Protozoa/Helminths

On this page
🦠 Parasitic Mastery: The Hidden World of Protozoa and Helminths
Parasites have shaped human history more than any war or empire, yet they remain invisible adversaries that infect billions today. You'll master the biology, transmission routes, and clinical presentations of protozoa and helminths, then build systematic approaches to diagnosis, treatment, and prevention that work across diverse global settings. From recognizing subtle symptom patterns to selecting targeted therapies and implementing public health strategies, you'll develop the clinical reasoning that transforms these complex organisms from diagnostic mysteries into manageable conditions.
📌 Remember: HELM for major parasite groups - Helminths (worms), Ectoparasites (external), Leishmania/blood protozoa, Malaria - covering >95% of clinically significant parasitic diseases worldwide
The parasitic kingdom encompasses over 300 species that infect humans, yet mastering the top 25 high-yield organisms provides the foundation for 85% of parasitic diagnoses encountered in clinical practice. These organisms have co-evolved with humans for millennia, developing sophisticated mechanisms to evade immune responses while causing predictable clinical syndromes.
| Parasite Class | Major Species | Primary Transmission | Global Prevalence | Mortality Rate | Key Diagnostic Feature |
|---|---|---|---|---|---|
| Protozoa | Plasmodium spp. | Mosquito vector | 250M cases/year | 2-15% | Intraerythrocytic forms |
| Cestodes | Taenia spp. | Undercooked meat | 50M infected | <1% | Segmented body structure |
| Trematodes | Schistosoma spp. | Freshwater contact | 240M infected | 5-10% | Terminal spine morphology |
| Nematodes | Ascaris lumbricoides | Fecal-oral route | 1.5B infected | <1% | Cylindrical unsegmented body |
| Tissue Protozoa | Toxoplasma gondii | Cat exposure/food | 2B infected | <1% | Intracellular cysts |
- Single-celled eukaryotic organisms with complex life cycles
- Reproduce through binary fission or sexual reproduction
- Size range: 2-100 micrometers in diameter
- Plasmodium: 1-5 μm (smallest)
- Balantidium: 50-100 μm (largest)
- Entamoeba: 10-25 μm (most common)
- Helminth Characteristics
- Multicellular worms with specialized organ systems
- Length varies from 2mm to 10 meters
- Lifespan: 1-30 years in human hosts
- Enterobius: 1-2 months (shortest)
- Schistosoma: 5-30 years (longest)
- Ascaris: 1-2 years (most common)
⭐ Clinical Pearl: Eosinophilia >500 cells/μL suggests helminth infection in 85% of cases, while <200 cells/μL makes helminth infection unlikely (negative predictive value 92%)
💡 Master This: Geographic exposure history within 5 years predicts 75% of exotic parasitic infections - always correlate travel patterns with incubation periods and endemic zones
Connect foundational parasite classification through transmission mechanisms to understand how environmental factors determine infection patterns and clinical presentations.
🦠 Parasitic Mastery: The Hidden World of Protozoa and Helminths
🔄 Transmission Dynamics: The Parasitic Pathway Network
📌 Remember: VIPS transmission routes - Vector-borne (25% of parasites), Ingestion (45%), Percutaneous (20%), Sexual/direct contact (10%) - covering all major parasitic transmission mechanisms
Vector-Borne Transmission Patterns:
- Anopheles mosquitoes: Active dusk to dawn (6 PM - 6 AM)
- Peak biting: 10 PM - 2 AM
- Flight range: 1-3 kilometers from breeding sites
- Altitude limit: <2000 meters elevation
- Sandflies (Phlebotomus): Active 2-4 hours post-sunset
- Flight range: <200 meters (poor fliers)
- Breeding sites: organic debris in arid regions
- Peak season: dry months with temperatures 20-35°C
| Transmission Route | Incubation Period | Geographic Risk | Prevention Efficacy | Diagnostic Window | Treatment Success |
|---|---|---|---|---|---|
| Vector-borne | 7-30 days | Tropical/subtropical | 85% with barriers | 48-72 hours | 90-95% |
| Food/Water | 1-14 days | Global distribution | 95% with sanitation | 24-48 hours | 95-99% |
| Percutaneous | 2-8 weeks | Freshwater regions | 90% with protection | 4-12 weeks | 70-85% |
| Direct Contact | 1-7 days | Crowded conditions | 80% with hygiene | 12-24 hours | 95-99% |
| Congenital | Birth to months | Global risk | 60% with screening | Variable | 50-80% |
- Temperature: Optimal range 25-30°C for most vectors
- Humidity: >60% required for vector survival
- Rainfall: 100-200mm annually supports breeding
- Drought conditions: Concentrate water sources, increase transmission
- Flooding: Disperse vectors, decrease immediate risk
- Seasonal patterns: Wet season = peak transmission for 80% of vector-borne diseases
- Socioeconomic Transmission Amplifiers
- Population density: >1000 people/km² increases transmission 3-5 fold
- Sanitation access: <50% coverage correlates with 10x higher parasite prevalence
- Water quality: >10 CFU/100mL bacterial contamination predicts parasitic co-infection
- Urban slums: 15-25x higher transmission rates
- Rural poverty: 5-10x increased infection risk
- Refugee populations: 20-40x elevated parasite burden
⭐ Clinical Pearl: Fever within 7 days of tropical travel suggests bacterial/viral causes (85% probability), while fever after 14 days increases parasitic probability to 45%, especially malaria
💡 Master This: Eosinophil count timing reveals transmission route - immediate elevation suggests ongoing tissue migration (Ascaris, hookworm), while delayed eosinophilia indicates chronic infection (Schistosoma, filarial worms)
Connect transmission understanding through diagnostic pattern recognition to identify the clinical presentations that distinguish different parasitic infections.
🔄 Transmission Dynamics: The Parasitic Pathway Network
🎯 Clinical Recognition: The Parasitic Syndrome Decoder
📌 Remember: SHIFT for parasitic fever patterns - Spiking (malaria), Hectic (amoebic liver), Intermittent (Kala-azar), Fluctuating (trypanosomiasis), Tertian/quartan (specific Plasmodium species)
Fever Pattern Recognition Framework:
-
Malaria (Plasmodium)
- P. falciparum: Continuous fever, no periodicity
- P. vivax/ovale: 48-hour cycles (tertian pattern)
- P. malariae: 72-hour cycles (quartan pattern)
- Associated: Rigors lasting 15-60 minutes, profuse sweating, severe headache
-
Visceral Leishmaniasis (Kala-azar)
- Double quotidian: Two fever spikes daily
- Duration: Weeks to months of irregular fever
- Associated: Massive splenomegaly (extends >5cm below costal margin)
- Pancytopenia: WBC <4000, platelets <100,000
| Clinical Syndrome | Primary Parasites | Key Symptoms | Timeline | Diagnostic Clue | Mortality Risk |
|---|---|---|---|---|---|
| Acute Fever | Plasmodium, Babesia | Rigors, sweats, headache | 1-4 weeks | Cyclical pattern | 5-20% |
| Chronic Diarrhea | Giardia, Cryptosporidium | Watery stools, malabsorption | >4 weeks | Fatty stools | <1% |
| Eosinophilia | Helminths (tissue phase) | Often asymptomatic | 2-8 weeks | >500 cells/μL | <1% |
| Hepatosplenomegaly | Schistosoma, Leishmania | Abdominal distension | Months-years | Portal hypertension | 10-30% |
| Neurologic | Toxoplasma, Cysticercus | Seizures, focal deficits | Variable | Ring-enhancing lesions | 20-50% |
- Acute bloody diarrhea: E. histolytica (85% of parasitic dysentery)
- >6 stools/day with blood and mucus
- Tenesmus and lower abdominal cramping
- Fever in 40% of cases
- Chronic watery diarrhea: Giardia lamblia (most common)
- Malabsorption with steatorrhea
- Weight loss >10% body weight
- Flatulence and sulfurous belching
- Malabsorption syndrome: Strongyloides or Giardia
- Vitamin B12 deficiency (Diphyllobothrium)
- Fat-soluble vitamin deficiency
- Protein-energy malnutrition
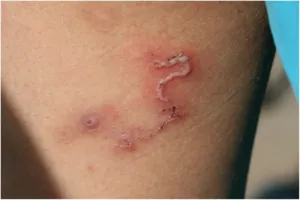
- Skin and Soft Tissue Manifestations
- Cutaneous leishmaniasis: Painless ulcer with raised borders
- Incubation: 2-8 weeks post-sandfly bite
- Self-healing in 6-18 months
- Satellite lesions in 15% of cases
- Larva migrans: Serpiginous tracks advancing 2-5cm/day
- Intense pruritus following tracks
- Eosinophilia >1000 cells/μL
- Self-limited in 4-8 weeks
- Cutaneous leishmaniasis: Painless ulcer with raised borders
⭐ Clinical Pearl: Swimmer's itch within 24 hours of freshwater exposure suggests cercarial dermatitis, while delayed symptoms >4 weeks indicate chronic schistosomiasis with different treatment requirements
💡 Master This: Eosinophil-to-neutrophil ratio >0.1 in febrile patients suggests parasitic infection with 78% sensitivity and 85% specificity - more reliable than absolute eosinophil count alone
Connect clinical pattern recognition through systematic diagnostic approaches to master the laboratory techniques that confirm parasitic infections.
🎯 Clinical Recognition: The Parasitic Syndrome Decoder
🔬 Diagnostic Mastery: The Parasitological Detective System
📌 Remember: MOST for diagnostic sample timing - Malaria (during fever spike), Ova and parasites (3 samples over 7 days), Serology (>4 weeks post-exposure), Tissue biopsy (for definitive species identification)
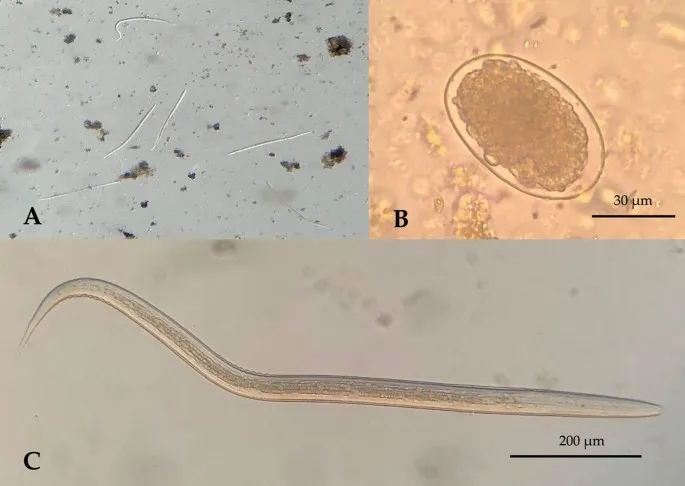
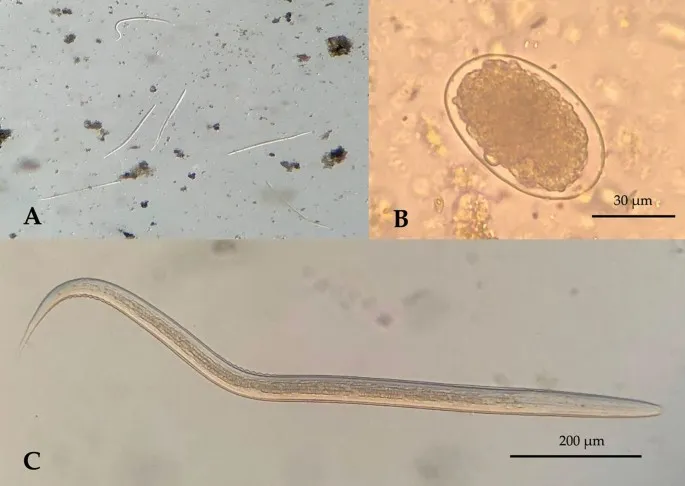
Microscopic Diagnostic Standards:
-
Stool Examination Protocol
- Three specimens collected on alternate days
- Fresh sample: Examined within 1 hour for motile trophozoites
- Preserved sample: 10% formalin for cyst morphology
- Concentration techniques: Formalin-ethyl acetate increases sensitivity 3-5 fold
- Sensitivity: 85-95% for 3 specimens, 60-70% for single specimen
-
Blood Smear Techniques
- Thick smear: Sensitivity 95% for >100 parasites/μL
- Thin smear: Species identification and parasitemia quantification
- Optimal timing: During fever spike or every 6 hours for 24 hours
- Parasitemia calculation: Parasites per 200 WBCs × WBC count/200
| Diagnostic Method | Sensitivity | Specificity | Time to Result | Cost Range | Clinical Application |
|---|---|---|---|---|---|
| Microscopy | 85-95% | >99% | 1-2 hours | $10-25 | First-line screening |
| Antigen Detection | 90-98% | 95-99% | 15-30 minutes | $15-40 | Rapid diagnosis |
| PCR/Molecular | >99% | >99% | 2-6 hours | $50-150 | Species confirmation |
| Serology | 80-95% | 85-95% | 1-3 days | $25-75 | Chronic/past infection |
| Culture | 70-90% | >99% | 3-7 days | $30-60 | Drug susceptibility |
- Malaria RDTs: Detect HRP-2, pLDH, or aldolase antigens
- Sensitivity: >95% for P. falciparum at >100 parasites/μL
- Specificity: >95% in non-endemic areas
- False positives: HRP-2 persists 2-4 weeks post-treatment
- Giardia/Cryptosporidium: Enzyme immunoassays
- Sensitivity: 90-95% for Giardia, 85-90% for Cryptosporidium
- Superior to microscopy for Cryptosporidium detection
- Results: Available in 15-30 minutes
- Molecular Diagnostics
- Real-time PCR: Gold standard for species identification
- Sensitivity: >99% for >1 parasite/μL
- Quantitative: Provides parasitemia levels
- Multiplex panels: Detect 15-20 pathogens simultaneously
- LAMP (Loop-mediated isothermal amplification)
- Field-deployable technology
- Results: 30-60 minutes without thermal cycling
- Sensitivity: 95-98% compared to PCR
- Real-time PCR: Gold standard for species identification
⭐ Clinical Pearl: Negative microscopy with high clinical suspicion requires molecular confirmation - PCR sensitivity is 10-100 fold higher than microscopy for low-level parasitemia
💡 Master This: Serology timing determines interpretation - IgM positive suggests recent infection (<3 months), while IgG positive indicates past exposure or chronic infection (may persist years)
Connect diagnostic mastery through treatment algorithms to understand how accurate identification guides therapeutic decision-making and monitoring.
🔬 Diagnostic Mastery: The Parasitological Detective System
⚕️ Treatment Algorithms: The Therapeutic Command Center
📌 Remember: RAPID treatment principles - Resistance patterns, Adverse effects, Pharmacology, Interaction potential, Dose optimization - ensuring >90% cure rates with minimal toxicity
Antimalarial Treatment Algorithms:
-
Uncomplicated P. falciparum
- First-line: Artemether-lumefantrine (Coartem)
- Dosing: 4 tablets twice daily for 3 days (adults)
- Cure rate: >95% in artemisinin-sensitive areas
- Take with food: Increases lumefantrine absorption 3-fold
- Alternative: Atovaquone-proguanil (Malarone)
- Dosing: 4 tablets daily for 3 days
- Cure rate: >98% globally
- Advantage: No resistance in most regions
- First-line: Artemether-lumefantrine (Coartem)
-
Severe/Complicated Malaria
- First-line: IV Artesunate 2.4 mg/kg at 0, 12, 24 hours, then daily
- Mortality reduction: 15-35% compared to quinine
- Follow-up: Oral artemisinin combination after IV treatment
- Monitoring: Parasitemia every 12 hours until <1%
| Parasite Class | First-Line Drug | Dosing | Duration | Cure Rate | Major Side Effects |
|---|---|---|---|---|---|
| Malaria (uncomplicated) | Artemether-lumefantrine | 4 tabs BID | 3 days | >95% | GI upset (15%) |
| Malaria (severe) | IV Artesunate | 2.4 mg/kg | 3+ days | >90% | Delayed hemolysis (5%) |
| Giardiasis | Metronidazole | 250 mg TID | 7 days | 85-95% | Metallic taste (30%) |
| Amoebiasis | Metronidazole + Paromomycin | 750 mg TID + 500 mg TID | 10 + 7 days | >95% | GI upset (20%) |
| Schistosomiasis | Praziquantel | 20 mg/kg BID | 1 day | 85-95% | Transient symptoms (40%) |
- Giardiasis Treatment Options
- Metronidazole: 250 mg TID × 7 days (85-95% cure)
- Tinidazole: 2g single dose (90-95% cure, better compliance)
- Nitazoxanide: 500 mg BID × 3 days (85-90% cure, pregnancy safe)
- Amebic Dysentery Protocol
- Tissue amebicide: Metronidazole 750 mg TID × 10 days
- Luminal amebicide: Paromomycin 500 mg TID × 7 days
- Cure verification: Stool examination at 2-4 weeks
- Antihelminthic Therapy
- Broad-spectrum: Albendazole 400 mg (single dose for most nematodes)
- Ascaris: Single dose, cure rate >95%
- Hookworm: Single dose, cure rate 85-90%
- Trichuris: 3 days, cure rate 70-80%
- Schistosomiasis: Praziquantel 20 mg/kg BID × 1 day
- All species: Single-day treatment
- Cure rate: 85-95% for all Schistosoma species
- Repeat treatment: 4-6 weeks if persistent symptoms
- Broad-spectrum: Albendazole 400 mg (single dose for most nematodes)
⭐ Clinical Pearl: Artemisinin resistance (delayed parasite clearance >72 hours) requires extended treatment and alternative combinations - monitor parasite clearance half-life as resistance marker
💡 Master This: Drug-food interactions significantly affect antiparasitic efficacy - lumefantrine requires fatty meal for absorption, while atovaquone needs food for bioavailability
Connect treatment understanding through prevention strategies to master the public health approaches that control parasitic transmission and reduce global disease burden.
⚕️ Treatment Algorithms: The Therapeutic Command Center
🛡️ Prevention Mastery: The Global Defense Network
📌 Remember: SHIELD prevention strategies - Sanitation improvement, Host protection, Insecticide use, Education programs, Larvicide application, Drug prophylaxis - achieving >80% transmission reduction when combined effectively
Vector Control Strategies:
-
Insecticide-Treated Nets (ITNs)
- Coverage target: >80% of at-risk population
- Effectiveness: 50-60% reduction in malaria incidence
- Lifespan: 3-5 years with proper maintenance
- Resistance management: Rotate insecticide classes every 3-5 years
-
Indoor Residual Spraying (IRS)
- Target coverage: >85% of structures in endemic areas
- Effectiveness: 45-55% reduction in transmission
- Duration: 6-12 months depending on insecticide class
- Cost-effectiveness: $2-8 per person protected annually
| Prevention Strategy | Target Diseases | Effectiveness | Cost per DALY | Implementation Scale | Sustainability |
|---|---|---|---|---|---|
| ITNs | Malaria, lymphatic filariasis | 50-60% | $2-24 | Population-wide | High |
| IRS | Malaria, Chagas disease | 45-55% | $8-32 | High-risk areas | Medium |
| Mass Drug Administration | Lymphatic filariasis, onchocerciasis | 70-90% | $1-15 | Endemic communities | High |
| Water/Sanitation | Intestinal parasites | 60-80% | $5-50 | Community-based | Very High |
| Chemoprophylaxis | Malaria | 85-95% | $15-100 | Individual | Low |
- Malaria Prevention by Region
- Chloroquine-sensitive areas: Chloroquine 300 mg weekly
- Chloroquine-resistant areas: Atovaquone-proguanil daily or doxycycline daily
- Mefloquine: 250 mg weekly (avoid if psychiatric history)
- Duration: Start 1-2 weeks before travel, continue 4 weeks after return
- Traveler's Diarrhea Prevention
- High-risk areas: Bismuth subsalicylate or probiotics
- Immunocompromised: Rifaximin 200 mg daily
- Food/water precautions: >95% effective when strictly followed
- Community-Based Interventions
- Water, Sanitation, and Hygiene (WASH)
- Improved water sources: 60-70% reduction in waterborne parasites
- Sanitation coverage: >75% needed for community-wide impact
- Hygiene education: 30-40% additional reduction when combined with infrastructure
- Mass Drug Administration (MDA)
- Lymphatic filariasis: Annual treatment for >5 years
- Coverage target: >80% of eligible population
- Drug combinations: Albendazole + DEC or Albendazole + ivermectin
- Water, Sanitation, and Hygiene (WASH)
⭐ Clinical Pearl: Combination prevention strategies achieve synergistic effects - ITNs + IRS provide 70-80% protection versus 50-60% for either intervention alone
💡 Master This: Resistance management requires rotation strategies - insecticide classes should be rotated every 3-5 years to maintain effectiveness and delay resistance development
Connect prevention strategies through rapid clinical reference tools to create a comprehensive mastery framework for immediate clinical application.
🛡️ Prevention Mastery: The Global Defense Network
🎯 Clinical Arsenal: The Parasitology Quick-Strike Reference
📌 Remember: STAT for parasitic emergencies - Severe malaria (cerebral/pulmonary), Toxoplasmosis (CNS), Amebic liver abscess (rupture risk), Trypanosomiasis (cardiac/CNS) - requiring immediate intervention within hours
Emergency Parasitic Syndromes:
- Severe Malaria Criteria (any one indicates severe disease)
- Cerebral malaria: GCS <11 or seizures
- Severe anemia: Hgb <7 g/dL with parasitemia >2%
- Pulmonary edema: ARDS with parasitemia
- Renal failure: Creatinine >3 mg/dL
- Hypoglycemia: Glucose <40 mg/dL
- Shock: SBP <80 mmHg with parasitemia
| Clinical Scenario | Key Features | Immediate Action | Drug of Choice | Monitoring | Prognosis |
|---|---|---|---|---|---|
| Cerebral Malaria | GCS <11, seizures, parasitemia | IV access, artesunate | Artesunate 2.4 mg/kg | Neuro checks q2h | Mortality 15-20% |
| Amebic Liver Abscess | RUQ pain, fever, hepatomegaly | Imaging, metronidazole | Metronidazole 750 mg TID | Size monitoring | Mortality <1% |
| Neurocysticercosis | Seizures, ring lesions | Anticonvulsants, steroids | Albendazole + prednisone | ICP monitoring | Variable |
| Strongyloides Hyperinfection | Eosinopenia, GI bleeding | Isolation, ivermectin | Ivermectin 200 μg/kg | Stool monitoring | Mortality 50-85% |
| Babesiosis | Hemolysis, parasitemia >10% | Exchange transfusion | Atovaquone + azithromycin | Parasitemia levels | Mortality 5-10% |
- Fever + Travel History
- <7 days: Bacterial/viral most likely (85%)
- 7-21 days: Malaria consideration (45% if endemic exposure)
- >21 days: Chronic parasites (Schistosoma, Leishmania)
- Eosinophilia + Symptoms
- >1500 cells/μL: Helminth infection (90% probability)
- 500-1500 cells/μL: Tissue migration phase (70% probability)
- <500 cells/μL: Protozoan infection more likely
- Treatment Quick Reference
- Life-Threatening Emergencies
- Severe malaria: IV artesunate immediately
- Amebic liver abscess: Metronidazole + drainage if >5cm
- Strongyloides hyperinfection: Ivermectin + supportive care
- Common Outpatient Infections
- Giardiasis: Tinidazole 2g single dose
- Pinworm: Albendazole 400mg single dose, repeat in 2 weeks
- Scabies: Permethrin 5% cream, whole body application
- Life-Threatening Emergencies
⭐ Clinical Pearl: Negative malaria smear does NOT rule out severe malaria - PCR sensitivity is 10-fold higher, and treatment should be initiated based on clinical suspicion in high-risk patients
💡 Master This: Steroid contraindications in parasitic infections - avoid steroids in Strongyloides (hyperinfection risk), amebic dysentery (perforation risk), and active malaria (cerebral edema risk)
This comprehensive parasitology framework transforms complex infectious disease knowledge into systematic clinical expertise, enabling rapid diagnosis and evidence-based treatment of parasitic infections across all clinical settings.
🎯 Clinical Arsenal: The Parasitology Quick-Strike Reference
Practice Questions: Protozoa/Helminths
Test your understanding with these related questions
A 30-year-old forest landscape specialist is brought to the emergency department with hematemesis and confusion. One week ago, she was diagnosed with influenza when she had fevers, severe headaches, myalgias, hip and shoulder pain, and a maculopapular rash. After a day of relative remission, she developed abdominal pain, vomiting, and diarrhea. A single episode of hematemesis occurred prior to admission. Two weeks ago she visited rainforests and caves in western Africa where she had direct contact with animals, including apes. She has no history of serious illnesses or use of medications. She is restless and her temperature is 38.0°C (100.4°F); pulse, 95/min; respirations, 20/min; and supine and upright blood pressure, 130/70 mm Hg and 100/65 mm Hg, respectively. Conjunctival suffusion is seen. Ecchymoses are observed on the lower extremities. She is bleeding from one of her intravenous lines. The peripheral blood smear is negative for organisms. The laboratory studies show the following: Hemoglobin 10 g/dL Leukocyte count 1,000/mm3 Segmented neutrophils 65% Lymphocytes 20% Platelet count 50,000/mm3 Partial thromboplastin time (activated) 60 seconds Prothrombin time 25 seconds Fibrin split products positive Serum Alanine aminotransferase (ALT) 85 U/L Aspartate aminotransferase (AST) 120 U/L γ-Glutamyltransferase (GGT) 83 U/L (N = 5–50 U/L) Creatinine 2 mg/dL Which of the following is the most likely causal pathogen?











