Microbiome in antibiotic-associated diarrhea US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Microbiome in antibiotic-associated diarrhea. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Microbiome in antibiotic-associated diarrhea US Medical PG Question 1: A 47-year-old man comes to the physician because of abdominal pain and foul-smelling, watery diarrhea for several days. He has not had nausea, vomiting, or blood in the stool. He has a history of alcohol use disorder and recently completed a 7-day course of clindamycin for pneumonia. He has not traveled out of the United States. Which of the following toxins is most likely to be involved in the pathogenesis of this patient's symptoms?
- A. Cereulide toxin
- B. Cholera toxin
- C. Clostridioides difficile cytotoxin (Correct Answer)
- D. Shiga toxin
- E. Alpha toxin
Microbiome in antibiotic-associated diarrhea Explanation: ***Clostridioides difficile cytotoxin***
- The patient's history of recent **clindamycin** use, followed by **abdominal pain** and **foul-smelling, watery diarrhea**, is highly suggestive of *Clostridioides difficile* infection.
- *C. difficile* produces **cytotoxin (TcdB)** and **enterotoxin (TcdA)**, which lead to colitis and diarrhea, often after antimicrobial therapy.
*Cereulide toxin*
- This preformed toxin is produced by *Bacillus cereus* and typically causes a **short-incubation** emetic type of food poisoning, characterized by **nausea and vomiting**.
- The patient's symptoms are primarily diarrhea, and nausea/vomiting are absent, making this less likely.
*Cholera toxin*
- Produced by *Vibrio cholerae*, this toxin causes profuse, **"rice-water" diarrhea** with rapid dehydration.
- The patient has not traveled to endemic areas, and there is no mention of the characteristic severe dehydration or "rice-water" stool.
*Shiga toxin*
- This toxin, produced by *Shigella dysenteriae* and enterohemorrhagic *E. coli* (EHEC), typically causes **bloody diarrhea** and can lead to **hemolytic uremic syndrome (HUS)**.
- The patient's diarrhea is watery and explicitly stated to be without blood, ruling out Shiga toxin as the cause.
*Alpha toxin*
- This toxin is produced by *Clostridium perfringens* and is primarily associated with **gas gangrene** (myonecrosis) and some forms of food poisoning.
- While *C. perfringens* can cause diarrhea, it's typically mild and self-limiting, and the clinical picture in this patient, especially with recent antibiotic use, points more strongly to *C. difficile*.
Microbiome in antibiotic-associated diarrhea US Medical PG Question 2: On the 4th day of hospital admission due to pneumonia, a 69-year-old woman develops non-bloody diarrhea and abdominal pain. She is currently treated with ceftriaxone. Despite the resolution of fever after the first 2 days of admission, her temperature is now 38.5°C (101.3°F). On physical examination, she has mild generalized abdominal tenderness without abdominal guarding or rebound tenderness. Laboratory studies show re-elevation of leukocyte counts. Ceftriaxone is discontinued. Given the most likely diagnosis in this patient, which of the following is the most sensitive test?
- A. Nucleic acid amplification test (Correct Answer)
- B. Stool culture for bacterial isolation and toxin presence
- C. Enzyme immunoassay glutamate dehydrogenase
- D. Gram stain of stool sample
- E. Endoscopy
Microbiome in antibiotic-associated diarrhea Explanation: ***Nucleic acid amplification test***
- **NAAT** (PCR) for *C. difficile* toxin genes is the most **sensitive** and specific test for routine clinical diagnosis of *C. difficile* infection.
- It detects the **DNA** of toxin-producing *C. difficile* (tcdB gene) and is highly reliable even with low bacterial loads.
- NAAT has become the **gold standard** in most clinical settings due to its rapid turnaround time (hours) and excellent sensitivity (~90-95%) and specificity (~95%).
*Enzyme immunoassay glutamate dehydrogenase*
- **EIA GDH** detects an antigen common to all *C. difficile* strains (both toxin-producing and non-toxin-producing).
- While it has **high sensitivity** (~85-95%), it has **low specificity** and requires confirmation with a toxin test or NAAT, as it cannot distinguish between toxigenic and non-toxigenic strains.
- Often used as part of a **two-step algorithm** for screening.
*Gram stain of stool sample*
- A **Gram stain** of stool is generally not helpful for diagnosing *C. difficile* infection.
- It would show a mix of **gut flora** and would not specifically identify *C. difficile* or its toxins.
*Stool culture for bacterial isolation and toxin presence*
- **Stool culture** for *C. difficile* is technically the most sensitive method (~95-100%) but does not differentiate toxin-producing from non-toxin-producing strains without subsequent **toxin testing**.
- It is also **time-consuming** (2-3 days) and labor-intensive, making it impractical for routine clinical diagnosis.
- Primarily used for **research** or **epidemiological typing**.
*Endoscopy*
- **Endoscopy** with visualization of **pseudomembranes** is highly specific for severe *C. difficile* infection.
- However, it is an **invasive procedure**, not sensitive for mild-to-moderate disease, and is usually reserved for cases where diagnosis is unclear or severe complications (toxic megacolon, fulminant colitis) are suspected.
Microbiome in antibiotic-associated diarrhea US Medical PG Question 3: A 42-year-old man with hypertension and type 2 diabetes mellitus is admitted to the hospital because of swelling and redness of the left leg for 3 days. He has chills and malaise. He is treated with intravenous clindamycin for 7 days. On the 8th day at the hospital, he has profuse, foul-smelling, and watery diarrhea. He has nausea and intermittent abdominal cramping. His temperature is 38°C (100.4°F), pulse is 97/min, and blood pressure is 110/78 mm Hg. Bowel sounds are hyperactive. Abdominal examination shows mild tenderness in the left lower quadrant. Rectal examination shows no abnormalities. His hemoglobin concentration is 14.3 g/dL, leukocyte count is 12,300/mm3, and C-reactive protein concentration is 62 mg/L (N=0.08–3.1). After discontinuing clindamycin, which of the following is the most appropriate pharmacotherapy for this patient's condition?
- A. Intravenous vancomycin
- B. Oral fidaxomicin (Correct Answer)
- C. Intravenous metronidazole
- D. Oral metronidazole
- E. Oral rifaximin
Microbiome in antibiotic-associated diarrhea Explanation: ***Oral fidaxomicin***
- The patient's presentation with profuse, foul-smelling, watery diarrhea, abdominal cramping, and fever after prolonged antibiotic use (clindamycin) is highly suggestive of **Clostridioides difficile infection (CDI)**.
- **Oral fidaxomicin** is a first-line agent for initial CDI episodes with **superior efficacy** in reducing recurrence rates compared to metronidazole and similar cure rates to oral vancomycin. It is preferred due to its **narrow spectrum**, **bactericidal activity against C. difficile**, and **minimal disruption to normal colonic flora**.
- Current IDSA/SHEA guidelines recommend fidaxomicin or oral vancomycin as first-line therapy for initial CDI episodes.
*Intravenous vancomycin*
- **Intravenous vancomycin** has poor penetration into the GI tract and is therefore **ineffective for C. difficile infection (CDI)**, which is an intraluminal infection.
- Oral vancomycin is effective for CDI, but intravenous administration will not treat the infection.
*Intravenous metronidazole*
- **Intravenous metronidazole** has limited efficacy in treating **Clostridioides difficile infection (CDI)** as first-line therapy.
- While it achieves some colonic concentration even when given intravenously, oral agents (fidaxomicin or vancomycin) are preferred for initial episodes.
- IV metronidazole may be used as adjunctive therapy in fulminant cases with ileus when oral agents cannot reach the colon.
*Oral metronidazole*
- **Oral metronidazole** was previously used for non-severe CDI but is **no longer recommended as first-line therapy** per updated IDSA/SHEA guidelines due to inferior cure rates and higher recurrence rates compared to vancomycin and fidaxomicin.
- It may be considered only when fidaxomicin and vancomycin are unavailable.
*Oral rifaximin*
- **Oral rifaximin** is sometimes used as **adjunctive therapy following standard treatment** to prevent recurrent C. difficile infection (CDI).
- It is **not recommended as initial monotherapy** for an active CDI episode.
Microbiome in antibiotic-associated diarrhea US Medical PG Question 4: A 21-year-old woman comes to the physician because of a 4-day history of abdominal cramps and bloody diarrhea 5 times per day. Her symptoms began after she ate an egg sandwich from a restaurant. Her vital signs are within normal limits. Physical examination shows diffuse abdominal tenderness. Stool culture shows gram-negative rods that produce hydrogen sulfide and do not ferment lactose. Which of the following effects is most likely to occur if she receives antibiotic therapy?
- A. Orange discoloration of bodily fluids
- B. Pruritic maculopapular rash on the extensor surface
- C. Self-limiting systemic inflammatory response
- D. Prolonged fecal excretion of the pathogen (Correct Answer)
- E. Thrombocytopenia and hemolytic anemia
Microbiome in antibiotic-associated diarrhea Explanation: ***Prolonged fecal excretion of the pathogen***
- The patient's symptoms (abdominal cramps, bloody diarrhea after eating an egg sandwich) and stool culture results (gram-negative rods, hydrogen sulfide producers, non-lactose fermenting) are highly suggestive of **Salmonella enterica** infection.
- Antibiotic treatment for non-typhoidal Salmonella gastroenteritis typically **prolongs fecal excretion** and does not shorten the illness, reserving antibiotics for severe cases or immunocompromised individuals.
*Orange discoloration of bodily fluids*
- **Orange discoloration of bodily fluids** (urine, sweat, tears) is a known side effect of **rifampin**, an antibiotic primarily used for tuberculosis and some bacterial meningitides.
- Rifampin is not indicated nor commonly used for Salmonella gastroenteritis.
*Pruritic maculopapular rash on the extensor surface*
- A **pruritic maculopapular rash on the extensor surfaces** is a common presentation of drug reactions, often associated with **penicillins** or **cephalosporins**, especially in viral infections (e.g., amoxicillin rash in mononucleosis).
- This is a general antibiotic side effect and not specifically linked to the outcome of treating Salmonella.
*Self-limiting systemic inflammatory response*
- A self-limiting systemic inflammatory response could be a general reaction to an active infection or a drug, but it's not the most likely or specific outcome of **antibiotic therapy in Salmonella gastroenteritis**.
- Worsening of symptoms can occur in some cases due to toxemia from bacterial lysis (e.g., Jarisch-Herxheimer reaction), but "self-limiting systemic inflammatory response" is too generic for this specific scenario.
*Thrombocytopenia and hemolytic anemia*
- **Thrombocytopenia and hemolytic anemia** in the setting of diarrheal illness strongly suggest **hemolytic uremic syndrome (HUS)**, which is typically associated with **Shiga toxin-producing E. coli** (STEC), particularly E. coli O157:H7.
- While Salmonella can cause severe disease, HUS is not a typical complication of its treatment, and antibiotics are often avoided in STEC infections due to increased risk of HUS.
Microbiome in antibiotic-associated diarrhea US Medical PG Question 5: A 55-year-old man presents to the physician with complaints of 5 days of watery diarrhea, fever, and bloating. He has not noticed any blood in his stool. He states that his diet has not changed recently, and his family has been spared from diarrhea symptoms despite eating the same foods that he has been cooking at home. He has no history of recent travel outside the United States. His only medication is high-dose omeprazole, which he has been taking daily for the past few months to alleviate his gastroesophageal reflux disease (GERD). Which of the following is the most appropriate initial test to work up this patient’s symptoms?
- A. Stool toxin assay (Correct Answer)
- B. Colonoscopy
- C. Fecal occult blood test
- D. Stool culture
- E. Stool ova and parasite
Microbiome in antibiotic-associated diarrhea Explanation: ***Stool toxin assay***
- The patient's presentation of **watery diarrhea** and fever, especially with a history of **high-dose omeprazole use**, strongly suggests **Clostridioides difficile infection**.
- **Omeprazole** (a proton pump inhibitor) reduces stomach acid, which can disrupt the normal gut flora and increase susceptibility to *C. difficile*; a **stool toxin assay** is the most direct diagnostic test for this infection.
*Colonoscopy*
- While a colonoscopy can visualize pseudomembranes associated with severe *C. difficile* colitis, it is an **invasive procedure** and not the initial diagnostic test of choice for suspected infectious diarrhea.
- It is usually reserved for cases with atypical presentations, suspected complications, or when other diagnostic tests are inconclusive.
*Fecal occult blood test*
- The patient describes **watery diarrhea** and specifically states he has **not noticed any blood in his stool**, making a fecal occult blood test unlikely to be helpful in this acute setting.
- This test is primarily used for screening **colorectal cancer** or identifying chronic gastrointestinal bleeding.
*Stool culture*
- A stool culture primarily identifies bacterial pathogens like *Salmonella*, *Shigella*, or *Campylobacter*, which typically cause diarrheal illnesses that may include **bloody stools** or have specific epidemiological links (e.g., foodborne outbreaks).
- Given the history of **omeprazole use** and the absence of blood, *C. difficile* is more likely than these common bacterial enteritides, and a stool culture does not detect *C. difficile* itself.
*Stool ova and parasite*
- This test is used to detect **parasitic infections** (e.g., Giardia, Cryptosporidium), which can cause watery diarrhea and bloating.
- However, given the specific risk factor of **omeprazole use**, **Clostridioides difficile** infection is a more probable diagnosis, making the stool toxin assay the more appropriate initial test.
Microbiome in antibiotic-associated diarrhea US Medical PG Question 6: A 70-year-old man with loose stools over the last 24 hours, accompanied by abdominal pain, cramps, nausea, and anorexia, was hospitalized. Previously, the man was diagnosed with a lung abscess and was treated with clindamycin for 5 days. Past medical history was significant for non-erosive antral gastritis and hypertension. He takes esomeprazole and losartan. Despite the respiratory improvement, fevers and leukocytosis persisted. Which of the following pathogenic mechanisms would you expect to find in this patient?
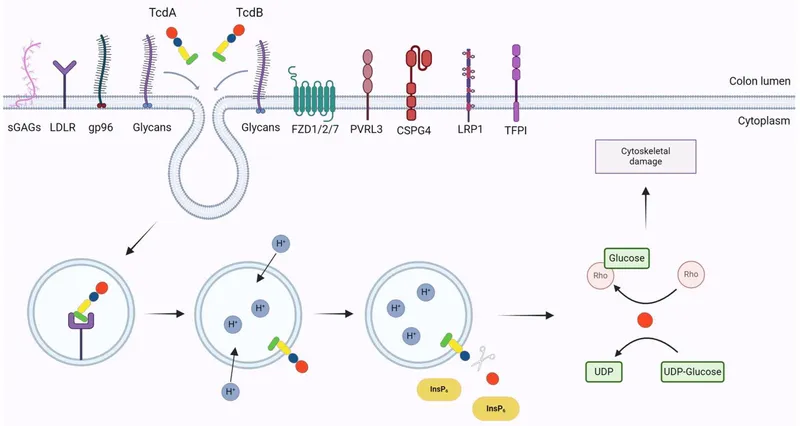
- A. Glucosylation of Rho family GTPases (Correct Answer)
- B. Inactivation of elongation factor EF-2
- C. Inactivation of the 60S ribosome subunit
- D. Cell membrane degradation by lecithinase
- E. ADP-ribosylation of Gs-alpha subunit of G-protein coupled receptors
Microbiome in antibiotic-associated diarrhea Explanation: ***Glucosylation of Rho family GTPases***
- The clinical presentation (clindamycin use, loose stools, abdominal pain, fever, leukocytosis) strongly suggests **_Clostridioides difficile_ infection (CDI)**. The **_C. difficile_ toxins A and B** are glucosyltransferases that modify and inactivate Rho family GTPases.
- **Inactivation of Rho GTPases** leads to disruption of the **cytoskeleton**, loss of tight junctions between enterocytes, and ultimately causes **cell death and colonic inflammation**, resulting in pseudomembranous colitis.
*Inactivation of elongation factor EF-2*
- This is the mechanism of action of **diphtheria toxin** (produced by **_Corynebacterium diphtheriae_**) and **_Pseudomonas aeruginosa_ exotoxin A**.
- These toxins **ADP-ribosylate elongation factor 2 (EF-2)**, inhibiting protein synthesis and leading to cell death. This does not align with the patient's symptoms or antibiotic history.
*Inactivation of the 60S ribosome subunit*
- This mechanism is associated with **Shiga toxin** (produced by **_Shigella dysenteriae_** and **enterohemorrhagic _E. coli_ (EHEC)**) and **ricin toxin**.
- These toxins enzymatically remove an adenine residue from the 28S rRNA of the 60S ribosomal subunit, thereby **halting protein synthesis** and causing cell damage.
*Cell membrane degradation by lecithinase*
- **Lecithinase (alpha-toxin)** produced by **_Clostridium perfringens_** is a phospholipase that degrades **lecithin** in cell membranes.
- This leads to **hemolysis, myonecrosis, and tissue destruction** characteristic of gas gangrene, which is not consistent with the patient's diarrheal illness.
*ADP-ribosylation of Gs-alpha subunit of G-protein coupled receptors*
- This is the mechanism of action of **cholera toxin** (produced by **_Vibrio cholerae_**) and **heat-labile enterotoxin (LT)** of **enterotoxigenic _E. coli_ (ETEC)**.
- **ADP-ribosylation of Gs-alpha subunit** permanently activates adenylate cyclase, leading to increased intracellular **cAMP**, which causes excessive **secretion of water and electrolytes** into the gut lumen, resulting in watery diarrhea, but without significant inflammation as seen in the patient.
Microbiome in antibiotic-associated diarrhea US Medical PG Question 7: A 72-year-old patient presents to the emergency department because of abdominal pain, diarrhea, and fever. He was started on levofloxacin for community-acquired pneumonia 2 weeks prior with resolution of his pulmonary symptoms. He has had hypertension for 20 years, for which he takes amlodipine. His temperature is 38.3°C (101.0°F), pulse is 90/min, and blood pressure is 110/70 mm Hg. On examination, mild abdominal distension with minimal tenderness was found. Laboratory tests reveal a peripheral white blood cell count of 12.000/mm3 and a stool guaiac mildly positive for occult blood. Which of the following best describe the mechanism of this patient illness?
- A. Damage to the gastrointestinal tract by enteropathogenic viruses
- B. Autoimmune inflammation of the rectum
- C. Disruption of normal bowel flora and infection by spore-forming rods (Correct Answer)
- D. Decreased blood flow to the gastrointestinal tract
- E. Presence of osmotically active, poorly absorbed solutes in the bowel lumen
Microbiome in antibiotic-associated diarrhea Explanation: ***Disruption of normal bowel flora and infection by spore-forming rods***
- This describes **Clostridioides difficile infection (CDI)**, which is strongly suggested by the patient's recent antibiotic use (levofloxacin, a fluoroquinolone) followed by abdominal pain, diarrhea, fever, and leukocytosis.
- Antibiotics disrupt the normal gut microbiome, allowing **C. difficile (spore-forming rods)** to proliferate and produce toxins that cause colitis.
*Damage to the gastrointestinal tract by enteropathogenic viruses*
- While viral gastroenteritis can cause these symptoms, the **recent history of antibiotic use** makes CDI a much more likely diagnosis.
- Viral infections typically resolve spontaneously and are less likely to cause a significant **leukocytosis** and **occult blood in stool** in this context.
*Autoimmune inflammation of the rectum*
- Conditions like **ulcerative colitis**, an autoimmune disease, can cause similar symptoms but typically have a **chronic or relapsing course** and are not usually triggered by recent antibiotic use.
- The acute presentation following antibiotics strongly points away from an autoimmune process.
*Decreased blood flow to the gastrointestinal tract*
- **Ischemic colitis** can cause abdominal pain and bloody diarrhea, especially in older patients with vascular risk factors (like hypertension).
- However, the prominent **fever** and **leukocytosis**, coupled with recent antibiotic use, are more indicative of an infectious process like CDI than ischemia.
*Presence of osmotically active, poorly absorbed solutes in the bowel lumen*
- This mechanism describes **osmotic diarrhea**, which can be caused by malabsorption (e.g., lactose intolerance) or certain laxatives.
- Osmotic diarrhea typically **resolves with fasting** and is not usually associated with fever, significant leukocytosis, or occult blood in the stool, which are present here.
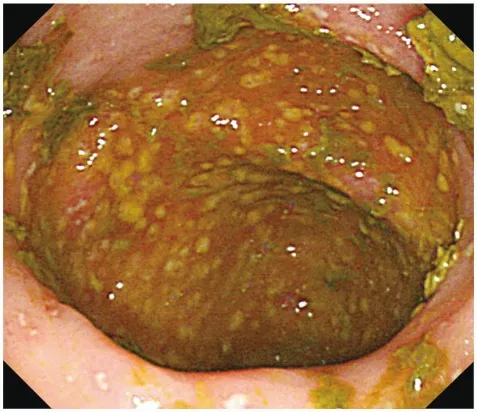
Microbiome in antibiotic-associated diarrhea US Medical PG Question 8: An 87-year-old male nursing home resident is currently undergoing antibiotic therapy for the treatment of a decubitus ulcer. One week into the treatment course, he experiences several episodes of watery diarrhea. Subsequent sigmoidoscopy demonstrates the presence of diffuse yellow plaques on the mucosa of the sigmoid colon. Which of the following is the best choice of treatment for this patient?
- A. Intravenous vancomycin
- B. Intravenous gentamicin
- C. Oral metronidazole (Correct Answer)
- D. Oral trimethoprim/sulfamethoxazole
- E. Oral morphine
Microbiome in antibiotic-associated diarrhea Explanation: ***Oral metronidazole***
- The patient's presentation with **watery diarrhea** and **yellow plaques (pseudomembranes) on sigmoidoscopy** after antibiotic therapy is classic for **Clostridioides difficile infection (CDI)**.
- Among the options provided, **oral metronidazole** is the best choice as it achieves therapeutic concentrations in the colonic lumen and has activity against C. difficile.
- Current **IDSA guidelines** recommend oral **vancomycin or fidaxomicin** as first-line therapy for CDI; however, metronidazole remains an acceptable alternative, particularly in resource-limited settings or when first-line agents are unavailable.
- Metronidazole has good **colonic penetration** when administered orally and is effective against anaerobic bacteria including C. difficile.
*Intravenous vancomycin*
- While **vancomycin** is highly effective against C. difficile, it **must be administered orally** to treat CDI because IV vancomycin does not achieve adequate concentrations in the gut lumen.
- Intravenous vancomycin is excreted primarily by the kidneys and does not reach the colonic mucosa in therapeutic amounts.
- IV vancomycin is appropriate for systemic infections like **MRSA bacteremia or endocarditis**, but not for intestinal infections like CDI.
*Intravenous gentamicin*
- **Gentamicin** is an aminoglycoside antibiotic effective against **gram-negative bacteria** but has **no activity against C. difficile**, which is a gram-positive anaerobic bacillus.
- Aminoglycosides carry significant risks of **nephrotoxicity and ototoxicity**, making them inappropriate for this clinical scenario.
- Use of gentamicin would not address the underlying CDI and could worsen outcomes.
*Oral trimethoprim/sulfamethoxazole*
- **Trimethoprim/sulfamethoxazole** is a broad-spectrum antibiotic effective for various infections (UTIs, Pneumocystis, etc.) but has **no significant activity against C. difficile**.
- Continued antibiotic use with agents ineffective against C. difficile could further disrupt normal gut flora and potentially **worsen the CDI**.
*Oral morphine*
- **Morphine** is an opioid analgesic with **no antibacterial properties** and therefore cannot treat bacterial infections like CDI.
- Opioids can actually **slow gastrointestinal motility**, which may worsen outcomes in CDI by prolonging exposure to toxins.
- While it might provide symptomatic relief of abdominal discomfort, it does not address the underlying infection and is contraindicated in infectious diarrhea.
Microbiome in antibiotic-associated diarrhea US Medical PG Question 9: A 28-year-old male presents to his primary care physician with complaints of intermittent abdominal pain and alternating bouts of constipation and diarrhea. His medical chart is not significant for any past medical problems or prior surgeries. He is not prescribed any current medications. Which of the following questions would be the most useful next question in eliciting further history from this patient?
- A. "Does the diarrhea typically precede the constipation, or vice-versa?"
- B. "Is the diarrhea foul-smelling?"
- C. "Please rate your abdominal pain on a scale of 1-10, with 10 being the worst pain of your life"
- D. "Are the symptoms worse in the morning or at night?"
- E. "Can you tell me more about the symptoms you have been experiencing?" (Correct Answer)
Microbiome in antibiotic-associated diarrhea Explanation: ***Can you tell me more about the symptoms you have been experiencing?***
- This **open-ended question** encourages the patient to provide a **comprehensive narrative** of their symptoms, including details about onset, frequency, duration, alleviating/aggravating factors, and associated symptoms, which is crucial for diagnosis.
- In a patient presenting with vague, intermittent symptoms like alternating constipation and diarrhea, allowing them to elaborate freely can reveal important clues that might not be captured by more targeted questions.
*Does the diarrhea typically precede the constipation, or vice-versa?*
- While knowing the sequence of symptoms can be helpful in understanding the **pattern of bowel dysfunction**, it is a very specific question that might overlook other important aspects of the patient's experience.
- It prematurely narrows the focus without first obtaining a broad understanding of the patient's overall symptomatic picture.
*Is the diarrhea foul-smelling?*
- Foul-smelling diarrhea can indicate **malabsorption** or **bacterial overgrowth**, which are important to consider in some gastrointestinal conditions.
- However, this is a **specific symptom inquiry** that should follow a more general exploration of the patient's symptoms, as it may not be relevant if other crucial details are missed.
*Please rate your abdominal pain on a scale of 1-10, with 10 being the worst pain of your life*
- Quantifying pain intensity is useful for assessing the **severity of discomfort** and monitoring changes over time.
- However, for a patient with intermittent rather than acute, severe pain, understanding the **character, location, and triggers** of the pain is often more diagnostically valuable than just a numerical rating initially.
*Are the symptoms worse in the morning or at night?*
- Diurnal variation can be relevant in certain conditions, such as inflammatory bowel diseases where nocturnal symptoms might be more concerning, or functional disorders whose symptoms might be stress-related.
- This is another **specific question** that should come after gathering a more complete initial picture of the patient's symptoms to ensure no key information is overlooked.
Microbiome in antibiotic-associated diarrhea US Medical PG Question 10: A hospital implements a bundle to reduce catheter-associated bloodstream infections. Components include: chlorhexidine bathing, antibiotic-impregnated catheters, antiseptic catheter site dressings, and daily line necessity assessment. After implementation, bloodstream infections with coagulase-negative staphylococci decrease by 60%, but Candida bloodstream infections increase by 40%. Evaluate the microbiological mechanisms underlying these divergent outcomes and synthesize an optimal prevention strategy.
- A. Antibiotic-impregnated catheters select for resistant Candida; use non-antibiotic catheters
- B. The bundle successfully reduced bacterial infections, revealing underlying fungal infections; add antifungal prophylaxis
- C. Multiple interventions disrupted skin flora creating ecological niche for Candida; modify bundle to preserve some commensal bacteria while maintaining antisepsis (Correct Answer)
- D. Chlorhexidine bathing eliminates bacterial skin flora but promotes fungal colonization; discontinue chlorhexidine
- E. Candida increase represents surveillance bias from increased culturing; no change needed
Microbiome in antibiotic-associated diarrhea Explanation: ***Multiple interventions disrupted skin flora creating ecological niche for Candida; modify bundle to preserve some commensal bacteria while maintaining antisepsis***
- Aggressive use of **chlorhexidine bathing** and **antibiotic-impregnated catheters** eliminates commensal bacterial flora that provide **colonization resistance** against opportunistic fungi.
- The reduction in **Coagulase-negative staphylococci** creates an available **ecological niche**, allowing *Candida* species to proliferate and colonize the catheter site more effectively.
*Antibiotic-impregnated catheters select for resistant Candida; use non-antibiotic catheters*
- While **antibiotic-impregnated catheters** reduce bacterial biofilm, they do not directly "select" for resistance in fungi, as antibiotics have no biochemical target in *Candida*.
- Removing them entirely may lead to a rebound in **staphylococcal infections**, failing to address the need for a balanced antiseptic strategy.
*The bundle successfully reduced bacterial infections, revealing underlying fungal infections; add antifungal prophylaxis*
- Adding **antifungal prophylaxis** as a routine measures increases the risk of developing **drug-resistant fungal strains** like *Candida auris*.
- This approach ignores the ecological disruption caused by the bundle and instead layers on more **antimicrobial pressure**, which is rarely a sustainable prevention strategy.
*Chlorhexidine bathing eliminates bacterial skin flora but promotes fungal colonization; discontinue chlorhexidine*
- Discontinuing **chlorhexidine bathing** would likely reverse the 60% reduction in **coagulase-negative staphylococcal** infections, which are a major source of morbidity.
- The goal should be optimization (e.g., targeted use or modified frequency) rather than total discontinuation of an effective **infection control** tool.
*Candida increase represents surveillance bias from increased culturing; no change needed*
- A 40% increase in **Candida bloodstream infections** is a significant clinical shift that requires a root-cause analysis rather than dismissal as **surveillance bias**.
- "No change needed" is incorrect because the bundle has created a new, clinically significant risk for **iatrogenic candidemia**.
More Microbiome in antibiotic-associated diarrhea US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.