and activate certain signaling pathways extracellularly, its primary role in the context of the insulin synthesis pathway is as a **byproduct** of proinsulin processing.
- Its clinical utility is primarily as a **biomarker** of endogenous insulin secretion, particularly useful in distinguishing between endogenous and exogenous insulin in diabetic patients.
Apoptosis pathways US Medical PG Question 4: A 29-year-old woman presents with shortness of breath and chest pain for the past week. She says her chest pain is aggravated by deep breathing and she becomes short of breath while walking upstairs in her home. She also has been feeling feverish and fatigued for the past week, as well as pain in her wrists, hands, and left knee. Review of systems is significant for a 4.5 kg (10.0 lb) weight loss over the previous month. Past medical history consists of 2 spontaneous abortions, both of which occurred in the 1st trimester. On physical examination, there is a pink rash present over her face, which is aggravated by exposure to sunlight. There are decreased breath sounds on the right. A chest radiograph is performed which reveals evidence of a right pleural effusion. Serum ANA and anti-dsDNA autoantibodies are positive. Urinalysis is unremarkable. Errors with which of the following is most likely to lead to her disease?
- A. Intrinsic pathway
- B. Cytotoxic CD8+ T cells
- C. Bcl-2 overexpression
- D. Necrosis
- E. Fas-FasL interaction (Correct Answer)
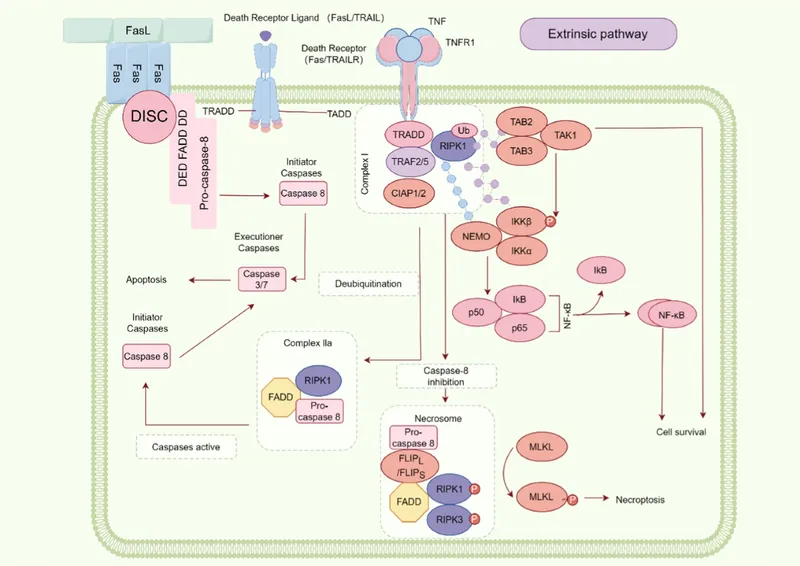
Apoptosis pathways Explanation: ***Fas-FasL interaction***
- This patient presents with multiple symptoms suggestive of **systemic lupus erythematosus (SLE)**, including photosensitive rash, arthritis, serositis (pleural effusion), weight loss, recurrent spontaneous abortions, and positive ANA/anti-dsDNA.
- Genetic defects in the **Fas** or **Fas ligand (FasL) apoptotic pathway** are strongly associated with increased risk of autoimmunity, particularly SLE, as they impair the deletion of autoreactive lymphocytes.
*Intrinsic pathway*
- The intrinsic apoptotic pathway is primarily activated by intracellular stress and mitochondria-dependent signals.
- While essential for cell death, defects in the intrinsic pathway are not as specifically implicated in the pathogenesis of SLE as the Fas-FasL (extrinsic) pathway.
*Cytotoxic CD8+ T cells*
- **CD8+ T cells** are primarily involved in killing virally infected or cancerous cells and are crucial for cellular immunity.
- While involved in some autoimmune processes, their dysfunction is not the primary or most common error leading to the development of SLE, which is largely mediated by autoantibodies.
*Bcl-2 overexpression*
- **Bcl-2** is an anti-apoptotic protein, and its overexpression inhibits apoptosis.
- While Bcl-2 overexpression could theoretically prevent the deletion of autoreactive cells, specific defects in the direct Fas-FasL signaling pathway are more directly and commonly linked to the immune dysregulation seen in SLE.
*Necrosis*
- **Necrosis** is an uncontrolled form of cell death often associated with inflammation and tissue damage.
- While certainly present in tissues affected by SLE due to inflammation, necrosis itself is a consequence of the disease process, not an upstream error in cell death regulation that leads to the autoimmunity of SLE.
Apoptosis pathways US Medical PG Question 5: Researchers are investigating the mechanism of cell apoptosis and host defense in mice. They have observed that mice with certain gene deletions are not able to fight the induced viral infection. They identify a cell that is able to destroy target cells infected with viruses by exocytosis of granule contents, which induces the activation of caspases. Which type of cell is responsible for this process?
- A. CD8+ lymphocytes (Correct Answer)
- B. CD4+ lymphocytes
- C. Macrophages
- D. Neutrophils
- E. Eosinophils
Apoptosis pathways Explanation: ***CD8+ lymphocytes***
- **CD8+ lymphocytes**, or **cytotoxic T lymphocytes (CTLs)**, are specialized to recognize and kill **virus-infected cells** and cancer cells.
- They achieve this by releasing cytotoxic granules containing **perforin** and **granzymes**, which enter the target cell and activate **caspases**, leading to **apoptosis**.
- Note: **Natural killer (NK) cells** also use a similar granule-mediated mechanism, but CD8+ T cells provide **antigen-specific** recognition via MHC class I.
*CD4+ lymphocytes*
- **CD4+ lymphocytes**, or **helper T cells**, primarily coordinate immune responses by secreting **cytokines** and activating other immune cells, rather than directly killing infected cells.
- They are crucial for both humoral and cell-mediated immunity but do not typically induce apoptosis via granule exocytosis.
*Macrophages*
- **Macrophages** are phagocytic cells that engulf and digest pathogens, cellular debris, and foreign substances.
- While they can present antigens and participate in immune responses, their primary role in antiviral defense is **phagocytosing infected cells** and presenting antigens, not inducing apoptosis via granule exocytosis.
*Neutrophils*
- **Neutrophils** are key components of the innate immune system, primarily involved in fighting bacterial infections through **phagocytosis**, degranulation, and formation of **neutrophil extracellular traps (NETs)**.
- They are not specialized for detecting and inducing apoptosis in virus-infected cells.
*Eosinophils*
- **Eosinophils** are primarily involved in the immune response against **parasitic infections** and allergic reactions.
- They release granules containing toxic proteins against parasites and contribute to inflammation, but they do not directly kill virus-infected cells via caspase activation.
Apoptosis pathways US Medical PG Question 6: During an autopsy of a decomposed body, the forensic pathologist notes marbling of the skin, bloating, and a green discoloration of the abdomen. Based on these findings, which of the following best estimates the postmortem interval?
- A. 7-10 days
- B. 1-2 months
- C. 2-3 weeks
- D. 3-5 days (Correct Answer)
Apoptosis pathways Explanation: ***3-5 days***
- The combination of **marbling of the skin**, **bloating**, and **green discoloration of the abdomen** are classic signs of early to moderate putrefaction. These changes typically become evident within **3 to 5 days** postmortem in temperate environments.
- **Green discoloration** of the abdomen is usually the first visible sign of putrefaction, appearing within 24-48 hours, followed by **bloating** due to gas production and then **marbling** as bacterial decomposition spreads through blood vessels.
*7-10 days*
- By **7-10 days**, decomposition would likely be more advanced, with prominent desquamation (**skin slipping**) and potentially the formation of **blisters** filled with putrefactive fluid, which are not explicitly mentioned here.
- While these changes can occur within this timeframe, the observed combination specifically points to an earlier stage than a full week.
*1-2 months*
- At **1-2 months**, the body typically enters the **skeletonization** stage, with significant loss of soft tissues due to insect activity and continued bacteria.
- The findings described (bloating, marbling, green discoloration) represent early putrefactive changes, not the advanced decomposition seen after several weeks or months.
*2-3 weeks*
- By **2-3 weeks**, extensive **bloating** and **tissue liquefaction** would be expected, and the body may begin to show signs of **maggot activity** if exposed to insects.
- The described findings are characteristic of a less advanced stage of decomposition compared to this longer interval.
Apoptosis pathways US Medical PG Question 7: A 40-year-old male presents to his primary care physician for a regularly scheduled check-up. Physical examination reveals nontender cervical lymphadenopathy. A biopsy of the lymph node reveals aggregates of follicular architecture, and cytogenic analysis shows a t(14;18) translocation. The protein most likely responsible for the patient’s condition does which of the following:
- A. Regulates passage through the cell cycle
- B. Activates DNA repair proteins
- C. Regulates cell growth through signal transduction
- D. Inhibits apoptosis (Correct Answer)
- E. Provides mitotic cytoskeleton
Apoptosis pathways Explanation: ***Inhibits apoptosis***
- The t(14;18) translocation is characteristic of **follicular lymphoma** and leads to the overexpression of the **BCL-2 protein**.
- **BCL-2** is an anti-apoptotic protein that prevents programmed cell death, allowing abnormal cells to accumulate.
*Regulates passage through the cell cycle*
- Proteins involved in **cell cycle regulation** (e.g., p53, Rb) control progression through different phases, but BCL-2's primary role is in cell survival, not direct cell cycle progression.
- Dysregulation of cell cycle proteins is seen in many cancers, but the specific BCL-2 translocation primarily affects apoptosis.
*Activates DNA repair proteins*
- **DNA repair proteins** (e.g., ATM, BRCA1/2) are crucial for maintaining genomic integrity and correcting DNA damage.
- While important in cancer development, their activation is not the direct function of the BCL-2 protein overexpressed due to the t(14;18) translocation.
*Regulates cell growth through signal transduction*
- **Signal transduction pathways** often involve growth factors and their receptors (e.g., RTKs) that regulate cell proliferation and differentiation.
- While BCL-2 indirectly impacts cell numbers by preventing apoptosis, its direct role is not in initiating or participating in growth-promoting signal transduction cascades.
*Provides mitotic cytoskeleton*
- The **mitotic cytoskeleton**, composed of microtubules, is essential for chromosome segregation during cell division.
- Proteins like tubulin are the primary components, and BCL-2 has no direct role in forming or organizing these structures.
Apoptosis pathways US Medical PG Question 8: During an experiment, an investigator attempts to determine the rates of apoptosis in various tissue samples. Injecting cytotoxic T cells into the cell culture of one of the samples causes the tissue cells to undergo apoptosis. Apoptosis is most likely due to secretion of which of the following substances in this case?
- A. Cytochrome C
- B. TNF-α
- C. Granzyme B (Correct Answer)
- D. Caspases
- E. Bcl-2
Apoptosis pathways Explanation: ***Granzyme B***
- **Granzyme B** is a serine protease released by **cytotoxic T cells** and **natural killer cells** that directly initiates apoptosis by cleaving and activating caspases within the target cell.
- Upon entry into the target cell, granzyme B activates executioner caspases, such as caspase-3 and caspase-7, leading to the **proteolytic cascade** that dismantles the cell.
*Cytochrome C*
- **Cytochrome c** is a mitochondrial protein that, when released into the cytoplasm, can trigger the **intrinsic pathway of apoptosis** by forming the apoptosome.
- While it's crucial for the intrinsic pathway, cytotoxic T cells primarily induce the **extrinsic pathway** of apoptosis.
*TNF-α*
- **TNF-α (Tumor Necrosis Factor-alpha)** is a cytokine that can induce apoptosis by binding to its receptor (TNFR1), activating adaptor proteins like TRADD and FADD, and subsequently initiating the extrinsic apoptotic pathway.
- However, while TNF-α can induce apoptosis, the scenario specifically mentions **cytotoxic T cells** as the cause, whose primary mechanism involves granzymes and perforin rather than TNF-α secretion.
*Caspases*
- **Caspases** are a family of cysteine proteases that are central to the apoptotic process, acting as both initiator and executioner enzymes.
- They are the *effectors* of apoptosis but are not the direct substances *secreted by cytotoxic T cells* to initiate the process in the target cell.
*Bcl-2*
- **Bcl-2** is an anti-apoptotic protein that inhibits the release of cytochrome c from mitochondria, thereby preventing the activation of the intrinsic pathway of apoptosis.
- It is a regulator *within the target cell* that prevents apoptosis, not a substance secreted by cytotoxic T cells to *induce* it.
Apoptosis pathways US Medical PG Question 9: A 38-year-old man is admitted to the hospital because of fever, yellowing of the skin, and nausea for 1 day. He recently returned from a backpacking trip to Brazil and Paraguay, during which he had a 3-day episode of high fever that resolved spontaneously. Physical examination shows jaundice, epigastric tenderness, and petechiae over his trunk. Five hours after admission, he develops dark brown emesis and anuria. Despite appropriate lifesaving measures, he dies. Postmortem liver biopsy shows eosinophilic degeneration of hepatocytes with condensed nuclear chromatin. This patient’s hepatocytes were most likely undergoing which of the following processes?
- A. Regeneration
- B. Steatosis
- C. Necrosis
- D. Apoptosis (Correct Answer)
- E. Proliferation
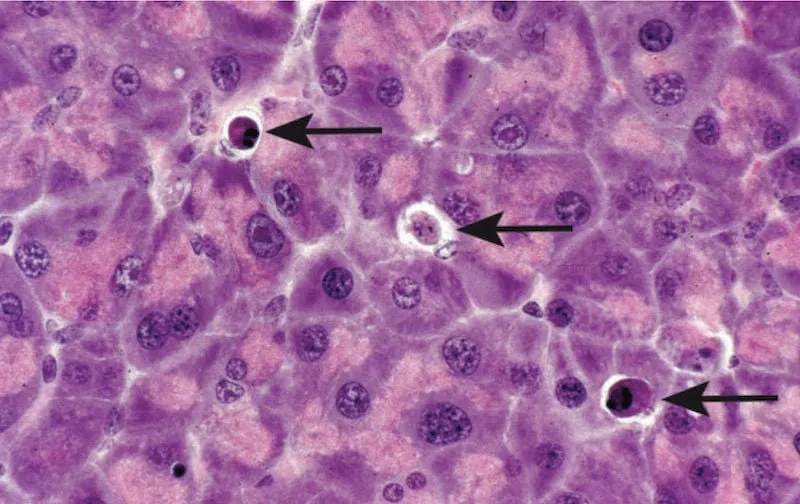
Apoptosis pathways Explanation: ***Apoptosis***
- The patient's symptoms (fever, jaundice, epigastric tenderness, petechiae, dark emesis, anuria) and history of travel to endemic areas are highly suggestive of **Yellow Fever**.
- **Eosinophilic degeneration of hepatocytes with condensed nuclear chromatin**, described as **Councilman bodies** or **apoptotic bodies**, is a characteristic histological finding in Yellow Fever and indicates programmed cell death.
*Regeneration*
- This process involves the replacement of damaged tissue with new, healthy tissue, which would contradict the patient's rapidly deteriorating condition and death.
- While regeneration can occur in the liver, the described histological findings of **eosinophilic degeneration** and **condensed nuclear chromatin** are indicative of cell death, not repair.
*Steatosis*
- **Steatosis** refers to the accumulation of fat droplets within hepatocytes, which is usually seen in conditions like alcoholic liver disease or non-alcoholic fatty liver disease.
- This is not consistent with the eosinophilic degeneration and condensed chromatin described, which point to a different type of cellular injury.
*Necrosis*
- **Necrosis** is a form of unregulated cell death often associated with inflammation and cellular swelling; the description of **eosinophilic degeneration** and **condensed nuclear chromatin** points specifically to apoptotic cell death rather than necrotic changes which would typically include cell swelling and rupture.
- While Yellow Fever does cause significant liver damage leading to cell death, the specific histological features (e.g., Councilman bodies) are characteristic of **apoptosis**, not typically seen in necrosis.
*Proliferation*
- **Proliferation** refers to an increase in the number of cells, typically in response to a stimulus or as part of a disease process like cancer.
- The patient's rapid decline and the histological findings of dying cells (eosinophilic degeneration, condensed chromatin) are antithetical to cellular proliferation.
Apoptosis pathways US Medical PG Question 10: A 37-year-old woman presents to the occupational health clinic for a new employee health screening. She has limited medical records prior to her immigration to the United States several years ago. She denies any current illness or significant medical history. Purified protein derivative (PPD) is injected on the inside of her left forearm for tuberculosis (TB) screening. Approximately 36 hours later, the patient comes back to the occupational health clinic and has an indurated lesion with bordering erythema measuring 15 mm in diameter at the site of PPD injection. Of the following options, which is the mechanism of her reaction?
- A. Type III and IV–mixed immune complex and cell-mediated hypersensitivity reactions
- B. Type III–immune complex-mediated hypersensitivity reaction
- C. Type I–anaphylactic hypersensitivity reaction
- D. Type II–cytotoxic hypersensitivity reaction
- E. Type IV–cell-mediated (delayed) hypersensitivity reaction (Correct Answer)
Apoptosis pathways Explanation: ***Type IV–cell-mediated (delayed) hypersensitivity reaction***
- The **PPD test** for tuberculosis is a classic example of a **Type IV hypersensitivity reaction**, also known as **delayed-type hypersensitivity (DTH)**. This reaction is orchestrated by **T lymphocytes** (specifically CD4+ T cells) that recognize antigens presented by antigen-presenting cells
- The **induration** at 36 hours is a hallmark of this type of reaction, as it typically peaks between **24 to 72 hours** after antigen exposure, reflecting the time required for T cells to migrate to the site and initiate an inflammatory response. The immune response involves the release of **cytokines** leading to macrophage accumulation and localized tissue damage.
*Type III and IV–mixed immune complex and cell-mediated hypersensitivity reactions*
- While immune complexes (Type III) and cell-mediated reactions (Type IV) can both lead to tissue damage, a PPD test is primarily a **cell-mediated response** and is not characterized by significant immune complex deposition.
- Mixed reactions are less common and usually involve a sustained presence of antigen leading to both types of responses, which is not the typical mechanism for an acute PPD skin test.
*Type III–immune complex-mediated hypersensitivity reaction*
- **Type III hypersensitivity** is characterized by the formation of **antigen-antibody immune complexes** that deposit in tissues, leading to inflammation and tissue damage, often seen in conditions like serum sickness or lupus nephritis.
- The PPD reaction is based on T-cell recognition of mycobacterial antigens, not the deposition of soluble antigen-antibody complexes.
*Type I–anaphylactic hypersensitivity reaction*
- **Type I hypersensitivity** is an **immediate allergic reaction** mediated by **IgE antibodies** binding to mast cells and basophils, leading to histamine release upon re-exposure to an allergen.
- This type of reaction typically occurs within minutes of exposure, not 36 hours later, and presents with symptoms like hives, angioedema, or anaphylaxis.
*Type II–cytotoxic hypersensitivity reaction*
- **Type II hypersensitivity** involves **antibodies (IgG or IgM)** binding to antigens on the surface of **host cells**, leading to cell lysis or dysfunction, often seen in transfusion reactions or autoimmune hemolytic anemia.
- The PPD test does not involve direct antibody-mediated destruction of host cells.
More Apoptosis pathways US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.
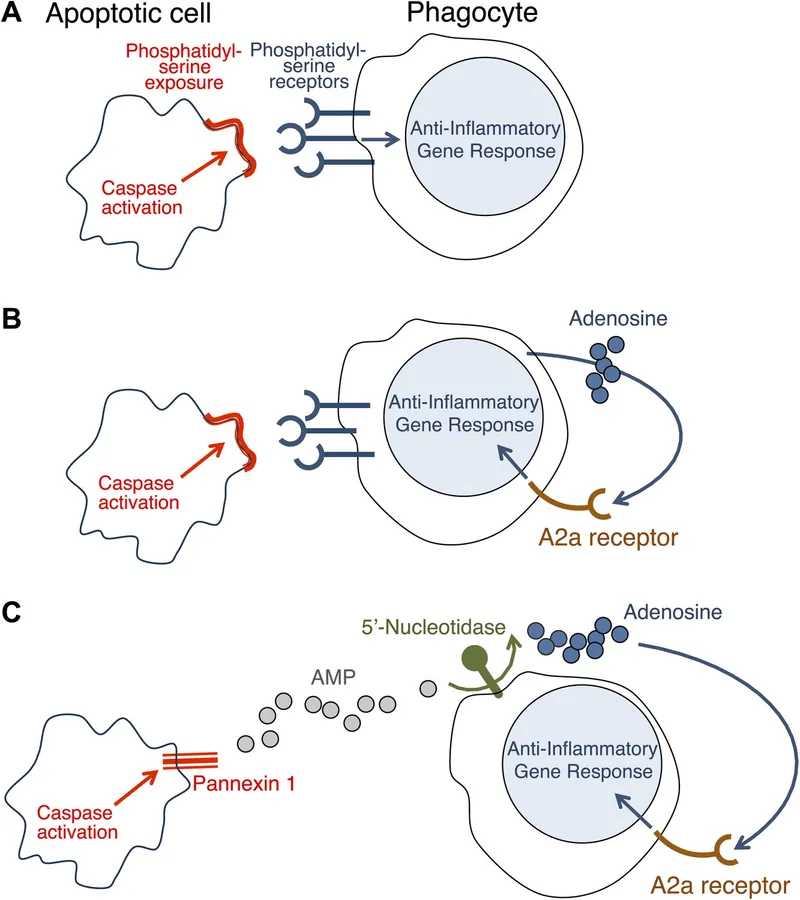
 \n\n> ⭐ Key Distinction: Unlike necrosis, apoptosis does not trigger an inflammatory response because cell contents are contained within apoptotic bodies and cleared by phagocytes.
\n\n> ⭐ Key Distinction: Unlike necrosis, apoptosis does not trigger an inflammatory response because cell contents are contained within apoptotic bodies and cleared by phagocytes.