Normal flora

On this page
🦠 The Microbial Metropolis: Your Body's Invisible Ecosystem
You harbor trillions of microbial allies whose collective influence shapes your immunity, metabolism, and resistance to infection in ways that rival any organ system. This lesson reveals how normal flora colonize every body surface, train your immune defenses, synthesize essential nutrients, and form living barriers against pathogens. You'll master the mechanisms behind colonization resistance, understand why disrupting this balance invites disease, and learn to predict clinical consequences when antibiotics or illness disturb your microbial ecosystem. By connecting foundational microbiology to real-world scenarios, you'll gain the diagnostic insight to recognize when normal flora protect versus when they betray their host.

The human microbiome represents our most intimate symbiotic relationship, where 99% of our microbial DNA outnumbers human genes by 150:1. These microscopic residents don't merely coexist-they actively shape immunity, metabolism, and disease resistance through 10,000+ biochemical interactions daily.
📌 Remember: HOMES for major body sites - Heart (sterile), Oral cavity (10^8 CFU/mL), Mouth to anus (increasing density), Eyes (minimal), Skin (10^6 CFU/cm²)
Normal flora distribution follows predictable anatomical patterns based on oxygen availability, pH gradients, and nutrient sources. The oral cavity harbors 700+ species at 10^8-10^9 CFU/mL, while the stomach maintains <10^3 CFU/mL due to pH 1.5-3.5 gastric acid. The small intestine shows progressive colonization from 10^4 CFU/mL proximally to 10^7 CFU/mL distally.
| Body Site | Bacterial Density | Dominant Phyla | pH Range | Oxygen Level | Clinical Significance |
|---|---|---|---|---|---|
| Skin | 10^6 CFU/cm² | Actinobacteria, Firmicutes | 4.5-6.5 | Aerobic | Barrier function, wound infection risk |
| Oral Cavity | 10^8-10^9 CFU/mL | Firmicutes, Bacteroidetes | 6.5-7.5 | Mixed | Dental caries, endocarditis source |
| Stomach | <10^3 CFU/mL | Proteobacteria | 1.5-3.5 | Microaerophilic | H. pylori colonization site |
| Small Intestine | 10^4-10^7 CFU/mL | Firmicutes, Proteobacteria | 6.0-7.4 | Anaerobic | SIBO development, malabsorption |
| Colon | 10^11-10^12 CFU/g | Bacteroidetes, Firmicutes | 5.5-7.0 | Strict anaerobic | C. diff overgrowth, metabolic functions |
The Firmicutes:Bacteroidetes ratio serves as a critical clinical biomarker, with healthy individuals maintaining 1:1 to 3:1 ratios. Ratios exceeding 10:1 correlate with obesity (BMI >30), metabolic syndrome (insulin resistance >2.5 HOMA-IR), and inflammatory bowel disease (calprotectin >250 μg/g).
💡 Master This: Normal flora density increases 1,000-fold from stomach to colon, creating distinct ecological niches that determine infection susceptibility and antibiotic resistance patterns
Connect these foundational patterns through colonization dynamics to understand how microbial communities establish territorial dominance and clinical significance.
🦠 The Microbial Metropolis: Your Body's Invisible Ecosystem
🏗️ Microbial Architects: Building Your Bacterial Blueprint
📌 Remember: SPACE for colonization mechanisms - Surface adhesion, PH modification, Antimicrobial production, Competitive exclusion, Enzyme secretion
The critical window hypothesis identifies birth to 3 years as the microbiome programming period. Cesarean delivery delays Bifidobacterium colonization by 6-12 months, increasing asthma risk by 20% and allergies by 15%. Antibiotic exposure during this window reduces microbial diversity by 25-40%, with effects persisting 2-4 years.
- Colonization Phases:
- Phase 1 (0-3 days): Facultative anaerobes establish (E. coli, Enterococcus)
- Phase 2 (4-7 days): Oxygen depletion enables strict anaerobes (Bacteroides, Clostridium)
- Phase 3 (1-4 weeks): Bifidobacterium dominance in breastfed infants (60-90% of total flora)
- Breast milk oligosaccharides serve as selective prebiotics
- Secretory IgA (0.5-1.0 g/L) provides passive immunity
- Phase 4 (6-24 months): Weaning transition introduces adult-like diversity
- Firmicutes increase from 20% to 60%
- Bacteroidetes stabilize at 20-30%

⭐ Clinical Pearl: Antibiotic-associated diarrhea occurs in 10-25% of patients because β-lactams reduce anaerobic bacteria by 100-1000 fold, eliminating colonization resistance against C. difficile spores
Biofilm formation enables persistent colonization through extracellular polymeric substances (EPS). Dental plaque biofilms reach 10^8-10^9 CFU/cm² within 24-48 hours, while gut biofilms maintain stable pH gradients and nutrient cycling. Quorum sensing coordinates population density through autoinducer molecules, triggering virulence factors when bacterial density exceeds 10^6-10^7 CFU/mL.
💡 Master This: Colonization resistance requires >10^8 CFU/g anaerobic bacteria in the colon-antibiotic disruption below this threshold enables pathogenic overgrowth within 24-72 hours
Connect these colonization principles through metabolic partnerships to understand how established communities maintain homeostasis and clinical stability.
🏗️ Microbial Architects: Building Your Bacterial Blueprint
🔬 Metabolic Powerhouses: The Biochemical Assembly Line
Short-chain fatty acid (SCFA) production represents the microbiome's most clinically significant metabolic function. Bacteroides and Clostridium species ferment dietary fiber into acetate (60-70%), propionate (20-25%), and butyrate (10-15%). Butyrate serves as the primary energy source for colonocytes, providing 60-70% of cellular ATP and maintaining intestinal barrier integrity.
📌 Remember: SCFA production ratios - Acetate (60-70%), Propionate (20-25%), Butyrate (10-15%) - APB like "Always Producing Benefits"
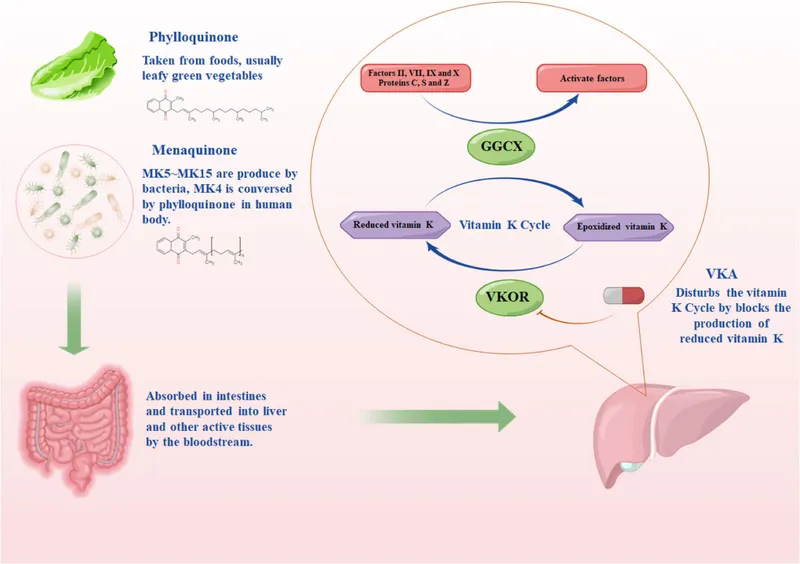
Vitamin synthesis by normal flora prevents nutritional deficiencies in healthy individuals. Bacteroides and Enterococcus produce vitamin K (menaquinones), essential for coagulation factors II, VII, IX, X. Deficiency develops within 7-14 days of broad-spectrum antibiotic therapy, increasing bleeding risk by 3-5 fold. Folate production by Lactobacillus and Bifidobacterium contributes 10-15% of daily requirements.
| Metabolic Function | Key Organisms | Products | Clinical Significance | Deficiency Timeline |
|---|---|---|---|---|
| Vitamin K Synthesis | Bacteroides, Enterococcus | Menaquinones | Coagulation factors | 7-14 days |
| Folate Production | Lactobacillus, Bifidobacterium | Folate, B12 | DNA synthesis, RBC formation | 2-4 weeks |
| SCFA Generation | Clostridium, Bacteroides | Acetate, Propionate, Butyrate | Colonocyte energy, barrier function | 3-7 days |
| Bile Acid Metabolism | Clostridium, Eubacterium | Secondary bile acids | Lipid absorption, signaling | 1-2 weeks |
| Xenobiotic Processing | Enterococcus, E. coli | Drug metabolites | Medication efficacy/toxicity | Variable |
Bile acid metabolism demonstrates sophisticated microbial biochemistry. Primary bile acids (cholic acid, chenodeoxycholic acid) undergo bacterial 7α-dehydroxylation by Clostridium species, producing secondary bile acids (deoxycholic acid, lithocholic acid). This process affects lipid absorption (85-95% efficiency) and cholesterol homeostasis (hepatic synthesis regulation).
- Metabolic Integration Networks:
- Tryptophan metabolism: Lactobacillus produces indole compounds affecting serotonin synthesis (90% of body serotonin originates in gut)
- Histamine regulation: Lactobacillus reuteri degrades histamine, reducing allergic responses by 20-30%
- Ammonia detoxification: Lactobacillus and Bifidobacterium convert ammonia to less toxic compounds
- Critical in hepatic encephalopathy prevention
- Lactulose therapy promotes beneficial bacteria growth
- Reduces serum ammonia by 30-50% in cirrhotic patients
💡 Master This: SCFA production requires 20-30g daily fiber intake-Western diets providing <15g reduce butyrate levels by 40-60%, compromising intestinal barrier function and increasing inflammatory markers
Connect these metabolic functions through immune system interactions to understand how microbial products shape host defense mechanisms and clinical outcomes.
🔬 Metabolic Powerhouses: The Biochemical Assembly Line
🛡️ Immune System Bootcamp: Training Your Cellular Army
Immune tolerance to normal flora develops through regulatory T-cell (Treg) induction and anti-inflammatory cytokine production. Bacteroides fragilis produces polysaccharide A (PSA), which activates CD4+ T-cells and promotes IL-10 secretion (>100 pg/mL). This mechanism prevents inappropriate inflammatory responses to commensal organisms while maintaining pathogen recognition capabilities.
📌 Remember: TRAIN for immune education - Tolerance induction, Regulatory T-cells, Anti-inflammatory cytokines, IgA production, Natural killer cell modulation
Secretory IgA production represents the primary adaptive immune response to normal flora. Plasma cells in Peyer's patches produce 3-5 grams of secretory IgA daily, coating bacterial surfaces without complement activation or inflammatory responses. Germ-free mice show 90% reduction in IgA levels and defective immune development.
Microbiome diversity correlates directly with immune system competence. Shannon diversity index >3.0 associates with reduced autoimmune disease risk (OR 0.3-0.5) and enhanced vaccine responses (2-3 fold higher antibody titers). Antibiotic-induced dysbiosis reduces vaccine efficacy by 50-70% for influenza and pneumococcal vaccines.
| Immune Function | Microbial Mediators | Mechanism | Clinical Impact | Quantitative Effect |
|---|---|---|---|---|
| Treg Induction | B. fragilis PSA | CD4+ T-cell activation | Autoimmune prevention | 3-5 fold ↑ Tregs |
| IgA Production | Segmented filamentous bacteria | Th17 stimulation | Mucosal immunity | 10-20 fold ↑ IgA |
| NK Cell Activity | Lactobacillus casei | Cytokine modulation | Antiviral defense | 40-60% ↑ activity |
| Macrophage Training | LPS exposure | TLR4 priming | Pathogen recognition | 2-4 fold ↑ response |
| Complement Regulation | Bacteroides thetaiotaomicron | C5a degradation | Inflammation control | 30-50% ↓ C5a levels |
Molecular mimicry between commensal antigens and pathogenic epitopes enables cross-protective immunity. Bifidobacterium surface proteins share structural homology with respiratory syncytial virus (RSV), providing partial protection against severe RSV disease in infants (30-40% reduction in hospitalization rates).
- Immune System Programming:
- Critical period: Birth to 2 years for immune education
- Microbial exposure shapes T-cell repertoire and cytokine profiles
- Cesarean delivery delays immune maturation by 6-12 months
- Th1/Th2 imbalance persists through childhood
- Allergy risk increases 15-20%
- Asthma prevalence rises 20-25%
- Antibiotic exposure disrupts immune programming
- Each course before age 2 increases asthma risk by 10-15%
- Broad-spectrum antibiotics show greatest impact
💡 Master This: Immune tolerance requires continuous microbial stimulation-germ-free conditions or excessive antibiotic use result in hyperactive immune responses and increased autoimmune disease risk (2-3 fold higher incidence)
Connect these immune interactions through pathogen resistance mechanisms to understand how normal flora provides active protection against infectious diseases.
🛡️ Immune System Bootcamp: Training Your Cellular Army
⚔️ The Bacterial Bodyguard Brigade: Frontline Defense Systems

Colonization resistance operates through four primary mechanisms: nutrient competition, niche occupation, antimicrobial production, and immune stimulation. C. difficile infection demonstrates this principle-normal anaerobic flora at >10^8 CFU/g prevents spore germination and toxin production. Antibiotic disruption reducing anaerobic density below 10^6 CFU/g enables C. diff overgrowth in 15-25% of hospitalized patients.
📌 Remember: CAMP for colonization resistance - Competitive exclusion, Antimicrobial production, Mucosal barrier enhancement, Pathogen recognition priming
Bacteriocin production represents sophisticated antimicrobial warfare between bacterial species. Lactobacillus acidophilus produces lactacin B, effective against Listeria monocytogenes at concentrations of 10-50 μg/mL. Enterococcus faecalis secretes enterocin, inhibiting vancomycin-resistant enterococci (VRE) growth by >99% at therapeutic concentrations.
pH modification creates hostile environments for pathogenic organisms. Lactobacillus species maintain vaginal pH 3.8-4.5 through lactic acid production (50-100 mM concentrations), preventing bacterial vaginosis in 85-90% of reproductive-age women. Gastric Helicobacter pylori colonization occurs only when pH rises above 4.0 due to urease activity or proton pump inhibitor therapy.
| Defense Mechanism | Key Organisms | Target Pathogens | Efficacy Rate | Clinical Application |
|---|---|---|---|---|
| Bacteriocin Production | Lactobacillus, Enterococcus | Listeria, VRE, MRSA | 90-99% inhibition | Probiotic therapy |
| pH Acidification | Lactobacillus acidophilus | Candida, E. coli, GBS | 85-95% prevention | Vaginal health |
| Nutrient Competition | Bacteroides, Clostridium | C. difficile, Salmonella | 95-99% exclusion | Post-antibiotic protection |
| Receptor Blocking | Bifidobacterium | Rotavirus, E. coli | 70-85% reduction | Pediatric infections |
| Immune Priming | Segmented filamentous bacteria | Salmonella, Citrobacter | 60-80% protection | Mucosal immunity |
Biofilm interference prevents pathogenic adhesion and invasion. Lactobacillus rhamnosus produces biosurfactants that disrupt Candida albicans biofilm formation by 80-90% and reduce urogenital tract infections by 40-50% in high-risk populations. Staphylococcus epidermidis on skin produces phenol-soluble modulins that inhibit S. aureus colonization.
- Pathogen-Specific Resistance Mechanisms:
- Salmonella: Bacteroides compete for iron and carbohydrate sources
- Microbiota-derived propionate inhibits Salmonella growth
- Restoration requires 48-72 hours post-antibiotic
- Candida overgrowth: Lactobacillus maintains acidic pH and produces antifungal compounds
- Fluconazole resistance correlates with lactobacilli depletion
- Probiotic restoration reduces recurrence by 50-70%
- Respiratory pathogens: Streptococcus salivarius produces bacteriocins active against S. pyogenes
- Nasal colonization reduces strep throat incidence by 40-60%
- Salmonella: Bacteroides compete for iron and carbohydrate sources
💡 Master This: Colonization resistance requires >48-72 hours to re-establish after antibiotic therapy-early probiotic intervention during this vulnerable window prevents opportunistic infections in 70-80% of high-risk patients
Connect these protective mechanisms through clinical dysbiosis patterns to understand how disrupted normal flora leads to predictable infectious complications and therapeutic opportunities.
⚔️ The Bacterial Bodyguard Brigade: Frontline Defense Systems
🌐 The Microbiome Network: Integration and Clinical Mastery
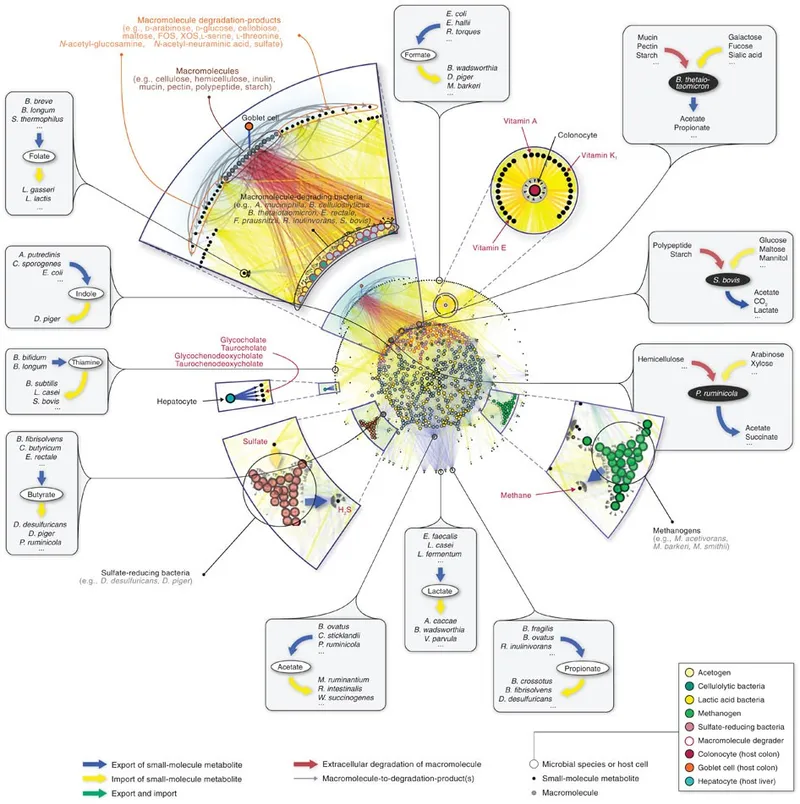
Cross-system communication occurs through microbial metabolites, immune mediators, and neural pathways. The gut-brain axis demonstrates this integration-gut bacteria produce >30 neurotransmitters including GABA (Lactobacillus), serotonin precursors (Enterococcus), and dopamine (Bacillus). Dysbiosis correlates with depression (OR 2.5-3.0) and anxiety disorders (OR 1.8-2.2).
📌 Remember: AXIS for microbiome integration - Autonomic nervous system, Xenobiotic metabolism, Immune modulation, Systemic inflammation
Microbiome-drug interactions represent an emerging clinical frontier. Eggerthella lenta metabolizes digoxin, reducing bioavailability by 30-40% in 10% of patients. Clostridium species activate sulfasalazine through azo-reduction, while depleted microbiomes show reduced efficacy of immune checkpoint inhibitors (response rates drop from 40% to 15%).
Personalized microbiome medicine requires understanding individual variation and therapeutic targets. Enterotype classification based on dominant genera (Bacteroides, Prevotella, Ruminococcus) predicts drug responses, dietary interventions, and disease susceptibilities. Bacteroides-dominant individuals show better responses to fiber supplementation (SCFA production increases 2-3 fold).
| Integration System | Mediators | Clinical Correlations | Therapeutic Targets | Success Rates |
|---|---|---|---|---|
| Gut-Brain Axis | Neurotransmitters, SCFAs | Depression, anxiety, autism | Psychobiotics, prebiotics | 60-70% improvement |
| Gut-Liver Axis | Bile acids, endotoxins | NAFLD, cirrhosis | Microbiome modulation | 40-50% reduction |
| Gut-Lung Axis | Immune mediators | Asthma, allergies | Probiotic therapy | 30-40% benefit |
| Microbiome-Cancer | Immune activation | Immunotherapy response | Microbiome optimization | 2-3 fold ↑ response |
| Drug Metabolism | Bacterial enzymes | Variable drug efficacy | Personalized dosing | 20-30% improvement |
Therapeutic microbiome modulation encompasses probiotics, prebiotics, synbiotics, and fecal microbiota transplantation (FMT). FMT achieves >90% cure rates for recurrent C. difficile infection and shows promise for inflammatory bowel disease (30-40% remission rates), metabolic disorders, and neuropsychiatric conditions.
- Clinical Mastery Framework:
- Assessment: Microbiome testing guides personalized interventions
- 16S rRNA sequencing identifies taxonomic composition
- Functional metagenomics reveals metabolic capacity
- Metabolomics measures microbial products and host responses
- Intervention: Targeted microbiome therapies based on individual profiles
- Strain-specific probiotics for defined clinical outcomes
- Prebiotic selection based on existing microbiome composition
- Antibiotic stewardship to preserve beneficial flora
- Monitoring: Longitudinal tracking of microbiome stability and clinical responses
- Diversity indices predict treatment success
- Functional markers correlate with symptom improvement
- Assessment: Microbiome testing guides personalized interventions
💡 Master This: Microbiome-based medicine requires systems thinking-single-organism approaches fail because clinical outcomes depend on community interactions, metabolic networks, and host-microbe crosstalk across multiple physiological systems
Understanding normal flora transforms from basic microbiology into precision medicine, where microbiome optimization becomes a therapeutic modality for preventing disease, enhancing treatments, and improving clinical outcomes across medical specialties.
🌐 The Microbiome Network: Integration and Clinical Mastery
🎯 Clinical Command Center: Rapid Microbiome Mastery
📌 Remember: FLORA for clinical assessment - Function (metabolic capacity), Location (site-specific), Organisms (key species), Resistance (colonization), Alterations (dysbiosis patterns)
Essential Clinical Thresholds for immediate application:
| Clinical Scenario | Key Threshold | Intervention Timing | Success Predictor | Monitoring Parameter |
|---|---|---|---|---|
| C. diff Prevention | Anaerobic flora <10^6 CFU/g | Within 72h of antibiotics | Probiotic strain selection | Toxin clearance |
| Vaginal Dysbiosis | pH >4.5, Lactobacilli <10^6 CFU/mL | Symptomatic presentation | Nugent score improvement | pH normalization |
| SIBO Diagnosis | Small bowel >10^5 CFU/mL | Breath test positivity | Antibiotic sensitivity | Symptom resolution |
| Antibiotic Stewardship | Diversity index <2.0 | Post-treatment assessment | Microbiome restoration | Shannon diversity |
| Immunocompromised Risk | IgA <0.5 g/L, Low diversity | Prophylactic intervention | Immune reconstitution | Infection rates |
Rapid Assessment Protocol:
- History: Antibiotic exposure (type, duration, timing), dietary patterns, delivery mode, early feeding
- Physical: Site-specific examination for dysbiosis indicators (oral thrush, vaginal discharge, skin infections)
- Laboratory: Targeted testing based on clinical presentation and risk factors
- Stool analysis: Calprotectin (>250 μg/g indicates inflammation)
- Vaginal pH: >4.5 suggests bacterial vaginosis
- Breath testing: Hydrogen/methane for SIBO (>20 ppm rise)
💡 Master This: Normal flora disruption follows predictable patterns-broad-spectrum antibiotics cause dysbiosis within 24-48 hours, recovery requires 2-4 weeks, and complications occur during the vulnerable restoration period
Clinical Decision Framework for microbiome-related presentations:
Therapeutic Arsenal for immediate clinical application:
- Lactobacillus GG: C. diff prevention (10^10 CFU daily)
- Saccharomyces boulardii: Antibiotic-associated diarrhea (250-500 mg BID)
- Lactobacillus crispatus: Vaginal health (10^8 CFU daily)
- Bifidobacterium longum: IBS symptoms (10^9 CFU daily)
Understanding normal flora transforms every clinical encounter from symptom management to microbiome optimization, enabling precision interventions that restore health through microbial community balance.
🎯 Clinical Command Center: Rapid Microbiome Mastery
Practice Questions: Normal flora
Test your understanding with these related questions
A medical student is reading about a specific type of T cells that plays an important role in immunologic tolerance. Most of these cells develop in the thymus, but some of them also develop in peripheral lymphoid organs. Usually, they are CD4+ cells and also express CD25 molecules. The functions of these cells are dependent on forkhead box P3 (Foxp3). Their function is to block the activation of lymphocytes that could react with self-antigens in a potentially harmful manner. Which of the following interleukins is secreted by these cells?










