Cardiovascular

On this page
🫀 The Cardiac Engine: Mastering Cardiovascular Foundations
You'll build a complete mental model of the cardiovascular system by mastering how the heart generates pressure, how blood flows through resistance networks, and how these mechanics produce the clinical patterns you'll encounter daily. This lesson moves beyond memorizing facts to understanding why murmurs sound different, why certain drugs target specific hemodynamic problems, and how to systematically differentiate chest pain, heart failure, and shock states. By integrating anatomy, physiology, and pathology with diagnostic reasoning and treatment algorithms, you'll develop the pattern recognition and clinical judgment that separates competent from exceptional cardiovascular care.
The cardiovascular system represents the body's most sophisticated hydraulic network, designed for continuous operation over 80+ years without maintenance shutdowns. This remarkable engineering feat depends on precise coordination between:
-
Cardiac pump mechanics - Four-chamber design with unidirectional flow
- Right ventricle: 25/5 mmHg systolic/diastolic pressures
- Left ventricle: 120/10 mmHg systolic/diastolic pressures
- Stroke volume: 70 mL per beat at rest
- Ejection fraction: 55-70% in healthy hearts
-
Vascular network architecture - Branching tree design optimizing flow distribution
- Aortic diameter: 2.5-3.0 cm at root
- Capillary diameter: 5-10 micrometers
- Total capillary surface area: 600 square meters
- Venous return capacity: 65% of total blood volume
📌 Remember: LAMP for cardiac output determinants - Load (preload/afterload), Afterload, Myocardial contractility, Preload. Each factor directly influences the 5-6 L/min cardiac output that sustains life.
| Cardiac Parameter | Normal Range | Clinical Significance | Pathological Threshold | Monitoring Method |
|---|---|---|---|---|
| Heart Rate | 60-100 bpm | Autonomic balance | <50 or >100 bpm | ECG, pulse |
| Blood Pressure | 120/80 mmHg | Vascular resistance | >140/90 mmHg | Sphygmomanometry |
| Cardiac Output | 4-8 L/min | Tissue perfusion | <4 L/min | Thermodilution |
| Ejection Fraction | 55-70% | Contractile function | <40% | Echocardiography |
| Central Venous Pressure | 2-8 mmHg | Preload status | >12 mmHg | Swan-Ganz catheter |
-
Systole (0.3 seconds duration)
- Isovolumetric contraction: 50 msec
- Ejection phase: 250 msec
- Peak aortic pressure: 120 mmHg
-
Diastole (0.5 seconds duration)
- Isovolumetric relaxation: 80 msec
- Ventricular filling: 420 msec
- End-diastolic pressure: 8-12 mmHg
⭐ Clinical Pearl: The first heart sound (S1) marks mitral/tricuspid closure at systole onset, while S2 signals aortic/pulmonary closure at systole termination. Abnormal splitting patterns indicate conduction defects or pressure overload with >95% sensitivity.
💡 Master This: Frank-Starling mechanism governs cardiac output through length-tension relationships. Increased venous return stretches myocardial fibers, enhancing contractility up to optimal sarcomere length (2.2 micrometers). Beyond this point, contractility decreases exponentially.
Understanding these cardiovascular foundations provides the mechanical framework for recognizing pathological deviations. Connect these normal parameters through hemodynamic principles to understand how pressure gradients and flow dynamics create the clinical presentations you'll encounter in cardiovascular disease.
🫀 The Cardiac Engine: Mastering Cardiovascular Foundations
⚡ Hemodynamic Powerhouse: The Pressure-Flow Command Center
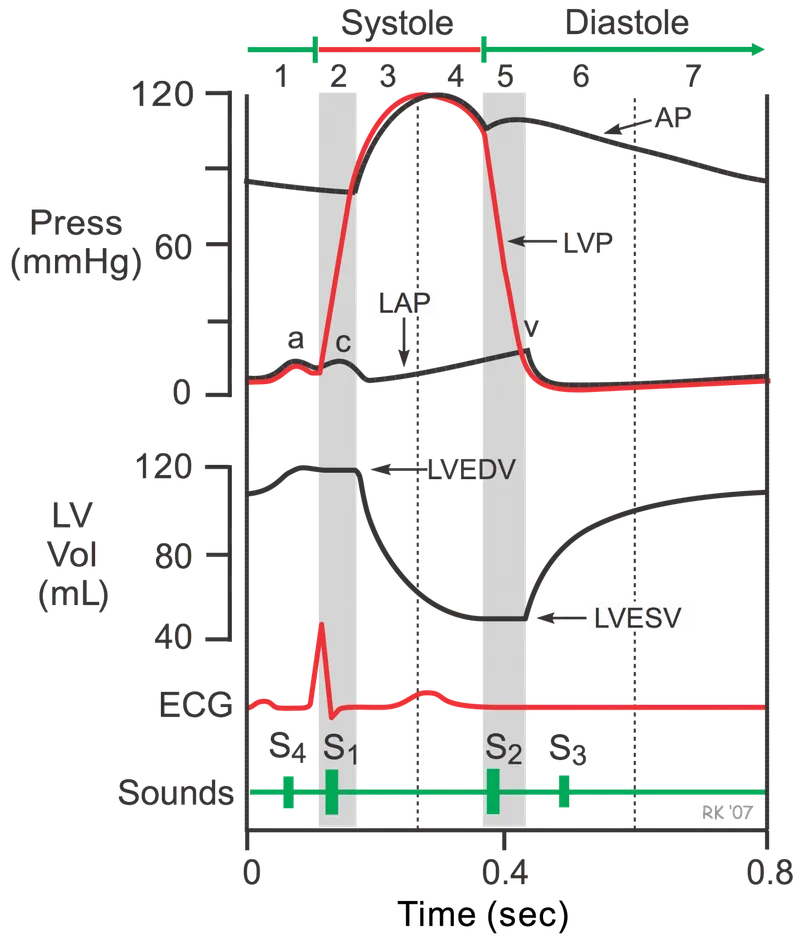
Hemodynamics governs every cardiovascular pathology through fundamental pressure-flow relationships. Master these principles, and you predict how stenotic lesions, regurgitant valves, and myocardial dysfunction alter the delicate balance maintaining tissue perfusion.
Poiseuille's Law defines vascular resistance with clinical precision:
$$Resistance = \frac{8 \times \eta \times L}{\pi \times r^4}$$
Where radius (r) exerts fourth-power influence on resistance. A 50% diameter reduction increases resistance 16-fold, explaining why coronary stenosis >70% produces flow-limiting symptoms.
-
Systemic vascular resistance: 800-1200 dynes⋅sec⋅cm⁻⁵
- Calculated: (MAP - CVP) × 80 / Cardiac Output
- Normal range maintains adequate perfusion pressure
- Elevated in hypertension and cardiogenic shock
-
Pulmonary vascular resistance: 150-250 dynes⋅sec⋅cm⁻⁵
- One-sixth of systemic resistance
- Increases dramatically in pulmonary hypertension
- >300 dynes⋅sec⋅cm⁻⁵ indicates pathological elevation
📌 Remember: MAPS for pressure determinants - Mean arterial pressure = Aortic compliance + Stroke volume + Peripheral resistance + Systemic factors. MAP = DBP + (SBP-DBP)/3 provides the driving pressure for organ perfusion.
| Hemodynamic Variable | Normal Value | Measurement Method | Pathological Range | Clinical Significance |
|---|---|---|---|---|
| Mean Arterial Pressure | 70-100 mmHg | Arterial line | <65 or >110 mmHg | Organ perfusion threshold |
| Pulse Pressure | 30-50 mmHg | SBP - DBP | >60 mmHg | Arterial stiffness marker |
| Stroke Volume | 60-80 mL | Echocardiography | <50 mL | Contractility assessment |
| Systemic Vascular Resistance | 800-1200 dynes | Calculated | >1500 dynes | Afterload quantification |
| Cardiac Index | 2.5-4.0 L/min/m² | Thermodilution | <2.2 L/min/m² | Size-adjusted cardiac output |
-
Cerebral autoregulation: MAP 60-150 mmHg
- Myogenic response: Smooth muscle contraction with ↑pressure
- Metabolic response: Vasodilation with ↑CO₂, ↓O₂
- Failure threshold: <50 mmHg or >180 mmHg
-
Coronary autoregulation: Diastolic pressure 60-120 mmHg
- Adenosine-mediated vasodilation during ↑oxygen demand
- Endothelial nitric oxide maintains basal tone
- Compromised in diabetes and hypertension
⭐ Clinical Pearl: Coronary perfusion pressure = Aortic diastolic pressure - Left ventricular end-diastolic pressure. Normal gradient >40 mmHg ensures adequate subendocardial perfusion. Aortic regurgitation reduces diastolic pressure, while heart failure elevates LVEDP, creating perfusion crisis.
💡 Master This: Ventricular-arterial coupling optimizes cardiac efficiency through impedance matching. Optimal coupling ratio (Ea/Ees) = 0.5-1.0 maximizes stroke work efficiency. Heart failure disrupts this relationship, requiring afterload reduction to restore mechanical efficiency.
These hemodynamic principles create the pressure-flow framework for understanding cardiovascular pathophysiology. Connect these fundamental relationships through clinical pattern recognition to identify how valvular lesions and myocardial dysfunction disrupt the delicate balance maintaining tissue perfusion.
⚡ Hemodynamic Powerhouse: The Pressure-Flow Command Center
🎯 Pattern Recognition Arsenal: Clinical Correlation Mastery
Master the "see this, think that" correlations that transform scattered findings into precise diagnoses. Every murmur characteristic, pulse abnormality, and pressure measurement provides diagnostic clues when interpreted through systematic pattern frameworks.
Murmur Recognition Patterns with hemodynamic correlations:
-
Systolic murmurs - Turbulent flow during ventricular ejection
-
Aortic stenosis: Crescendo-decrescendo, radiates to carotids
- Peak gradient >40 mmHg indicates severe stenosis
- Delayed carotid upstroke (pulsus tardus)
- Paradoxical S2 splitting with severe disease
-
Mitral regurgitation: Holosystolic, radiates to axilla
- V-wave prominence in pulmonary capillary wedge pressure
- Hyperdynamic precordium with volume overload
- S3 gallop indicates decompensated disease
-
-
Diastolic murmurs - Always pathological, require immediate evaluation
- Aortic regurgitation: High-pitched, decrescendo
- Wide pulse pressure (>60 mmHg)
- Duroziez sign: Femoral artery systolic/diastolic bruits
- Austin Flint murmur: Functional mitral stenosis
- Aortic regurgitation: High-pitched, decrescendo
📌 Remember: PASS for murmur analysis - Pitch (frequency), Audibility (intensity), Shape (configuration), Site (location/radiation). Grade 4-6/6 murmurs have palpable thrills indicating significant hemodynamic disturbance.
| Clinical Finding | Hemodynamic Significance | Associated Conditions | Diagnostic Accuracy | Quantitative Threshold |
|---|---|---|---|---|
| Pulsus Paradoxus | Ventricular interdependence | Tamponade, severe asthma | >95% sensitivity | >10 mmHg abnormal |
| Pulsus Alternans | Contractile dysfunction | Severe heart failure | >90% specificity | >20 mmHg variation |
| Jugular Venous Distension | Elevated right heart pressures | Heart failure, tamponade | >85% sensitivity | >8 cm H₂O abnormal |
| S3 Gallop | Volume overload | Systolic dysfunction | >90% specificity | EF <40% correlation |
| S4 Gallop | Reduced compliance | Diastolic dysfunction | >80% sensitivity | LVEDP >15 mmHg |
-
Typical angina (85-95% CAD probability)
- Substernal pressure/squeezing lasting 2-10 minutes
- Exertional provocation with rest relief
- Radiation to left arm, jaw, or epigastrium
-
Atypical angina (15-85% CAD probability)
- Two of three typical characteristics
- Atypical triggers: emotional stress, cold exposure
- Prolonged duration (>20 minutes) suggests ACS
-
Non-cardiac chest pain (<15% CAD probability)
- Sharp, stabbing quality
- Positional or respiratory variation
- Reproducible with palpation
⭐ Clinical Pearl: Diamond-Forrester classification stratifies pre-test probability for coronary artery disease based on age, gender, and symptom characteristics. Men >50 years with typical angina have >90% CAD probability, while women <40 years with atypical symptoms have <15% probability.
💡 Master This: Levine's sign (clenched fist over chest) indicates ischemic chest pain with >80% sensitivity. Combined with diaphoresis and nausea, this triad suggests acute coronary syndrome requiring immediate evaluation and antiplatelet therapy within 30 minutes.
These pattern recognition frameworks provide the clinical correlation tools for rapid cardiovascular assessment. Connect these diagnostic patterns through systematic evaluation approaches to distinguish life-threatening emergencies from benign conditions with confidence and precision.
🎯 Pattern Recognition Arsenal: Clinical Correlation Mastery
🔍 Diagnostic Discrimination Matrix: Systematic Differentiation
Heart Failure Classification with hemodynamic profiles:
-
Heart Failure with Reduced Ejection Fraction (HFrEF)
- Ejection fraction <40%
- Systolic dysfunction predominates
- Dilated ventricle with ↓contractility
- BNP >400 pg/mL or NT-proBNP >1600 pg/mL
-
Heart Failure with Preserved Ejection Fraction (HFpEF)
- Ejection fraction ≥50%
- Diastolic dysfunction predominates
- Normal/small ventricle with ↓compliance
- E/e' ratio >15 indicates ↑filling pressures
-
Heart Failure with Mildly Reduced Ejection Fraction (HFmrEF)
- Ejection fraction 40-49%
- Mixed systolic/diastolic dysfunction
- Intermediate prognosis and treatment response
📌 Remember: DEFEAT for diastolic dysfunction assessment - Doppler E/A ratio, E'/e ratio, Filling pressures, Echocardiographic parameters, Atrial size, Tissue Doppler velocities. E/e' >15 predicts PCWP >18 mmHg with >90% accuracy.
| Condition | Key Discriminator | Quantitative Threshold | Diagnostic Test | Treatment Implication |
|---|---|---|---|---|
| Acute MI vs Unstable Angina | Troponin elevation | >99th percentile | Serial troponins | Reperfusion urgency |
| STEMI vs NSTEMI | ST elevation | >1mm in 2+ leads | 12-lead ECG | Primary PCI <90min |
| Pericarditis vs MI | PR depression | >0.8mm in lead II | ECG + echo | Anti-inflammatory |
| Tamponade vs Restriction | Respiratory variation | >25% vs <10% | Cardiac catheterization | Surgical vs medical |
| AS vs MR | Murmur timing | Systolic vs holosystolic | Echocardiography | Valve replacement |
-
STEMI - ST elevation + troponin ↑
- Primary PCI within 90 minutes (door-to-balloon)
- Fibrinolysis if PCI unavailable within 30 minutes
- Peak troponin at 12-24 hours
-
NSTEMI - No ST elevation + troponin ↑
- Risk stratification using TIMI or GRACE scores
- Early invasive strategy if high-risk features
- Troponin rise within 3-6 hours
-
Unstable Angina - No ST elevation + troponin normal
- High-sensitivity troponin rules out MI with >99% NPV
- Stress testing after symptom resolution
- Medical management unless high-risk anatomy
⭐ Clinical Pearl: High-sensitivity troponin protocols enable 1-hour rule-out using <5 ng/L cutoff with >99.5% negative predictive value. Delta troponin >20% between 0 and 1 hour indicates acute MI with >95% specificity.
💡 Master This: Sgarbossa criteria diagnose STEMI in left bundle branch block with >90% specificity: ST elevation ≥1mm in concordant leads (5 points), ST depression ≥1mm in V1-V3 (3 points), ST elevation ≥5mm in discordant leads (2 points). Score ≥3 indicates acute MI.
These discrimination frameworks provide the systematic tools for precise cardiovascular diagnosis. Connect these quantitative thresholds through evidence-based treatment algorithms to optimize therapeutic decisions and patient outcomes with measurable precision.
🔍 Diagnostic Discrimination Matrix: Systematic Differentiation
⚖️ Treatment Command Center: Evidence-Based Intervention Algorithms
Acute Coronary Syndrome Management with time-critical interventions:
Heart Failure Treatment Optimization using guideline-directed medical therapy:
-
ACE Inhibitors/ARBs - Mortality reduction 15-20%
- Target dose: Lisinopril 20-40mg daily
- Contraindications: Creatinine >3.0 mg/dL, K+ >5.5 mEq/L
- Monitoring: Creatinine/K+ at 1-2 weeks
-
Beta-blockers - Mortality reduction 25-35%
- Evidence-based agents: Metoprolol succinate, carvedilol, bisoprolol
- Target heart rate: 60-70 bpm
- Contraindications: Decompensated HF, severe bradycardia
-
Aldosterone antagonists - Mortality reduction 15-30%
- Spironolactone 25-50mg or eplerenone 25-50mg
- EF <35% despite optimal therapy
- Monitor K+ - discontinue if >5.5 mEq/L
📌 Remember: GDMT for heart failure optimization - Guideline-directed Drug therapy includes Maximally Tolerated doses of ACE-I/ARB + Beta-blocker + Aldosterone antagonist. Sequential titration every 2-4 weeks until target doses or limiting side effects.
| Intervention | Indication | Time Window | Success Rate | Monitoring Parameter |
|---|---|---|---|---|
| Primary PCI | STEMI | <90 minutes | >95% patency | TIMI 3 flow |
| Fibrinolysis | STEMI, no PCI | <30 minutes | >80% patency | ST resolution >50% |
| Dual Antiplatelet | All ACS | Loading dose | >30% RRR | Bleeding complications |
| High-intensity Statin | ACS/CAD | Within 24hrs | >25% LDL reduction | LDL <70 mg/dL |
| Cardiac Rehabilitation | Post-MI | <30 days | >20% mortality reduction | Exercise capacity |
-
Stage 1 Hypertension (130-139/80-89 mmHg)
- Lifestyle modifications for 3 months
- Single agent if cardiovascular risk factors
- Target <130/80 mmHg in most patients
-
Stage 2 Hypertension (≥140/90 mmHg)
- Combination therapy with 2 agents
- Different classes: ACE-I + CCB or ACE-I + Thiazide
- Target <130/80 mmHg achieved in >80% patients
-
Hypertensive Crisis (≥180/120 mmHg)
- Immediate evaluation for end-organ damage
- Gradual reduction: 10-20% in first hour
- Avoid sublingual nifedipine - stroke risk
⭐ Clinical Pearl: SPRINT trial demonstrated intensive BP control (<120 mmHg systolic) reduces cardiovascular events by 25% and mortality by 27% in high-risk patients without diabetes. Number needed to treat = 61 over 3.3 years.
💡 Master This: Antiplatelet therapy duration after PCI depends on stent type and bleeding risk. Bare metal stents require 1 month dual therapy, drug-eluting stents require 6-12 months. DAPT score >2 favors extended therapy if low bleeding risk.
These evidence-based algorithms provide the treatment frameworks for optimal cardiovascular outcomes. Connect these intervention protocols through systematic monitoring approaches to achieve guideline-recommended targets and measurable clinical improvements with precision and safety.
⚖️ Treatment Command Center: Evidence-Based Intervention Algorithms
🔗 Cardiovascular Integration Network: Multi-System Connections
Cardiorenal Syndrome - Bidirectional organ dysfunction:
-
Type 1 - Acute cardiac → acute renal dysfunction
- Cardiogenic shock reduces renal perfusion
- GFR decline >25% within 48-72 hours
- Creatinine rise >0.3 mg/dL indicates kidney injury
-
Type 2 - Chronic cardiac → chronic renal dysfunction
- Heart failure causes progressive CKD
- eGFR <60 mL/min/1.73m² in >50% of HF patients
- Neurohormonal activation drives renal fibrosis
-
Type 3 - Acute renal → acute cardiac dysfunction
- Fluid overload precipitates pulmonary edema
- Electrolyte imbalances cause arrhythmias
- Uremic toxins depress myocardial contractility
📌 Remember: RAAS activation in heart failure - Renin-Angiotensin-Aldosterone System creates vicious cycle of vasoconstriction + fluid retention + cardiac remodeling. ACE inhibitors break this cycle, improving survival by 20-25%.
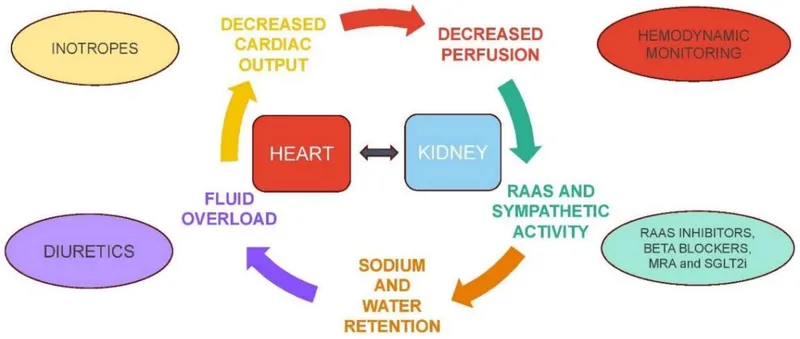
| System Integration | Primary Mechanism | Clinical Manifestation | Quantitative Marker | Therapeutic Target |
|---|---|---|---|---|
| Cardiopulmonary | Pressure transmission | Pulmonary edema | PCWP >18 mmHg | Preload reduction |
| Cardiorenal | Perfusion pressure | Acute kidney injury | Creatinine >0.3 mg/dL rise | RAAS inhibition |
| Cardiohepatic | Venous congestion | Hepatic dysfunction | Bilirubin >2.0 mg/dL | Diuresis |
| Cardiometabolic | Insulin resistance | Diabetes progression | HbA1c >7.0% | Glycemic control |
| Cardiocerebral | Embolic events | Stroke | CHA₂DS₂-VASc score | Anticoagulation |
-
Diabetes mellitus accelerates atherosclerosis through:
- Advanced glycation end products (AGEs) promote endothelial dysfunction
- Hyperglycemia increases oxidative stress and inflammation
- HbA1c >7.0% doubles cardiovascular risk
-
Metabolic syndrome components synergistically increase CVD risk:
- Waist circumference >40 inches (men) or >35 inches (women)
- Triglycerides >150 mg/dL
- HDL <40 mg/dL (men) or <50 mg/dL (women)
- Blood pressure >130/85 mmHg
- Fasting glucose >100 mg/dL
Pulmonary-Cardiovascular Integration in heart failure:
-
Pulmonary hypertension develops in >60% of chronic HF patients
- Passive component: Elevated left atrial pressure
- Reactive component: Pulmonary vasoconstriction
- mPAP >25 mmHg at rest indicates pulmonary hypertension
-
Right heart failure complicates advanced left heart disease
- Tricuspid regurgitation develops with RV dysfunction
- Hepatic congestion and peripheral edema
- BNP elevation reflects biventricular dysfunction
⭐ Clinical Pearl: Cardiac cachexia affects 10-15% of advanced heart failure patients, defined as >6% weight loss over 6 months. Inflammatory cytokines (TNF-α, IL-6) drive muscle wasting and predict poor prognosis with 50% mortality at 18 months.
💡 Master This: CHA₂DS₂-VASc score stratifies stroke risk in atrial fibrillation: Congestive HF, Hypertension, Age ≥75 (2 points), Diabetes, Stroke history (2 points), Vascular disease, Age 65-74, Sex category (female). Score ≥2 warrants anticoagulation with >65% stroke reduction.
These integration networks reveal how cardiovascular disease affects multiple organ systems simultaneously. Connect these multi-system relationships through comprehensive management strategies to address primary cardiac pathology while preventing secondary organ dysfunction and optimizing overall patient outcomes.
🔗 Cardiovascular Integration Network: Multi-System Connections
🎯 Cardiovascular Mastery Toolkit: Clinical Excellence Framework
This clinical arsenal transforms complex cardiovascular presentations into systematic assessments with measurable outcomes. Master these rapid-reference tools, and you possess the framework for expert-level cardiovascular practice.
Essential Clinical Thresholds - Memorize for immediate application:
- Cardiac Output: 4-8 L/min (normal), <4 L/min (shock)
- Ejection Fraction: ≥55% (normal), <40% (reduced)
- Troponin: >99th percentile (MI), <5 ng/L (rule-out)
- BNP: <100 pg/mL (excludes HF), >400 pg/mL (confirms HF)
- Coronary Stenosis: >70% (flow-limiting), >90% (critical)
📌 Remember: MONA for acute MI management - Morphine (if needed), Oxygen (if SpO₂ <90%), Nitroglycerin (if no hypotension), Aspirin (162-325mg chewed). Add P-2Y12 inhibitor and Statin for MONA-PS protocol.
Rapid Assessment Framework for cardiovascular emergencies:
-
Primary Survey (<2 minutes)
- Airway: Patent, no stridor
- Breathing: Rate 12-20, SpO₂ >94%
- Circulation: Pulse rate/quality, BP, perfusion
- Disability: Mental status, neurological deficits
-
Secondary Survey (<5 minutes)
- 12-lead ECG: ST changes, arrhythmias, conduction blocks
- Chest X-ray: Pulmonary edema, cardiomegaly, pneumothorax
- Laboratory: Troponin, BNP, electrolytes, CBC
- Echocardiogram: Wall motion, EF, valvular function
| Clinical Scenario | Key Decision Point | Time Target | Success Metric | Alternative Strategy |
|---|---|---|---|---|
| STEMI | Reperfusion strategy | <90 min PCI | TIMI 3 flow | Fibrinolysis <30 min |
| Cardiogenic Shock | Hemodynamic support | <60 min | CI >2.2 L/min/m² | Mechanical support |
| Acute HF | Volume assessment | <30 min | Symptom relief | Ultrafiltration |
| Hypertensive Crisis | BP reduction rate | 10-20%/hour | No end-organ damage | IV antihypertensives |
| Cardiac Arrest | ROSC achievement | <20 min | Neurological intact | ECMO consideration |
-
Antiplatelet Agents
- Aspirin: 81mg daily (maintenance), 162-325mg (loading)
- Clopidogrel: 75mg daily (maintenance), 600mg (loading)
- Ticagrelor: 90mg BID (maintenance), 180mg (loading)
-
Anticoagulants
- Heparin: 60 units/kg bolus, 12 units/kg/hr (target aPTT 60-80 sec)
- Enoxaparin: 1mg/kg BID (treatment), 30mg BID (prophylaxis)
- Warfarin: Target INR 2.0-3.0 (most indications), 2.5-3.5 (mechanical valves)
⭐ Clinical Pearl: Door-to-balloon time <90 minutes reduces mortality by 42% in STEMI patients. Every 30-minute delay increases mortality by 7.5%. Pre-hospital activation of catheterization lab saves average 15-20 minutes.
💡 Master This: GRACE score predicts 6-month mortality in ACS patients with C-statistic 0.83. Score >140 indicates high risk (>3% mortality) warranting early invasive strategy within 24 hours. Score <109 indicates low risk (<1% mortality) suitable for conservative management.
These mastery tools provide the clinical framework for expert cardiovascular practice. Apply these systematic approaches and evidence-based thresholds to achieve optimal diagnostic accuracy and therapeutic outcomes in every cardiovascular encounter.
🎯 Cardiovascular Mastery Toolkit: Clinical Excellence Framework
Practice Questions: Cardiovascular
Test your understanding with these related questions
A 48-year-old male accountant presents to the family practice clinic for his first health check-up in years. He has no complaints, and as far as he is concerned, he is well. He does not have any known medical conditions. His blood pressure is 140/89 mm Hg and his heart rate is 89/min. Physical examination is otherwise unremarkable. What is the single best initial management for this patient?













