Myocardial infarction pathology US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Myocardial infarction pathology. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Myocardial infarction pathology US Medical PG Question 1: A 74-year-old man presents with complaints of sudden severe crushing retrosternal pain. The pain radiated to his left arm shortly after it began, and he was subsequently rushed to the emergency department for evaluation. His troponins and creatine kinase-MB (CK-MB) were elevated. Unfortunately, the patient died within the next 2 hours and an autopsy was performed immediately. The gross examination of the heart will show?
- A. White, patchy, non-contractile scar
- B. Normal heart tissue (Correct Answer)
- C. Pallor of the infarcted tissue
- D. Abundant neutrophils
- E. Red granulation tissue surrounding the infarction
Myocardial infarction pathology Explanation: ***Normal heart tissue***
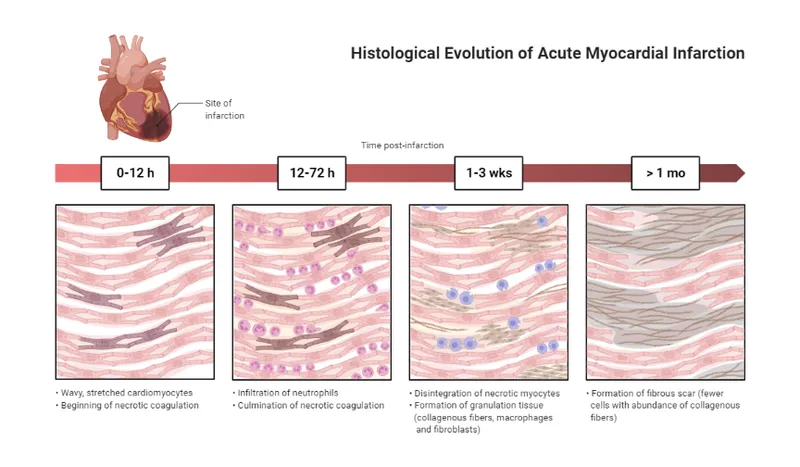
- At **0-4 hours** following a myocardial infarction, the heart muscle shows **no gross changes** on autopsy examination.
- Although **coagulative necrosis** begins at the cellular level within minutes, these microscopic changes are **not visible** to the naked eye during gross examination.
- The patient died within **2 hours** of symptom onset, which falls within this early window where the heart appears **grossly normal** despite the acute infarction.
- Elevated **cardiac enzymes** (troponins, CK-MB) confirm myocardial injury has occurred, but gross pathological changes lag behind biochemical and microscopic changes.
*Pallor of the infarcted tissue*
- **Pallor** (pale discoloration) of infarcted myocardium typically becomes visible on gross examination at **4-12 hours** post-infarction.
- At 2 hours, this change has not yet developed sufficiently to be visible on gross inspection.
- Pallor results from **edema** and the accumulation of dead cells, which takes several hours to manifest grossly.
*White, patchy, non-contractile scar*
- A **white fibrotic scar** is characteristic of a **healed myocardial infarction**, which takes **several weeks to months** to form.
- This represents complete replacement of necrotic tissue by **collagenous scar tissue** (fibrosis).
- This is a chronic finding, not an acute one.
*Abundant neutrophils*
- **Neutrophil infiltration** is a microscopic finding that typically begins around **12-24 hours** after infarction, becoming abundant over the following days.
- Even when present, neutrophils are not visible on **gross examination**—they require microscopic evaluation.
- At 2 hours post-infarction, neutrophils have not yet migrated to the infarcted area.
*Red granulation tissue surrounding the infarction*
- **Granulation tissue** formation begins around **3-7 days** after infarction and involves proliferation of **capillaries** and **fibroblasts**.
- Grossly, this appears as a **hyperemic border** with central yellow softening.
- This represents the healing phase and would not be present within 2 hours of symptom onset.
Myocardial infarction pathology US Medical PG Question 2: A 49-year-old man was brought to the emergency department by ambulance with complaints of sudden-onset chest pain that radiates into his neck and down his left arm. This substernal pain started 2 hours ago while he was having dinner. His past medical history is remarkable for hypercholesterolemia that is responsive to therapy with statins and coronary artery disease. His temperature is 37.0°C (98.6°F), blood pressure is 155/90 mm Hg, pulse is 112/min, and respiratory rate is 25/min. Troponin I levels are elevated. A 12-lead ECG was performed (see image). What is the most likely etiology of this patient’s presentation?
- A. Coronary vasospasm
- B. Right coronary artery occlusion (Correct Answer)
- C. Left circumflex artery occlusion
- D. Left anterior descending artery occlusion
- E. Left main coronary artery occlusion
Myocardial infarction pathology Explanation: ***Right coronary artery occlusion***
- The ECG shows significant **ST elevation in inferior leads (II, III, aVF)** and **ST depression in anterior leads (V1-V4)**, which is characteristic of an **inferior wall myocardial infarction**.
- **Inferior wall MIs** are typically caused by occlusion of the **right coronary artery (RCA)**. The reciprocal changes (ST depression in anterior leads) support this, indicating involvement of the posterolateral wall often supplied by the RCA.
*Coronary vasospasm*
- While coronary vasospasm (e.g., in **Prinzmetal angina**) can cause ST elevation, it usually presents with more transient symptoms that resolve with vasodilators, and the ST segment elevations are typically regional but often more widespread or dynamic.
- The patient's history of **coronary artery disease (CAD)** and persistent symptoms with elevated troponin point towards a fixed obstruction rather than vasospasm.
*Left circumflex artery occlusion*
- **Left circumflex artery occlusion** typically causes changes in leads I, aVL, V5, and V6 (high lateral or lateral wall MI), and sometimes posterior leads.
- The predominant ST elevation in leads II, III, and aVF is not characteristic of a primary **left circumflex artery occlusion**.
*Left anterior descending artery occlusion*
- **Left anterior descending (LAD) artery occlusion** usually results in **anterior or anteroseptal MI**, characterized by ST elevation in leads V1-V4 and potentially I and aVL.
- The ECG shows ST depression in V1-V4, which are reciprocal changes rather than direct signs of an **LAD occlusion**.
*Left main coronary artery occlusion*
- **Left main coronary artery occlusion** is a catastrophic event, often presenting with widespread ST depression in multiple leads with ST elevation in aVR (and sometimes V1).
- While life-threatening, the ECG pattern here with prominent inferior ST elevation and reciprocal anterior depression is more indicative of an **RCA occlusion** than a left main occlusion.
Myocardial infarction pathology US Medical PG Question 3: A 72-year-old man presents to the emergency department because of difficulty breathing and sharp chest pain. The chest pain increases in intensity with lying down, and it radiates to the scapular ridge. Approximately 3 weeks ago, he had an anterior ST-elevation myocardial infarction, which was treated with intravenous alteplase. He was discharged home in a stable condition. Current vital signs include a temperature of 38.1 (100.5°F), blood pressure of 131/91 mm Hg, and pulse of 99/min. On examination, heart sounds are distant and a scratching sound is heard on the left sternal border. ECG reveals widespread concave ST elevations in the precordial leads and PR depressions in leads V2-V6. Which of the following is the most likely cause of this patient condition?
- A. Recurrent infarction
- B. Myocarditis
- C. Aortic dissection
- D. Dressler’s syndrome (Correct Answer)
- E. Ventricular aneurysm
Myocardial infarction pathology Explanation: ***Dressler’s syndrome***
- This syndrome, also known as **post-myocardial infarction syndrome**, typically presents weeks to months after an MI and is characterized by pleuritic chest pain, fever, and pericardial friction rub.
- The **widespread ST elevations (concave)** and **PR depressions** on ECG are classic findings of pericarditis, which is the underlying pathology of Dressler's syndrome.
*Recurrent infarction*
- While an MI can cause chest pain, the pain associated with infarction is typically **retrosternal, crushing**, and does not improve with leaning forward or worsen with lying down.
- ECG findings of recurrent MI would show **convex ST elevations** in a specific coronary artery territory, not widespread concave ST elevation.
*Myocarditis*
- Myocarditis can cause chest pain, fever, and ECG changes (including ST elevations), but it is primarily an **inflammation of the heart muscle** often due to viral infection.
- In this case, the **pericardial friction rub** and history of recent MI strongly point towards pericardial inflammation, not primarily myocardial inflammation.
*Aortic dissection*
- Aortic dissection presents with **severe, tearing chest pain** that often radiates to the back, but it typically has an abrupt onset and is not associated with a pericardial friction rub or widespread ST elevations.
- The ECG findings of pericarditis do not support acute aortic dissection.
*Ventricular aneurysm*
- A ventricular aneurysm is a late complication of MI and can lead to symptoms like heart failure or arrhythmias, but it does **not typically cause acute pericarditic chest pain** or associated ECG findings.
- While it can cause persistent ST elevation, it would not be widespread and concave, and it wouldn't be associated with a friction rub.
Myocardial infarction pathology US Medical PG Question 4: A 32-year-old woman comes to the physician because of worsening fatigue and shortness of breath. Her symptoms began 8 months ago and have progressively worsened since then. She had recurrent episodes of joint pain and fever during childhood. She does not smoke or drink alcohol. She emigrated from the Congo with her parents when she was 12 years old. Her temperature is 37.4°C (99.3°F), pulse is 90/min and regular, respirations are 18/min, and blood pressure is 140/90 mm Hg. There is an opening snap followed by a diastolic murmur at the fifth left intercostal space in the midclavicular line. If left untreated, this patient is at greatest risk for which of the following complications?
- A. Pulmonary edema
- B. Systemic thromboembolism
- C. Pulmonary hypertension
- D. Right heart failure
- E. Atrial fibrillation (Correct Answer)
Myocardial infarction pathology Explanation: ***Atrial fibrillation***
- The patient's history of **recurrent joint pain and fever in childhood**, originating from the Congo, points towards a diagnosis of **rheumatic heart disease** causing **mitral stenosis**.
- **Mitral stenosis** leads to increased left atrial pressure and dilation, which are significant risk factors for developing **atrial fibrillation**. The presence of an **opening snap and diastolic murmur** further supports mitral stenosis.
*Pulmonary edema*
- While **pulmonary edema** can be a complication of severe **mitral stenosis** due to increased left atrial pressure and elevated pulmonary venous pressures, it is often precipitated by an acute event or occurs later in the disease course.
- **Atrial fibrillation** significantly exacerbates intra-atrial pressures and worsens symptoms, often preceding or coexisting with pulmonary edema, making it a more immediate and common long-term risk.
*Systemic thromboembolism*
- **Systemic thromboembolism** is a serious complication of untreated **mitral stenosis**, particularly when complicated by **atrial fibrillation**, due to stasis of blood in the dilated left atrium.
- However, the primary mechanism leading to an increased risk of thromboembolism in this context is the development of **atrial fibrillation**, which sets the stage for clot formation.
*Pulmonary hypertension*
- **Pulmonary hypertension** is a common consequence of chronic **mitral stenosis** as elevated left atrial pressures are transmitted to the pulmonary vasculature.
- While a severe complication that contributes to symptoms like shortness of breath, **atrial fibrillation** is a critical arrhythmia that can both worsen pulmonary hypertension and lead to other life-threatening complications.
*Right heart failure*
- **Right heart failure** eventually develops in severe, long-standing **mitral stenosis** due to sustained **pulmonary hypertension**, which increases the afterload on the right ventricle.
- While it represents an advanced stage of the disease, **atrial fibrillation** is a more immediate and common rhythm disturbance that contributes to the hemodynamic compromise and often predates overt right heart failure.
Myocardial infarction pathology US Medical PG Question 5: A 78-year-old man with a history of myocardial infarction status post coronary artery bypass grafting and a 60-pack-year history of smoking is found deceased in his apartment after not returning calls to his family for the last 2 days. The man was last known to be alive 3 days ago, when his neighbor saw him getting his mail. The family requests an autopsy. On autopsy, the man is found to have a 100% blockage of his left anterior descending artery of his heart and likely passed from sudden cardiac death 2 days prior. Which of the following findings is expected to be found on histologic examination of his damaged myocardium?
- A. Fat saponification
- B. Cellular debris and lymphocytes
- C. Cystic cavitation
- D. Cellular debris and macrophages
- E. Uniform binding of acidophilic dyes (Correct Answer)
Myocardial infarction pathology Explanation: ***Uniform binding of acidophilic dyes***
- This finding, often described as **coagulative necrosis**, is characteristic of myocardial infarction 1-3 days after onset, as enzymes denature and bind to eosin more uniformly.
- The patient was found deceased 2 days after his suspected death, placing the myocardial changes within this timeframe.
*Fat saponification*
- **Fat saponification** is a type of fat necrosis, typically seen in the pancreas or breast, resulting from the enzymatic destruction of fat cells.
- It does not occur in the myocardium following an ischemic event.
*Cellular debris and lymphocytes*
- **Lymphocytes** are generally not the predominant inflammatory cells in the initial stages of a myocardial infarction.
- While cellular debris would be present, the primary inflammatory infiltrate in the first 3 days after an MI is typically **neutrophils**, not lymphocytes.
*Cystic cavitation*
- **Cystic cavitation** is a characteristic feature of liquefactive necrosis, which occurs in the brain following an ischemic stroke, but not in the heart.
- The heart undergoes **coagulative necrosis** after an MI.
*Cellular debris and macrophages*
- **Macrophages** become prominent later in the healing process of a myocardial infarction, typically starting around **3-7 days** after the event.
- At the 2-day mark, the dominant cells would still be neutrophils and necrotic myocytes.
Myocardial infarction pathology US Medical PG Question 6: Two days after being admitted for acute myocardial infarction, a 61-year-old man has sharp, substernal chest pain that worsens with inspiration and improves when leaning forward. Cardiac examination shows a scratchy sound best heard over the left sternal border. Histopathological examination of the infarcted myocardial tissue is most likely to show which of the following findings?
- A. Neutrophilic infiltration
- B. Collagenous scar tissue
- C. Normal myocardium
- D. Coagulative necrosis (Correct Answer)
- E. Granulation tissue with macrophages
Myocardial infarction pathology Explanation: ***Coagulative necrosis***
- The patient's clinical presentation (sharp, substernal chest pain, worsening with inspiration, improving while leaning forward, and a scratchy pericardial friction rub) indicates **post-MI fibrinous pericarditis**, a common complication occurring 2-4 days after myocardial infarction.
- At **day 2 post-MI**, the infarcted myocardium demonstrates **coagulative necrosis** as the primary and most characteristic histopathological finding, representing irreversible ischemic cell death with preserved tissue architecture.
- While neutrophilic infiltration is also present at this timepoint, coagulative necrosis of the cardiomyocytes themselves is the defining pathological feature that distinguishes irreversible myocardial injury.
*Neutrophilic infiltration*
- **Neutrophilic infiltration** is indeed present at day 2 post-MI (peaks at days 1-3) as part of the acute inflammatory response to clear necrotic debris.
- However, neutrophils represent the **reactive inflammatory response** rather than the primary pathological change in the infarcted cardiomyocytes themselves.
- The question asks about the most characteristic histopathological finding, which is the **coagulative necrosis** of the myocardial cells, not the secondary inflammatory infiltrate.
*Collagenous scar tissue*
- **Collagenous scar tissue** forms much later during the remodeling phase, typically **7 weeks or more** after MI, representing the final stage of healing.
- At day 2, the tissue is still in the acute phase of coagulative necrosis and early inflammation, far too early for mature fibrous scar formation.
*Normal myocardium*
- The patient has sustained an **acute myocardial infarction** with irreversible injury to cardiac tissue.
- Histopathological examination of the infarcted region would show clear abnormalities, not **normal myocardium**.
*Granulation tissue with macrophages*
- **Granulation tissue** with fibroblasts, new capillaries, and macrophages begins forming during the proliferative phase, typically starting around **days 5-7** post-MI.
- At day 2, it is too early for granulation tissue formation; the tissue is still dominated by coagulative necrosis and acute neutrophilic inflammation.
Myocardial infarction pathology US Medical PG Question 7: A 73-year-old man with coronary artery disease and hypertension is brought to the emergency department by ambulance 90 minutes after the acute onset of substernal chest pain and dyspnea. He has smoked 2 packs of cigarettes daily for 52 years. Shortly after arriving at the hospital, he loses consciousness and is pulseless. Despite attempts at cardiopulmonary resuscitation, he dies. Examination of the heart at autopsy shows complete occlusion of the left anterior descending artery with a red thrombus overlying a necrotic plaque. Which of the following pathophysiologic mechanisms is most likely responsible for this patient's acute coronary condition?
- A. Influx of lipids into the endothelium
- B. Secretion of matrix metalloproteinases (Correct Answer)
- C. Release of platelet-derived growth factor
- D. Type III collagen deposition
- E. Proliferation of smooth muscle cells
Myocardial infarction pathology Explanation: ***Secretion of matrix metalloproteinases***
- **Matrix metalloproteinases (MMPs)** degrade the **extracellular matrix** within the fibrous cap of an atherosclerotic plaque, leading to its **destabilization and rupture**.
- Plaque rupture then exposes the highly thrombogenic lipid core, initiating thrombus formation and acute coronary events like the **red thrombus** seen in the **left anterior descending artery (LAD)**.
*Influx of lipids into the endothelium*
- This process is characteristic of the **initial stages of atherosclerosis**, leading to **fatty streak formation**, not the acute plaque rupture and thrombosis described.
- While essential for plaque development, lipid influx alone does not directly explain aggressive plaque rupture and acute thrombus formation.
*Release of platelet-derived growth factor*
- **Platelet-derived growth factor (PDGF)** is primarily involved in **smooth muscle cell proliferation** and migration, contributing to plaque growth and thickening.
- Its role is more chronic and proliferative, not immediate plaque destabilization and rupture leading to acute thrombosis.
*Type III collagen deposition*
- **Type III collagen** is characteristic of early, developing atherosclerotic plaques and granulation tissue, contributing to plaque stability.
- Plaque vulnerability associated with rupture involves a **thin fibrous cap** with reduced **collagen content**, often due to increased collagen degradation.
*Proliferation of smooth muscle cells*
- **Smooth muscle cell proliferation** occurs during chronic atherosclerosis, contributing to the **fibrous cap formation** and overall plaque stability.
- In the context of acute plaque rupture, it is the *erosion* of the fibrous cap, often due to degradation, rather than proliferation, that is the immediate cause.
Myocardial infarction pathology US Medical PG Question 8: A 69-year-old man is scheduled to undergo radical retropubic prostatectomy for prostate cancer in 2 weeks. He had a myocardial infarction at the age of 54 years. He has a history of GERD, unstable angina, hyperlipidemia, and severe osteoarthritis in the left hip. He is unable to climb up stairs or walk fast because of pain in his left hip. He had smoked one pack of cigarettes daily for 30 years but quit 25 years ago. He drinks one glass of wine daily. Current medications include aspirin, metoprolol, lisinopril, rosuvastatin, omeprazole, and ibuprofen as needed. His temperature is 36.4°C (97.5°F), pulse is 90/min, and blood pressure is 136/88 mm Hg. Physical examination shows no abnormalities. A 12-lead ECG shows Q waves and inverted T waves in leads II, III, and aVF. His B-type natriuretic protein is 84 pg/mL (N < 125). Which of the following is the most appropriate next step in management to assess this patient's perioperative cardiac risk?
- A. No further testing
- B. 24-hour ambulatory ECG monitoring
- C. Radionuclide myocardial perfusion imaging (Correct Answer)
- D. Treadmill stress test
- E. Resting echocardiography
Myocardial infarction pathology Explanation: ***Radionuclide myocardial perfusion imaging***
- This patient requires **perioperative cardiac risk assessment** before intermediate-risk surgery (radical prostatectomy).
- Key factors include: history of **myocardial infarction**, current cardiac risk factors, and **inability to exercise** due to severe osteoarthritis.
- Since he cannot perform exercise stress testing, **pharmacologic stress testing** with radionuclide myocardial perfusion imaging (using agents like adenosine, dipyridamole, or regadenoson) is the most appropriate test to assess for **inducible myocardial ischemia**.
- This provides functional assessment of coronary perfusion under pharmacologic stress, helping guide perioperative risk stratification and management.
- *Note: The presence of unstable angina would typically require cardiac stabilization first; this question focuses on selecting the appropriate stress test modality for a patient unable to exercise.*
*No further testing*
- This patient has significant cardiac risk factors including **prior MI**, ongoing cardiac medications, and ECG changes suggesting old infarction.
- Proceeding directly to surgery without functional cardiac assessment would be **inappropriate** given his risk profile and the intermediate-risk nature of the planned surgery.
*24-hour ambulatory ECG monitoring*
- Holter monitoring detects arrhythmias and silent ischemic episodes but does not provide **functional capacity assessment** or evaluation of inducible ischemia under stress conditions.
- It is not the primary tool for **perioperative cardiac risk stratification** before major surgery.
*Treadmill stress test*
- The patient's **severe osteoarthritis** prevents him from climbing stairs or walking fast, making him unable to achieve adequate exercise workload for a treadmill stress test.
- This functional limitation makes **exercise stress testing contraindicated**; pharmacologic stress testing is required instead.
*Resting echocardiography*
- Resting echocardiography assesses **baseline left ventricular function**, wall motion abnormalities from prior infarction, and valvular disease.
- While useful for structural assessment, it does **not evaluate for exercise-induced or stress-induced ischemia**, which is critical for perioperative risk assessment in patients with coronary artery disease.
- His normal BNP (84 pg/mL) suggests adequate baseline ventricular function, making functional ischemia assessment more relevant than structural evaluation alone.
Myocardial infarction pathology US Medical PG Question 9: A 50-year-old man presents the emergency department for intense chest pain, profuse sweating, and shortness of breath. The onset of these symptoms was 3 hours ago. The chest pain began after a heated discussion with a colleague at the community college where he is employed. Upon arrival, he is found conscious and responsive; the vital signs include a blood pressure of 130/80 mm Hg, a heart rate at 90/min, a respiratory rate at 20/min, and a body temperature of 36.4°C (97.5°F). His medical history is significant for hypertension diagnosed 7 years ago, which is well-controlled with a calcium channel blocker. The initial electrocardiogram (ECG) shows ST-segment depression in multiple consecutive leads, an elevated cardiac troponin T level, and normal kidney function. Which of the following would you expect to find in this patient?
- A. Subendocardial necrosis (Correct Answer)
- B. Transmural necrosis
- C. Incomplete occlusion of a coronary artery
- D. Coronary artery spasm
- E. Ventricular pseudoaneurysm
Myocardial infarction pathology Explanation: ***Subendocardial necrosis***
- This patient's presentation with **ST-segment depression** and **elevated troponin T** indicates a **Non-ST-segment Elevation Myocardial Infarction (NSTEMI)**, which typically results from subendocardial ischemia and necrosis.
- Subendocardial tissue is most vulnerable to ischemia due to its high oxygen demand and distal location from the coronary arteries, making it the first region to suffer damage when oxygen supply is compromised.
*Transmural necrosis*
- **Transmural necrosis** is characteristic of a **ST-segment Elevation Myocardial Infarction (STEMI)**, which presents with persistent **ST-segment elevation** on ECG.
- This patient's ECG shows **ST-segment depression**, ruling out transmural involvement at the time of presentation.
*Incomplete occlusion of a coronary artery*
- While an NSTEMI usually involves an **incomplete occlusion** or **critical stenosis** of a coronary artery, the question asks what would be *found* in the patient's heart tissue, not the mechanism.
- The direct tissue consequence of incomplete occlusion leading to NSTEMI is **subendocardial necrosis**, which is a more specific answer about the pathological finding.
*Coronary artery spasm*
- Although **coronary artery spasm (Prinzmetal angina)** can cause chest pain and ECG changes, it typically presents with **transient ST-segment elevation** (not depression) and often resolves spontaneously.
- The elevated troponin T indicates myocardial necrosis, which is not typically a feature of uncomplicated coronary artery spasm, and the duration of symptoms (3 hours) suggests a more sustained event than a transient spasm.
*Ventricular pseudoaneurysm*
- A **ventricular pseudoaneurysm** is a **late complication of myocardial infarction**, typically occurring weeks to months after the acute event, due to rupture of the ventricular free wall contained by pericardium.
- Given the 3-hour symptom onset, it is highly unlikely to be present in the acute phase of myocardial infarction.
Myocardial infarction pathology US Medical PG Question 10: A 55-year-old man comes to the physician because of a 4-month history of episodic, pressure-like chest pain. The chest pain occurs when he is walking up stairs and improves with rest. He has hypertension and type 2 diabetes mellitus. His father died from a myocardial infarction at the age of 50 years. Current medications include hydrochlorothiazide and metformin. His pulse is 85/min, respirations are 12/min, and blood pressure is 140/90 mm Hg. Cardiac examination shows normal heart sounds without any murmurs, rubs, or gallops. An ECG shows high amplitude of the S wave in lead V3. An exercise stress test is performed but stopped after 4 minutes because the patient experiences chest pain. An ECG obtained during the stress test shows sinus tachycardia and ST-segment depressions in leads V1–V4. Which of the following is the most appropriate long-term pharmacotherapy to reduce the frequency of symptoms in this patient?
- A. Metoprolol (Correct Answer)
- B. Clopidogrel
- C. Aspirin
- D. Nitroglycerin
- E. Isosorbide mononitrate
Myocardial infarction pathology Explanation: ***Metoprolol***
- **Beta-blockers** like metoprolol are first-line agents for **symptom relief** in stable angina by reducing myocardial oxygen demand.
- They decrease **heart rate**, **blood pressure**, and **myocardial contractility**, thereby reducing the frequency and severity of anginal episodes.
*Clopidogrel*
- **Clopidogrel** is an antiplatelet agent used primarily to prevent **thrombotic events** in patients with established cardiovascular disease or acute coronary syndromes.
- It does not directly reduce the frequency of anginal symptoms, but rather prevents progression to **myocardial infarction** or **stroke**.
*Aspirin*
- **Aspirin** is an antiplatelet medication used for **secondary prevention** of cardiovascular events by inhibiting platelet aggregation.
- While crucial for reducing cardiovascular risk, it does not directly alleviate the **frequency of anginal symptoms** themselves.
*Nitroglycerin*
- **Nitroglycerin** is a short-acting nitrate used to provide **immediate relief** of anginal pain during an acute episode.
- It is not a long-term pharmacotherapy for reducing the *frequency* of symptoms.
*Isosorbide mononitrate*
- **Isosorbide mononitrate** is a long-acting nitrate used to *prevent* angina, but it is typically a **second-line agent** after beta-blockers due to potential for **tolerance** and side effects.
- While it can reduce symptom frequency, beta-blockers are generally preferred as initial long-term therapy for symptom control.
More Myocardial infarction pathology US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.