HIV

On this page
🦠 HIV: The Retroviral Mastermind
HIV stands apart as medicine's most studied retrovirus, a pathogen that hijacks human immunity with molecular precision while evading detection through clinical disguise. You'll trace its journey from viral architecture through cellular invasion, decode the diagnostic algorithms that unmask infection despite its masquerade of nonspecific symptoms, and master the antiretroviral strategies that have transformed a death sentence into a manageable chronic condition. This lesson builds your understanding of how structure dictates pathogenesis, why timing of testing matters, and how combination therapy achieves viral suppression to preserve immune function and prevent transmission.
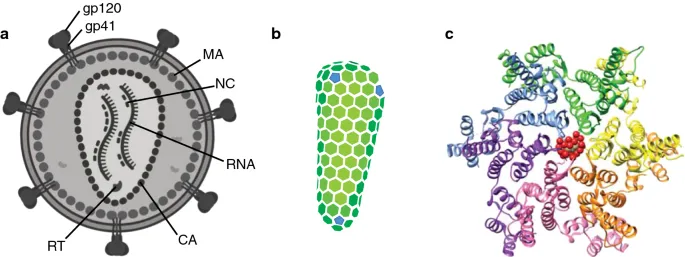
📌 Remember: HIV-1-2-3 - High viral load, Immune destruction, Viral persistence; 1 reverse transcriptase, 2 envelope proteins (gp120/gp41), 3 structural genes (gag/pol/env)
HIV represents a paradigm shift in infectious disease management, where chronic viral suppression replaces acute cure. The virus targets CD4+ T-helper cells, the orchestrators of adaptive immunity, creating progressive immunodeficiency that enables opportunistic infections and malignancies.
-
HIV-1 Global Dominance
- Accounts for >95% of global infections
- 9 subtypes (A-K) with geographic clustering
- Subtype B: 12% globally, >90% in North America/Europe
- Subtype C: 48% globally, dominant in sub-Saharan Africa
- CRFs (Circulating Recombinant Forms): 18% of infections
-
HIV-2 Regional Distribution
- Primarily West Africa (1-2 million infections)
- Lower transmission rate (2-9x less than HIV-1)
- Slower disease progression (median 20+ years to AIDS)
- Lower viral loads (often undetectable)
- Natural resistance to NNRTIs and enfuvirtide
| Parameter | HIV-1 | HIV-2 | Clinical Significance |
|---|---|---|---|
| Global Prevalence | 36.7 million | 1-2 million | HIV-1 pandemic strain |
| Transmission Rate | 0.1-3% per exposure | 0.01-0.3% per exposure | HIV-2 less infectious |
| Disease Progression | 8-10 years to AIDS | 15-20 years to AIDS | HIV-2 slower decline |
| Viral Load | 10³-10⁷ copies/mL | Often <400 copies/mL | HIV-2 naturally suppressed |
| Drug Resistance | NRTI/PI susceptible | NNRTI resistant | Different treatment algorithms |
💡 Master This: HIV's retroviral lifecycle enables permanent integration into host DNA, creating latent reservoirs that persist despite effective ART - understanding this explains why HIV requires lifelong treatment rather than short-term cure
The virus demonstrates remarkable genetic diversity, with 1% sequence variation occurring annually within individual patients. This quasispecies nature enables rapid drug resistance development and immune escape, requiring combination therapy and resistance monitoring for optimal outcomes.
Connect this foundational understanding through viral structure analysis to understand how HIV's sophisticated architecture enables its clinical persistence and therapeutic challenges.
🦠 HIV: The Retroviral Mastermind
⚙️ HIV Architecture: The Viral Engineering Marvel
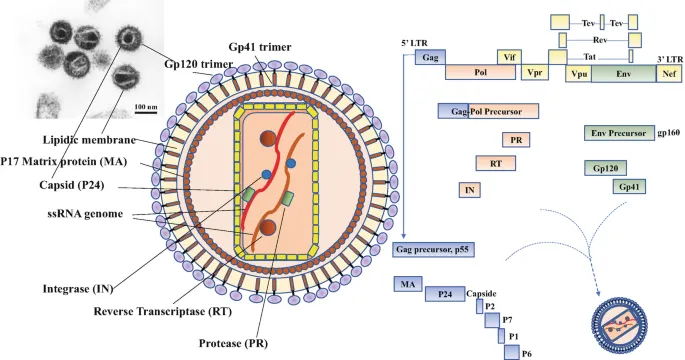
📌 Remember: GAG-POL-ENV structural trinity - Gag makes the Guts (structural proteins), Pol Produces enzymes, Env Enables entry through envelope proteins
-
Structural Gene Products (Gag Polyprotein)
- p24 (Capsid): 2,000 copies per virion
- Forms hexagonal lattice protecting viral RNA
- Antigen target for early HIV detection
- Half-life 6 hours - rapid turnover marker
- p17 (Matrix): 1,000-1,500 copies per virion
- Membrane association and nuclear import
- Contains myristoylation signal for membrane binding
- p7/p6 (Nucleocapsid): RNA binding and packaging
- Zinc finger motifs ensure genomic RNA incorporation
- p24 (Capsid): 2,000 copies per virion
-
Enzymatic Gene Products (Pol Polyprotein)
- Reverse Transcriptase: 50-100 copies per virion
- Error rate 1 in 10,000 nucleotides (vs. 1 in 10⁹ for DNA polymerase)
- RNase H activity degrades RNA template
- Integrase: 10-50 copies per virion
- 3' processing and strand transfer activities
- Target site duplication creates 5bp repeats
- Protease: 5-10 copies per virion
- Aspartyl protease cleaves Gag/Pol polyproteins
- Active site requires homodimer formation
- Reverse Transcriptase: 50-100 copies per virion
| Viral Component | Copy Number | Function | Clinical Target |
|---|---|---|---|
| gp120 | 7-14 trimers | CD4/coreceptor binding | Entry inhibitors |
| gp41 | 7-14 trimers | Membrane fusion | Fusion inhibitors |
| Reverse Transcriptase | 50-100 | RNA→DNA conversion | NRTIs/NNRTIs |
| Integrase | 10-50 | DNA integration | INSTIs |
| Protease | 5-10 | Polyprotein processing | Protease inhibitors |
| p24 Capsid | ~2,000 | Core structure | Diagnostic antigen |
- Envelope Protein Complexity
- gp120 Surface Protein: Variable loops enable immune escape
- V3 loop determines coreceptor tropism (CCR5 vs. CXCR4)
- CD4 binding site conserved across all HIV strains
- Glycan shield covers 50% of surface area
- gp41 Transmembrane Protein: Fusion machinery
- Fusion peptide inserts into target cell membrane
- Heptad repeats form six-helix bundle during fusion
- Membrane-proximal external region - neutralization target
- gp120 Surface Protein: Variable loops enable immune escape
💡 Master This: HIV's envelope protein variability creates strain-specific immunity but prevents universal vaccine development - this explains why natural infection doesn't provide cross-protective immunity and why vaccine development remains challenging
The virus packages two copies of genomic RNA plus cellular tRNAs that serve as reverse transcription primers. This diploid genome enables recombination between different viral strains during co-infection, accelerating genetic diversity and drug resistance evolution.
Connect this architectural mastery through replication mechanisms to understand how HIV's sophisticated lifecycle creates multiple therapeutic targets while enabling persistent infection.
⚙️ HIV Architecture: The Viral Engineering Marvel
🔄 Viral Hijacking: The Replication Masterclass
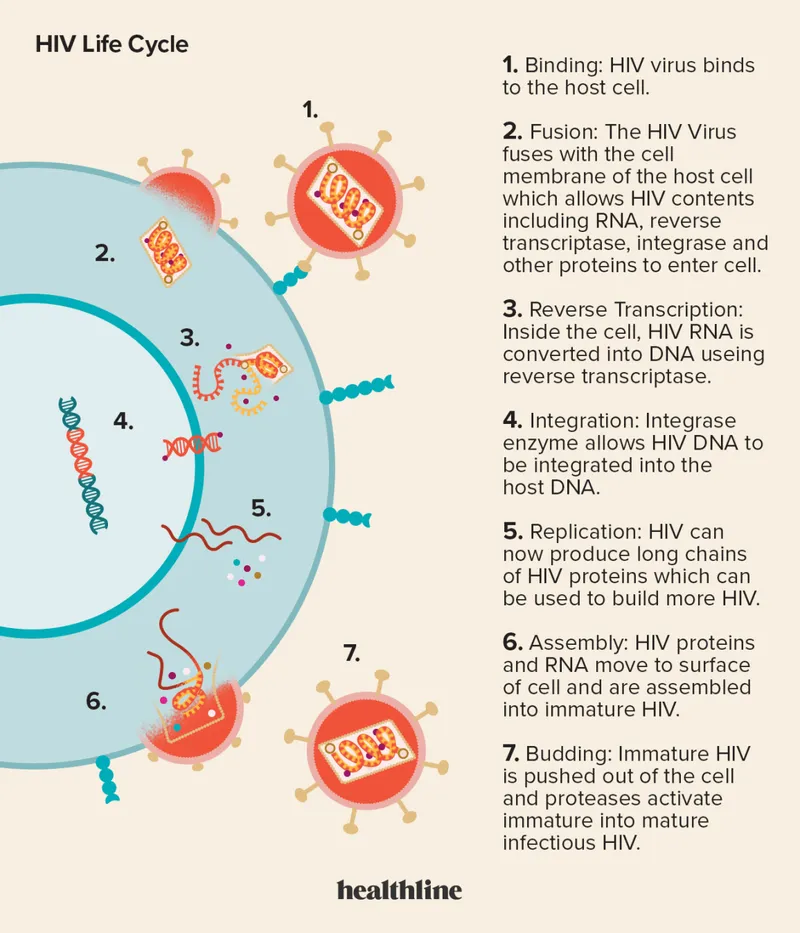
📌 Remember: ERIC-PAB replication sequence - Entry, Reverse transcription, Integration, Cell activation, Protein synthesis, Assembly, Budding
-
Entry and Uncoating Phase
- Binding Kinetics: gp120-CD4 interaction (Kd ~10 nM)
- Conformational change exposes coreceptor binding site
- CCR5 tropism: 90% of transmitted viruses
- CXCR4 tropism: 50% of late-stage infections
- Fusion Process: gp41-mediated membrane merger
- Fusion peptide insertion within <1 second
- Six-helix bundle formation provides fusion energy
- Viral core release into cytoplasm
- Binding Kinetics: gp120-CD4 interaction (Kd ~10 nM)
-
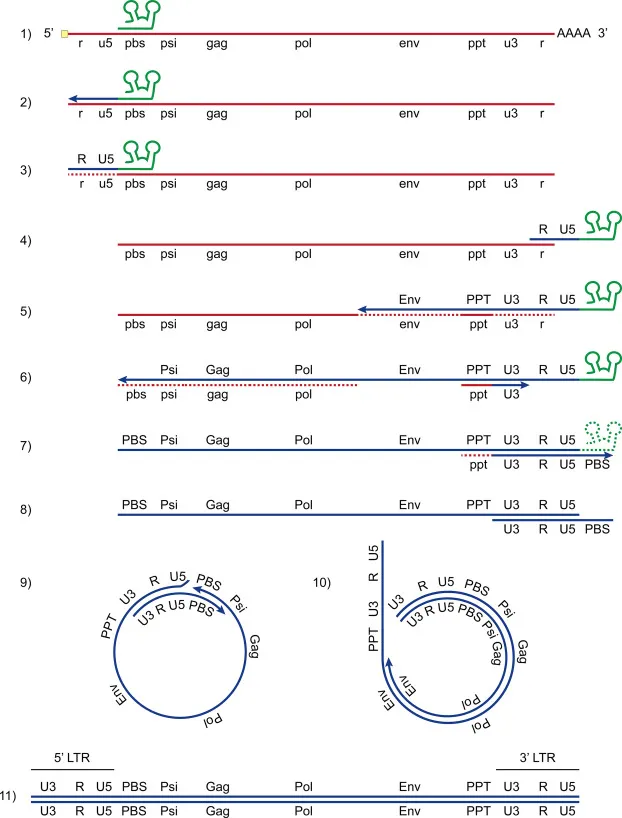
Reverse Transcription Dynamics
- Template Switching: 2-3 recombination events per cycle
- Error rate: 3 × 10⁻⁵ per nucleotide per cycle
- Mutation rate: 1-5 mutations per 9kb genome
- RNase H degrades RNA template progressively
- DNA Synthesis: 6-12 hours for completion
- Minus-strand DNA: tRNA primer extension
- Plus-strand DNA: polypurine tract priming
- Long Terminal Repeats (LTRs) created by template switching
- Template Switching: 2-3 recombination events per cycle
| Replication Phase | Duration | Key Events | Therapeutic Targets |
|---|---|---|---|
| Entry | <30 minutes | gp120/gp41 fusion | Maraviroc, Enfuvirtide |
| Reverse Transcription | 6-12 hours | RNA→DNA conversion | NRTIs, NNRTIs |
| Integration | 12-48 hours | Proviral DNA insertion | INSTIs |
| Transcription | Variable | Viral RNA synthesis | Latency reversing agents |
| Translation | 2-6 hours | Viral protein synthesis | Protease inhibitors |
| Assembly/Budding | 1-2 hours | Virion maturation | Maturation inhibitors |
- Integration and Proviral Establishment
- Nuclear Entry: Pre-integration complex (PIC) transport
- Integrase and matrix proteins contain nuclear localization signals
- Nuclear pores allow PIC passage in non-dividing cells
- Chromatin targeting favors transcriptionally active regions
- Integration Mechanism: Strand transfer chemistry
- 3' processing removes 2 nucleotides from viral DNA ends
- Strand transfer creates staggered cuts in host DNA
- Host repair machinery fills gaps and ligates DNA
- Nuclear Entry: Pre-integration complex (PIC) transport
💡 Master This: HIV's integration strategy creates permanent infection because proviral DNA becomes part of host chromosome - this explains why ART interruption leads to viral rebound and why cure strategies must eliminate integrated proviruses
- Transcriptional Control and Latency
- LTR Regulation: Tat-dependent transcriptional activation
- Basal transcription: 10-fold lower without Tat
- Tat binding: TAR RNA secondary structure recognition
- P-TEFb recruitment: RNA polymerase II elongation
- Latency Mechanisms: Multiple silencing pathways
- Chromatin modifications: H3K9me3 and H3K27me3 marks
- DNA methylation: CpG sites in 5' LTR
- Transcriptional interference: Host gene promoters
- LTR Regulation: Tat-dependent transcriptional activation
The virus produces 10⁹-10¹⁰ virions daily during untreated infection, with viral half-life of 6 hours in plasma. This rapid turnover combined with high error rate generates 10⁴-10⁵ mutations daily, enabling drug resistance within weeks of monotherapy.
Connect this replication mastery through clinical presentation patterns to understand how viral dynamics translate into recognizable disease manifestations and diagnostic opportunities.
🔄 Viral Hijacking: The Replication Masterclass
🎭 Clinical Masquerade: The Great Diagnostic Deception
📌 Remember: FLAMES acute HIV syndrome - Fever (80-90%), Lymphadenopathy (40-70%), Arthralgia/myalgia (50-70%), Mucocutaneous lesions (40-80%), Esophageal ulcers (10-20%), Sore throat (50-70%)
-
Acute Retroviral Syndrome Manifestations
- Constitutional Symptoms (Days 10-28 post-exposure)
- Fever: 38.5-40°C in 80-90% of patients
- Fatigue: Severe exhaustion lasting 2-4 weeks
- Weight loss: 2-5 kg during acute phase
- Night sweats: Drenching in 50-70% of cases
- Lymphoid System Involvement
- Generalized lymphadenopathy: >1cm nodes in ≥2 sites
- Splenomegaly: Palpable in 10-20% of patients
- Lymphocyte count: Initial ↑ then dramatic ↓
- Constitutional Symptoms (Days 10-28 post-exposure)
-
Mucocutaneous Manifestations
- Maculopapular Rash: 40-80% of acute infections
- Distribution: Trunk and face predominantly
- Characteristics: Non-pruritic, 5-10mm lesions
- Duration: 5-14 days with spontaneous resolution
- Oral Ulcerations: 10-30% of patients
- Aphthous-like lesions on tongue/buccal mucosa
- Painful and shallow with erythematous borders
- Esophageal extension in severe cases
- Maculopapular Rash: 40-80% of acute infections
| Clinical Feature | Frequency | Timing | Differential Diagnosis |
|---|---|---|---|
| Fever >38.5°C | 80-90% | Days 10-28 | EBV, CMV, influenza |
| Lymphadenopathy | 40-70% | Days 14-35 | EBV, toxoplasmosis |
| Pharyngitis | 50-70% | Days 7-21 | Streptococcal, viral |
| Rash | 40-80% | Days 14-28 | Drug reaction, viral exanthem |
| Myalgia/Arthralgia | 50-70% | Days 10-35 | Viral syndrome, autoimmune |
| Oral Ulcers | 10-30% | Days 14-42 | HSV, aphthous, Behçet's |
- Neurological Manifestations
- Aseptic Meningitis: 5-10% of acute infections
- CSF pleocytosis: 10-300 cells/μL (predominantly lymphocytic)
- Elevated protein: 40-100 mg/dL
- Normal glucose: >60 mg/dL
- HIV RNA detectable in CSF
- Peripheral Neuropathy: Rare in acute phase
- Facial palsy: Bell's palsy-like presentation
- Brachial plexopathy: Acute onset weakness
- Guillain-Barré syndrome: Ascending paralysis
- Aseptic Meningitis: 5-10% of acute infections
💡 Master This: Acute HIV diagnosis requires high clinical suspicion because antibody tests are negative during peak infectivity - only HIV RNA or p24 antigen testing can identify acute infection when transmission risk is highest
- Laboratory Abnormalities
- Hematologic Changes
- Lymphopenia: <1,000 cells/μL in 70% of patients
- Thrombocytopenia: <150,000/μL in 45% of patients
- Atypical lymphocytes: >10% on peripheral smear
- Biochemical Abnormalities
- Elevated LDH: >250 U/L in 60% of patients
- Transaminase elevation: ALT/AST 2-5x normal
- Low albumin: <3.5 g/dL due to acute inflammation
- Hematologic Changes
The window period between infection and antibody detection averages 22 days (range 10-42 days) with 3rd generation tests and 18 days with 4th generation tests. During this period, HIV RNA levels exceed 100,000 copies/mL in >95% of patients, making nucleic acid testing the diagnostic gold standard.
Connect this clinical presentation mastery through diagnostic strategies to understand how laboratory testing algorithms can reliably identify HIV infection across all stages of disease progression.
🎭 Clinical Masquerade: The Great Diagnostic Deception
🔬 Diagnostic Precision: The Testing Algorithm Arsenal
📌 Remember: RAPID-HIV testing sequence - Rapid screening, Antigen/antibody combo, Positive requires confirmation, Indeterminate needs RNA, Differentiation HIV-1/HIV-2
-
4th Generation Combination Assays
- Simultaneous Detection: HIV-1/HIV-2 antibodies + p24 antigen
- Sensitivity: 99.6% for established infection
- Specificity: 99.8% in low-prevalence populations
- Window period: 13-15 days (vs. 22 days for 3rd generation)
- p24 antigen: Detectable 1-3 weeks before antibodies
- Assay Methodology: Chemiluminescent immunoassays (CLIA)
- Signal-to-cutoff ratio: >1.0 indicates reactive
- Gray zone: 0.8-1.2 requires repeat testing
- Automated platforms: 150-300 tests/hour capacity
- Simultaneous Detection: HIV-1/HIV-2 antibodies + p24 antigen
-
Confirmatory Testing Algorithm
- HIV-1/HIV-2 Differentiation Assay
- Multispot rapid test: Results in 15 minutes
- Differentiates: HIV-1, HIV-2, or undifferentiated
- Sensitivity: >99% for HIV-1, >95% for HIV-2
- Cross-reactivity: <1% between HIV-1/HIV-2
- HIV RNA Quantification (for indeterminate results)
- Lower limit: 20-40 copies/mL (assay-dependent)
- Dynamic range: 20-10,000,000 copies/mL
- Turnaround time: 4-6 hours for urgent cases
- HIV-1/HIV-2 Differentiation Assay
| Test Type | Window Period | Sensitivity | Specificity | Clinical Use |
|---|---|---|---|---|
| 4th Generation | 13-15 days | 99.6% | 99.8% | Primary screening |
| 3rd Generation | 22-25 days | 99.3% | 99.5% | Resource-limited |
| HIV RNA | 7-10 days | >99.9% | >99.9% | Acute infection |
| Rapid Tests | 18-90 days | 92-99% | 98-99.8% | Point-of-care |
| Oral Fluid | 20-40 days | 91-98% | 99.2-99.8% | Non-invasive |
| Home Tests | 23-90 days | 92-99.3% | 99.1-99.9% | Self-testing |
- Specialized Testing Scenarios
- Acute Infection Diagnosis
- HIV RNA: Gold standard for window period detection
- p24 antigen: Alternative when RNA unavailable
- Pooled RNA testing: Cost-effective for high-volume screening
- Viral load: >100,000 copies/mL in >95% of acute cases
- Perinatal Testing Algorithm
- Maternal antibodies: Persist 12-18 months in uninfected infants
- HIV DNA PCR: Preferred test for infants <18 months
- Testing schedule: 14-21 days, 1-2 months, 4-6 months
- Definitive exclusion: 2 negative tests after 1 month of age
- Acute Infection Diagnosis
💡 Master This: Modern HIV testing achieves near-perfect accuracy through sequential algorithms that combine different detection methods - understanding test limitations and appropriate use prevents diagnostic errors and ensures optimal patient care
- Point-of-Care Testing Applications
- Rapid Test Characteristics
- Turnaround time: 15-20 minutes for results
- Sensitivity range: 92-99% (varies by manufacturer)
- Specificity range: 98-99.8% in routine populations
- CLIA-waived: Minimal training required
- Clinical Implementation
- Emergency departments: Immediate results for high-risk patients
- Labor and delivery: Rapid testing for unknown status
- Outreach programs: Community-based testing initiatives
- Resource-limited: Single-test diagnosis in low-resource settings
- Rapid Test Characteristics
The CDC algorithm recommends 4th generation screening followed by HIV-1/HIV-2 differentiation for reactive results. Indeterminate differentiation requires HIV RNA testing to distinguish true HIV-1 infection from false positive screening results.
Connect this diagnostic mastery through treatment principles to understand how accurate HIV diagnosis enables evidence-based therapeutic interventions that transform HIV from fatal disease to manageable chronic condition.
🔬 Diagnostic Precision: The Testing Algorithm Arsenal
⚕️ Therapeutic Command Center: The ART Revolution
📌 Remember: NRTI-NNRTI-PI-INSTI-EI drug classes - Nucleoside Reverse Transcriptase Inhibitors, Non-Nucleoside RTIs, Protease Inhibitors, Integrase STrand Transfer Inhibitors, Entry Inhibitors
-
First-Line ART Regimens (2023 Guidelines)
- Integrase-Based Combinations (Preferred)
- Bictegravir/TAF/FTC: Single tablet daily (Biktarvy)
- Dolutegravir + TAF/FTC: 2 tablets daily
- Dolutegravir + ABC/3TC: Alternative for TDF/TAF-intolerant
- Efficacy: >95% achieve viral suppression at 48 weeks
- Protease Inhibitor Alternatives
- Darunavir/cobicistat + TAF/FTC: Resistance barrier
- Reserved for INSTI-intolerant or drug interactions
- Efficacy: 85-90% viral suppression (non-inferiority)
- Integrase-Based Combinations (Preferred)
-
NRTI Backbone Selection
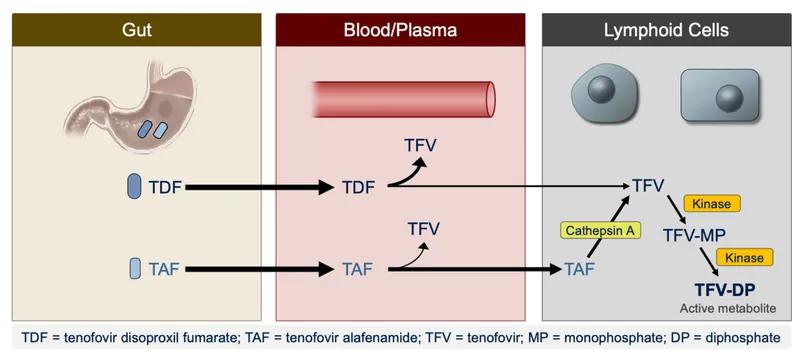
- Tenofovir-Based (Preferred)
- TAF (Tenofovir alafenamide): 25mg daily
- 90% lower plasma exposure vs. TDF
- Improved bone/renal safety profile
- Higher intracellular tenofovir concentrations
- TDF (Tenofovir disoproxil): 300mg daily
- Generic availability reduces cost
- Renal monitoring required (eGFR, proteinuria)
- TAF (Tenofovir alafenamide): 25mg daily
- Emtricitabine/Lamivudine: Cytidine analogs
- FTC: 200mg daily (longer half-life)
- 3TC: 300mg daily (twice-daily alternative)
- Cross-resistance: M184V mutation affects both drugs
- Tenofovir-Based (Preferred)
| Regimen | Pill Burden | Efficacy (48 weeks) | Key Advantages | Major Limitations |
|---|---|---|---|---|
| Bictegravir/TAF/FTC | 1 tablet daily | >95% | High barrier, well-tolerated | Drug interactions |
| Dolutegravir + TAF/FTC | 2 tablets daily | >95% | Pregnancy safe, generic DTG | Weight gain |
| Darunavir/c + TAF/FTC | 3 tablets daily | 85-90% | High resistance barrier | GI intolerance |
| Efavirenz/TDF/FTC | 1 tablet daily | 80-85% | Low cost, extensive data | CNS toxicity |
| Rilpivirine/TAF/FTC | 1 tablet daily | 85-90% | Minimal interactions | VL <100K requirement |
- Treatment Monitoring Parameters
- Viral Load Targets
- Goal: <50 copies/mL by 12-24 weeks
- Undetectable = Untransmittable (U=U)
- Virologic failure: >200 copies/mL on 2 consecutive tests
- Blips: 50-1000 copies/mL followed by <50 (usually benign)
- CD4 Recovery Patterns
- Initial rise: 50-150 cells/μL in first 3 months
- Continued recovery: 100-200 cells/μL/year for 2-3 years
- Plateau: Most patients reach >500 cells/μL
- Immune reconstitution: Faster with earlier treatment
- Viral Load Targets
💡 Master This: ART success depends on >95% adherence because suboptimal drug levels enable viral replication and resistance development - single-tablet regimens improve adherence and reduce treatment failure compared to multi-pill combinations
- Special Population Considerations
- Pregnancy Management
- Dolutegravir-based regimens preferred (safety data)
- Efavirenz avoided (teratogenicity risk)
- Viral suppression goal: <50 copies/mL by delivery
- Cesarean delivery if VL >1000 copies/mL
- Renal Impairment Adjustments
- eGFR <30 mL/min: Avoid TDF, dose-adjust renally-cleared drugs
- TAF preferred over TDF for bone/renal safety
- Integrase inhibitors: Minimal renal elimination
- Protease inhibitors: Hepatic metabolism (no dose adjustment)
- Pregnancy Management
Modern ART achieves viral suppression in >95% of adherent patients, with treatment failure primarily due to poor adherence rather than drug resistance. Single-tablet regimens and long-acting formulations continue improving treatment outcomes and quality of life.
Connect this therapeutic mastery through advanced clinical integration to understand how comprehensive HIV care addresses complex comorbidities, drug interactions, and long-term complications in the modern treatment era.
⚕️ Therapeutic Command Center: The ART Revolution
🎯 Clinical Mastery Arsenal: The HIV Care Continuum
📌 Remember: CARDIAC-HIV comorbidity priorities - Cardiovascular disease, Acceleratd aging, Renal disease, Diabetes, Immune activation, AIDS-defining cancers, Cognitive impairment
-
Essential Clinical Monitoring Framework
- Routine Laboratory Surveillance
- HIV RNA: Every 3-6 months (goal <50 copies/mL)
- CD4 count: Every 6-12 months if >300 cells/μL
- Complete metabolic panel: Every 6 months (renal/hepatic function)
- Lipid profile: Annually (cardiovascular risk assessment)
- HbA1c: Annually (diabetes screening)
- Preventive Care Integration
- Cervical cancer screening: Annually for women
- Anal cancer screening: Annually for MSM and women
- Bone density: Every 2-3 years after age 50
- Cardiovascular risk: 10-year ASCVD calculator
- Depression screening: PHQ-9 at routine visits
- Routine Laboratory Surveillance
-
Drug Interaction Management
- High-Risk Medication Classes
- Acid-reducing agents: Reduce INSTI absorption
- Separate dosing: 2 hours before or 6 hours after
- Dolutegravir: Take with food if PPI co-administered
- Anticonvulsants: Induce CYP3A4 (reduce PI/INSTI levels)
- Carbamazepine/phenytoin: Avoid with most ART
- Levetiracetam: Preferred seizure medication
- Antimycobacterials: Rifampin significantly reduces ART levels
- Rifabutin: Preferred alternative (dose adjustment required)
- Acid-reducing agents: Reduce INSTI absorption
- High-Risk Medication Classes
| Drug Class | ART Interaction | Management Strategy | Clinical Impact |
|---|---|---|---|
| Proton Pump Inhibitors | ↓ INSTI absorption | Separate dosing | Virologic failure |
| Anticonvulsants | ↓ PI/INSTI levels | Alternative agents | Resistance development |
| Rifamycins | ↓ Most ART levels | Rifabutin substitution | Treatment failure |
| Warfarin | Variable effects | INR monitoring | Bleeding/thrombosis |
| Statins | ↑ Statin levels | Dose reduction | Myopathy/rhabdomyolysis |
| Immunosuppressants | Variable levels | TDM recommended | Rejection/toxicity |
- Comorbidity Management Priorities
- Cardiovascular Disease Prevention
- Risk assessment: Framingham or ASCVD calculator
- Statin therapy: Consider for ASCVD risk >7.5%
- Blood pressure: Target <130/80 mmHg
- Smoking cessation: Reduces CVD risk by 50%
- Exercise: 150 minutes/week moderate activity
- Bone Health Optimization
- DEXA scan: Baseline and every 2-3 years
- Vitamin D: Target 25(OH)D >30 ng/mL
- Calcium: 1200mg daily for adults >50
- Bisphosphonates: T-score <-2.5 or fragility fracture
- Cardiovascular Disease Prevention
💡 Master This: Modern HIV care focuses on preventing non-AIDS complications because viral suppression eliminates AIDS risk but chronic inflammation accelerates aging and comorbidity development - comprehensive preventive care is essential for optimal outcomes
- Advanced Care Considerations
- Aging with HIV (>50 years: 54% of HIV population)
- Polypharmacy: Average 6-8 medications
- Frailty syndrome: 2-3x higher prevalence
- Cognitive impairment: 30-50% have HAND
- Social isolation: Depression in 40% of patients
- Long-Acting ART Options
- Cabotegravir/rilpivirine: Monthly injections
- Efficacy: Non-inferior to daily oral ART
- Adherence: Eliminates daily pill burden
- Candidates: Virally suppressed patients on stable regimen
- Aging with HIV (>50 years: 54% of HIV population)
Treatment success requires >95% adherence, regular monitoring, proactive comorbidity management, and patient education. Undetectable viral load eliminates transmission risk and enables normal life expectancy with appropriate comprehensive care.
🎯 Clinical Mastery Arsenal: The HIV Care Continuum
Practice Questions: HIV
Test your understanding with these related questions
A 32-year-old man comes to the physician for a follow-up examination 1 week after being admitted to the hospital for oral candidiasis and esophagitis. His CD4+ T lymphocyte count is 180 cells/μL. An HIV antibody test is positive. Genotypic resistance assay shows the virus to be susceptible to all antiretroviral therapy regimens and therapy with dolutegravir, tenofovir, and emtricitabine is initiated. Which of the following sets of laboratory findings would be most likely on follow-up evaluation 3 months later? $$$ CD4 +/CD8 ratio %%% HIV RNA %%% HIV antibody test $$$









