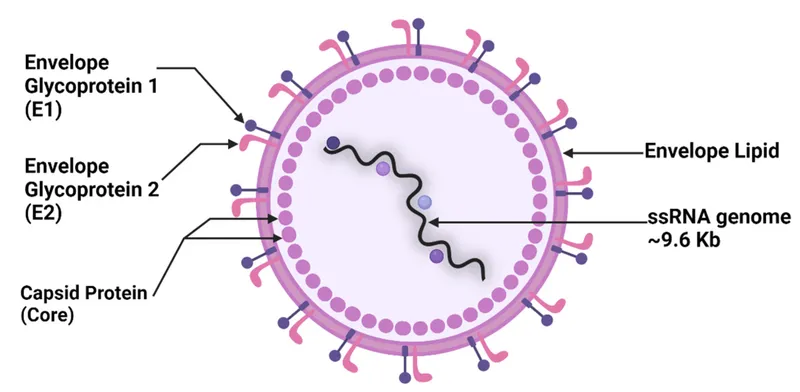
HCV structure and genotypes US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for HCV structure and genotypes. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
HCV structure and genotypes US Medical PG Question 1: A 28-year-old woman with a history of intravenous drug use is brought to the emergency department because of a 1-day history of fatigue, yellow eyes, confusion, and blood in her stools. She appears ill. Her temperature is 38.1°C (100.6°F). Physical examination shows pain in the right upper quadrant, diffuse jaundice with scleral icterus, and bright red blood in the rectal vault. Further evaluation demonstrates virions in her blood, some of which have a partially double-stranded DNA genome while others have a single-stranded RNA genome. They are found to share an identical lipoprotein envelope. This patient is most likely infected with which of the following pathogens?
- A. Deltavirus (Correct Answer)
- B. Filovirus
- C. Calicivirus
- D. Hepevirus
- E. Herpesvirus
HCV structure and genotypes Explanation: ***Deltavirus***
- The presence of both **partially double-stranded DNA virions** (Hepatitis B virus) and **single-stranded RNA virions** (Hepatitis D virus) sharing an identical lipoprotein envelope is pathognomonic for **coinfection with Hepatitis B and Deltavirus (HDV)** [1].
- **Hepatitis D (Deltavirus)** is a **defective RNA virus** that is obligately dependent on **Hepatitis B surface antigen (HBsAg)** for its replication, assembly, and transmission—explaining why both viruses share the same lipoprotein envelope [1].
- This coinfection or superinfection with HDV causes **more severe acute hepatitis** and higher rates of **fulminant hepatic failure** compared to HBV alone, consistent with the patient's presentation of jaundice, confusion (hepatic encephalopathy), and GI bleeding [1].
- The patient's **IV drug use** is a key risk factor for both HBV and HDV transmission [1].
*Filovirus*
- **Filoviruses** (e.g., Ebola, Marburg) cause **viral hemorrhagic fever** with severe bleeding manifestations and can present with bloody stools.
- However, they are **single-stranded RNA viruses** only and do not involve coinfection with a DNA virus, nor do they share envelopes with DNA viruses.
- Geographic exposure and clinical context (African hemorrhagic fever outbreaks) would be expected.
*Calicivirus*
- **Caliciviruses** (e.g., Norovirus, Sapovirus) are **non-enveloped, single-stranded RNA viruses** that cause acute **gastroenteritis** with vomiting and diarrhea.
- They do not cause hepatitis with jaundice and hepatic encephalopathy, nor do they involve DNA virus coinfection or envelope sharing.
*Hepevirus*
- **Hepatitis E virus (Hepevirus)** is a **non-enveloped, single-stranded RNA virus** that causes acute hepatitis, primarily through **fecal-oral transmission** (contaminated water).
- While it causes liver disease, it does not involve coinfection with a DNA virus or envelope sharing, and it is not typically associated with IV drug use.
- It can cause severe disease in pregnant women but does not explain the dual genome findings.
*Herpesvirus*
- **Herpesviruses** are **enveloped, double-stranded DNA viruses** that cause various infections (HSV, VZV, EBV, CMV).
- While they have DNA genomes and envelopes, they do not coinfect with RNA viruses sharing the same envelope structure, nor are they typically associated with acute severe hepatitis in IV drug users.
- Hepatitis from CMV or EBV would have different serologic and molecular findings.
HCV structure and genotypes US Medical PG Question 2: A scientist in Chicago is studying a new blood test to detect Ab to EBV with increased sensitivity and specificity. So far, her best attempt at creating such an exam reached 82% sensitivity and 88% specificity. She is hoping to increase these numbers by at least 2 percent for each value. After several years of work, she believes that she has actually managed to reach a sensitivity and specificity much greater than what she had originally hoped for. She travels to China to begin testing her newest blood test. She finds 2,000 patients who are willing to participate in her study. Of the 2,000 patients, 1,200 of them are known to be infected with EBV. The scientist tests these 1,200 patients' blood and finds that only 120 of them tested negative with her new exam. Of the patients who are known to be EBV-free, only 20 of them tested positive. Given these results, which of the following correlates with the exam's specificity?
- A. 82%
- B. 90%
- C. 84%
- D. 86%
- E. 98% (Correct Answer)
HCV structure and genotypes Explanation: ***98%***
- **Specificity** measures the proportion of **true negatives** among all actual negatives.
- In this case, 800 patients are known to be EBV-free (actual negatives), and 20 of them tested positive (false positives). This means 800 - 20 = 780 tested negative (true negatives). Specificity = (780 / 800) * 100% = **98%**.
*82%*
- This value represents the *original sensitivity* before the scientist’s new attempts to improve the test.
- It does not reflect the *newly calculated specificity* based on the provided data.
*90%*
- This value represents the *newly calculated sensitivity* of the test, not the specificity.
- Out of 1200 EBV-infected patients, 120 tested negative (false negatives), meaning 1080 tested positive (true positives). Sensitivity = (1080 / 1200) * 100% = 90%.
*84%*
- This percentage is not directly derived from the information given for either sensitivity or specificity after the new test results.
- It does not correspond to any of the calculated values for the new test's performance.
*86%*
- This percentage is not directly derived from the information given for either sensitivity or specificity after the new test results.
- It does not correspond to any of the calculated values for the new test's performance.
HCV structure and genotypes US Medical PG Question 3: A scientist is researching the long term effects of the hepatitis viruses on hepatic tissue. She finds that certain strains are oncogenic and increase the risk of hepatocellular carcinoma. However, they appear to do so via different mechanisms. Which of the following answer choices correctly pairs the hepatitis virus with the correct oncogenic process?
- A. Hepatitis A virus - chronic inflammation
- B. Hepatitis C virus - chronic inflammation
- C. Hepatitis E virus - integration of viral DNA into host hepatocyte genome
- D. Hepatitis B virus - integration of viral DNA into host hepatocyte genome (Correct Answer)
- E. Hepatitis A virus - integration of viral DNA into host hepatocyte genome
HCV structure and genotypes Explanation: ***Hepatitis B virus - integration of viral DNA into host hepatocyte genome***
- **Hepatitis B virus (HBV)** is a **DNA virus** that can integrate its genetic material into the host hepatocyte genome, leading to genomic instability and promoting oncogenesis.
- This integration, along with chronic inflammation and the production of viral regulatory proteins, contributes significantly to the development of **hepatocellular carcinoma (HCC)**.
*Hepatitis A virus - chronic inflammation*
- **Hepatitis A virus (HAV)** is an **RNA virus** that causes **acute hepatitis** but does not lead to chronic infection or chronic inflammation.
- Due to its acute and self-limiting nature, HAV is **not associated with hepatocellular carcinoma**.
*Hepatitis C virus - integration of viral DNA into host hepatocyte genome*
- **Hepatitis C virus (HCV)** is an **RNA virus** and therefore does not integrate its DNA into the host genome (as it has no DNA phase).
- HCV causes HCC primarily through **chronic inflammation**, **fibrosis**, and **cirrhosis**, not DNA integration.
*Hepatitis E virus - integration of viral DNA into host hepatocyte genome*
- **Hepatitis E virus (HEV)** is an **RNA virus** that typically causes acute, self-limiting hepatitis and does not integrate its genetic material into the host genome.
- While HEV can cause chronic infection in immunocompromised individuals, it is **not generally recognized as an oncogenic virus** leading to HCC.
*Hepatitis A virus - integration of viral DNA into host hepatocyte genome*
- **Hepatitis A virus (HAV)** is an **RNA virus**, meaning it does not have a DNA stage and therefore cannot integrate DNA into the host genome.
- HAV causes **acute, self-limiting infections** and is definitively **not associated with hepatocellular carcinoma**.
HCV structure and genotypes US Medical PG Question 4: A 30-year-old woman presents with generalized fatigue, joint pain, and decreased appetite. She says that symptoms onset a year ago and have not improved. The patient’s husband says he has recently noticed that her eyes and skin are yellowish. The patient denies any history of smoking or alcohol use, but she admits to using different kinds of intravenous illicit drugs during her college years. The patient is afebrile and vital signs are within normal limits. Physical examination is unremarkable, except for moderate scleral icterus. A polymerase chain reaction (PCR) of a blood sample is positive for a viral infection that reveals a positive-sense RNA virus, that is small, enveloped, and single-stranded. The patient is started on a drug that resembles a purine RNA nucleotide. She agrees not to get pregnant before or during the use of this medication. Which of the following is the drug that was most likely given to this patient?
- A. Sofosbuvir
- B. Cidofovir
- C. Ribavirin (Correct Answer)
- D. Simeprevir
- E. Interferon-alpha
HCV structure and genotypes Explanation: ***Ribavirin***
- The patient's history of **intravenous drug use**, fatigue, joint pain, decreased appetite, and **scleral icterus** are highly suggestive of **chronic Hepatitis C virus (HCV) infection**. The description of the virus as a **small, enveloped, single-stranded positive-sense RNA virus** confirms HCV. The patient is started on a drug that resembles a **purine RNA nucleotide** and is instructed not to get pregnant, which is characteristic of Ribavirin.
- **Ribavirin** is a **guanosine analog** that interferes with viral RNA synthesis and is known to be **teratogenic**, necessitating strict contraception during and after treatment.
*Sofosbuvir*
- While **Sofosbuvir** is used to treat Hepatitis C and is a **nucleotide analog** (specifically a uridine analog), it is a **prodrug** that mimics a uridine nucleotide, not a purine, and it is **not associated with the severe teratogenicity** that requires a two-contraception rule like Ribavirin.
- Sofosbuvir is a **direct-acting antiviral (DAA)** that inhibits the HCV RNA-dependent RNA polymerase, but the description of a purine RNA nucleotide points away from this drug.
*Cidofovir*
- **Cidofovir** is a **cytosine nucleotide analog** primarily used to treat **cytomegalovirus (CMV)** retinitis in HIV/AIDS patients.
- It works by inhibiting viral DNA polymerase, and it is **not used for Hepatitis C infection**.
*Simeprevir*
- **Simeprevir** is an **HCV protease inhibitor**, not a nucleotide analog. It specifically targets the **NS3/4A protease** of the Hepatitis C virus.
- Although it is an effective DAA for HCV, its mechanism of action and class are different from the described "purine RNA nucleotide."
*Interferon-alpha*
- **Interferon-alpha** was historically used to treat Hepatitis C, but it is a **cytokine** that modulates the immune response, not a nucleoside/nucleotide analog.
- Its use has largely been replaced by more effective and better-tolerated direct-acting antivirals due to significant side effects and lower efficacy.
HCV structure and genotypes US Medical PG Question 5: An investigator is studying the rate of multiplication of hepatitis C virus in hepatocytes. The viral genomic material is isolated, enzymatically cleaved into smaller fragments and then separated on a formaldehyde agarose gel membrane. Targeted probes are then applied to the gel and visualized under x-ray. Which of the following is the most likely structure being identified by this test?
- A. Lipid-linked oligosaccharides
- B. Transcription factors
- C. Polypeptides
- D. Ribonucleic acids (Correct Answer)
- E. Deoxyribonucleic acids
HCV structure and genotypes Explanation: ***Ribonucleic acids***
- The description of isolating "viral genomic material," which is then "enzymatically cleaved" and run on a "formaldehyde agarose gel," followed by the application of "targeted probes" and X-ray visualization, perfectly matches the technique of **Northern blotting**.
- Northern blotting is used to detect and quantify specific **RNA sequences**, which is consistent with the hepatitis C virus being an RNA virus.
*Lipid-linked oligosaccharides*
- These molecules are involved in protein glycosylation and are typically analyzed using techniques like **mass spectrometry** or **chromatography**, not Northern blotting.
- They are not nucleic acid material, which is implied by "viral genomic material" and enzymatic cleavage steps.
*Transcription factors*
- **Transcription factors** are proteins that regulate gene expression and would typically be identified using techniques like **Western blotting** (for protein detection) or Electrophoretic Mobility Shift Assay (EMSA) for DNA binding.
- They are not directly "genomic material" that would be cleaved and run on an agarose gel in this manner.
*Polypeptides*
- **Polypeptides** are chains of amino acids, i.e., proteins, which are normally detected using **Western blotting** after separation on an SDS-PAGE gel.
- The use of "formaldehyde agarose gel" and "enzymatic cleavage" points specifically to nucleic acid analysis, not protein analysis.
*Deoxyribonucleic acids*
- While DNA is genomic material and is often analyzed similarly, the use of a **formaldehyde agarose gel** is characteristic of RNA electrophoresis because formaldehyde prevents RNA from forming secondary structures.
- Furthermore, hepatitis C is a **single-stranded RNA virus**, meaning its genome is RNA, not DNA.
HCV structure and genotypes US Medical PG Question 6: A 23-year-old male with a homozygous CCR5 mutation is found to be immune to HIV infection. The patient’s CCR5 mutation interferes with the function of which viral protein?
- A. gp41
- B. Reverse transcriptase
- C. pp17
- D. gp120 (Correct Answer)
- E. p24
HCV structure and genotypes Explanation: ***gp120***
- The **gp120 protein** on the HIV envelope is responsible for binding to the **CD4 receptor** and the **coreceptor CCR5** or CXCR4 on host cells, which is the initial step for viral entry.
- A homozygous **CCR5 mutation** (specifically the **CCR5-Δ32** deletion) prevents HIV from using this coreceptor, thereby blocking the binding of gp120 and subsequent viral entry.
*gp41*
- **gp41** is another envelope protein that, after gp120 binds to CD4 and a coreceptor, undergoes a conformational change to mediate **fusion** of the viral and host cell membranes.
- While essential for entry, gp41 acts downstream of gp120's primary binding to the coreceptor, so a CCR5 mutation primarily affects gp120's ability to engage with the cell.
*Reverse transcriptase*
- **Reverse transcriptase** is a viral enzyme responsible for **converting viral RNA into DNA** once the virus has already entered the host cell cytoplasm.
- A CCR5 mutation prevents viral entry, thus the activity of reverse transcriptase is not directly interfered with by the mutation itself but rather by the lack of cellular access.
*pp17*
- **pp17**, also known as **matrix protein**, is an internal structural protein that plays a role in the assembly of new virions and guiding the **reverse transcribed DNA** into the nucleus.
- This protein is involved in later stages of the viral life cycle *after* entry and integration, and its function is not directly blocked by a CCR5 mutation.
*p24*
- **p24** is the major **capsid protein** that forms the core of the HIV virus, enclosing the viral RNA and enzymes.
- It is critical for maintaining the structural integrity of the virus and is a key target for diagnostic tests, but it does not directly participate in the initial binding and entry process that is affected by a CCR5 mutation.
HCV structure and genotypes US Medical PG Question 7: A 52-year-old man presents to his physician after his routine screening revealed that he has elevated liver enzymes. He complains of occasional headaches during the past year, but otherwise feels well. The patient reports that he was involved in a serious car accident in the 1980s. He does not smoke or drink alcohol. He has no history of illicit intravenous drug use. He does not currently take any medications and has no known allergies. His father had a history of alcoholism and died of liver cancer. The patient appears thin. His temperature is 37.8°C (100°F), pulse is 100/min, and blood pressure is 110/70 mm Hg. The physical examination reveals no abnormalities. The laboratory test results show the following:
Complete blood count
Hemoglobin 14 g/dL
Leukocyte count 10,000/mm3
Platelet count 146,000/mm3
Comprehensive metabolic profile
Glucose 150 mg/dL
Albumin 3.2 g/dL
Total bilirubin 1.5 mg/dL
Alkaline phosphatase 75 IU/L
AST 95 IU/L
ALT 73 IU/L
Other lab tests
HIV negative
Hepatitis B surface antigen negative
Hepatitis C antibody positive
HCV RNA positive
HCV genotype 1
A liver biopsy is performed and shows mononuclear infiltrates localized to portal tracts that reveal periportal hepatocyte necrosis. Which of the following is the most appropriate next step in management?
- A. Peginterferon alpha therapy
- B. Interferon and ribavirin therapy
- C. Sofosbuvir and ledipasvir therapy (Correct Answer)
- D. Tenofovir and entecavir therapy
- E. Tenofovir and velpatasvir therapy
HCV structure and genotypes Explanation: ***Sofosbuvir and ledipasvir therapy***
- This patient has chronic **Hepatitis C (HCV) infection** (HCV antibody positive, HCV RNA positive). **Sofosbuvir/ledipasvir** is an effective **direct-acting antiviral (DAA)** regimen for **genotype 1 HCV**, which is indicated for treatment-naïve patients without cirrhosis.
- The liver biopsy findings of **mononuclear infiltrates** and **periportal necrosis** confirm active hepatitis and the need for antiviral treatment to prevent progression to cirrhosis.
*Peginterferon alpha therapy*
- **Peginterferon alpha** was historically used for HCV, but its use has largely been replaced by **DAAs** due to significant side effects and lower efficacy.
- This therapy is associated with numerous adverse effects, including **flu-like symptoms**, **depression**, and **bone marrow suppression**.
*Interferon and ribavirin therapy*
- This combination was a standard treatment for HCV before the advent of DAAs, but it is associated with a high burden of **side effects** like **hemolytic anemia** (from ribavirin) and **flu-like symptoms** (from interferon).
- Given the availability of highly effective and well-tolerated DAAs, this regimen is no longer considered first-line for chronic HCV.
*Tenofovir and entecavir therapy*
- **Tenofovir** and **entecavir** are antiviral medications primarily used for the treatment of **chronic Hepatitis B (HBV) infection**.
- This patient's **Hepatitis B surface antigen is negative**, ruling out chronic HBV infection as the primary issue requiring these specific drugs.
*Tenofovir and velpatasvir therapy*
- While **velpatasvir** is a DAA used for HCV, its combination with **tenofovir** is not a standard HCV treatment for genotype 1.
- **Tenofovir** is primarily an anti-HBV drug; for HCV, velpatasvir is typically combined with **sofosbuvir** (as in Epclusa) for pan-genotypic coverage.
HCV structure and genotypes US Medical PG Question 8: A 45-year-old man presents for follow-up to monitor his chronic hepatitis C treatment. The patient was infected with hepatitis C genotype 1, one year ago. He has been managed on a combination of pegylated interferon-alpha and ribavirin, but a sustained viral response has not been achieved. Past medical history is significant for non-alcoholic fatty liver disease for the last 5 years. Which of the following, if added to the patient’s current treatment regimen, would most likely benefit this patient?
- A. Emtricitabine
- B. Entecavir
- C. Simeprevir (Correct Answer)
- D. Tenofovir
- E. Telbivudine
HCV structure and genotypes Explanation: ***Simeprevir***
- Simeprevir is a **first-generation direct-acting antiviral (DAA)**, specifically a **protease inhibitor (NS3/4A inhibitor)**, highly effective against **HCV genotype 1**.
- Adding simeprevir to a regimen of **pegylated interferon-alpha and ribavirin** significantly increases the likelihood of achieving a **sustained virologic response** for patients who previously failed interferon-based therapy.
- **Note:** While this triple therapy approach was standard practice historically, current guidelines (as of 2024-2025) favor **interferon-free DAA combination regimens** (such as sofosbuvir/ledipasvir or glecaprevir/pibrentasvir) as first-line treatment for HCV genotype 1. However, among the options provided, simeprevir remains the only appropriate HCV-specific antiviral agent.
*Emtricitabine*
- This is a **nucleoside reverse transcriptase inhibitor (NRTI)** primarily used in the treatment of **HIV infection** and sometimes for hepatitis B.
- It has **no significant role** in the treatment of **hepatitis C viral infection**.
*Entecavir*
- Entecavir is an **antiviral agent** specifically used for the treatment of **chronic hepatitis B virus (HBV)** infection.
- It has **no established efficacy** against the **hepatitis C virus (HCV)**.
*Tenofovir*
- Tenofovir is a **nucleotide reverse transcriptase inhibitor** primarily used for treating **HIV infection** and **chronic hepatitis B virus (HBV)** infection.
- It is **not effective** against **hepatitis C virus (HCV)**.
*Telbivudine*
- Telbivudine is an **oral antiviral agent** indicated specifically for the treatment of **chronic hepatitis B virus (HBV)** infection.
- It does **not have antiviral activity** against the **hepatitis C virus (HCV)**.
HCV structure and genotypes US Medical PG Question 9: A 53-year-old man presents to an urgent care center with severe fever that began during the day along with muscle and joint pains. He states that he felt fine the day before but then developed a fever to 103°F (39.4°C) and had to leave work after which he developed a headache and body pains. The patient states that he was recently in South Asia for a business trip and was otherwise feeling well since returning 2 weeks ago. On exam, the patient’s temperature is 103.3°F (39.6°C), blood pressure is 110/84 mmHg, pulse is 94/min, and respirations are 14/min. On physical exam, the patient appears flushed and has a rash that blanches when touched. On laboratory workup, the pathogen was identified as an enveloped virus with an icosahedral capsid and had positive-sense, single-stranded linear RNA. Which of the following is the most likely cause of this patient's presentation?
- A. Dengue virus (Correct Answer)
- B. Norovirus
- C. Coronavirus
- D. Marburg virus
- E. Saint Louis encephalitis virus
HCV structure and genotypes Explanation: ***Dengue virus***
- The patient's presentation with **acute onset of high fever**, severe **muscle and joint pains** ("breakbone fever"), headache, and a **blanching rash** after recent travel to **South Asia** is highly characteristic of dengue fever.
- The description of the pathogen as an **enveloped virus** with an **icosahedral capsid** and **positive-sense, single-stranded linear RNA** perfectly matches the **Flaviviridae family** to which the dengue virus belongs.
- Among the options, only dengue virus and Saint Louis encephalitis virus have these exact structural characteristics (both are flaviviruses), but the **clinical presentation** with severe myalgia/arthralgia and travel to South Asia clearly points to dengue.
*Norovirus*
- Norovirus typically causes **gastroenteritis**, characterized primarily by **vomiting, diarrhea**, and abdominal cramps, which are not the dominant symptoms in this patient.
- While fever can occur, it's usually **mild** and not as prominent as the high fever and severe myalgia/arthralgia seen in dengue.
- **Structurally**, norovirus is **non-enveloped** (naked capsid), which does not match the pathogen description.
*Coronavirus*
- Coronaviruses are associated with **respiratory illnesses** (e.g., common cold, SARS, MERS, COVID-19) causing symptoms like cough, shortness of breath, and sore throat.
- While fever and body aches can occur, the **severe joint pains** and typical rash are not hallmarks of coronavirus infections.
- **Structurally**, coronaviruses have **helical nucleocapsid symmetry**, not icosahedral, which excludes this option based on the pathogen description.
*Marburg virus*
- Marburg virus causes a severe **hemorrhagic fever** with symptoms including high fever, severe headache, malaise, followed by gastrointestinal symptoms, and eventually **hemorrhagic manifestations** (e.g., bleeding from orifices, petechiae, purpura).
- The patient's presentation does not describe any hemorrhagic signs, and the rash is blanching, not petechial or purpuric.
- **Structurally**, Marburg is a filovirus with **helical symmetry** and **negative-sense ssRNA**, not positive-sense with icosahedral capsid, which excludes this option.
*Saint Louis encephalitis virus*
- Saint Louis encephalitis virus causes a **neuroinvasive disease** characterized by encephalitis, presenting with altered mental status, seizures, and focal neurological deficits, although some patients may have milder fever and headache.
- While it shares the **same viral structure** as dengue (both are flaviviruses with enveloped, icosahedral, (+)ssRNA), the **clinical presentation** differs significantly—this patient lacks neurological symptoms.
- The prominent **severe myalgia, arthralgia**, typical blanching rash, and **travel history to dengue-endemic South Asia** distinguish dengue from Saint Louis encephalitis.
HCV structure and genotypes US Medical PG Question 10: An investigator is studying the mechanism of HIV infection in cells obtained from a human donor. The effect of a drug that impairs viral fusion and entry is being evaluated. This drug acts on a protein that is cleaved off of a larger glycosylated protein in the endoplasmic reticulum of the host cell. The protein that is affected by the drug is most likely encoded by which of the following genes?
- A. gag
- B. env (Correct Answer)
- C. tat
- D. pol
- E. rev
HCV structure and genotypes Explanation: ***env***
- The **env (envelope) gene** of HIV encodes for the precursor protein **gp160**, which is then cleaved by host cellular proteases into **gp120** and **gp41** within the endoplasmic reticulum.
- **gp120** and **gp41** together form the viral envelope glycoproteins responsible for viral binding to host cells and **fusion/entry**, making them the target of drugs that impair these processes.
*gag*
- The **gag (group-specific antigen) gene** encodes for structural proteins of the viral core, such as **p24 (capsid protein)**, p17 (matrix protein), and p7 (nucleocapsid protein).
- These proteins are primarily involved in the assembly of new virions and do not directly mediate viral fusion and entry.
*tat*
- The **tat (trans-activator of transcription) gene** encodes a regulatory protein that significantly enhances the transcription of viral genes.
- It plays a crucial role in the viral life cycle by increasing the efficiency of HIV gene expression, but it is not directly involved in viral fusion or entry.
*pol*
- The **pol (polymerase) gene** encodes for essential viral enzymes, including **reverse transcriptase**, integrase, and protease.
- These enzymes are critical for converting viral RNA into DNA, integrating viral DNA into the host genome, and cleaving viral polyproteins, respectively, but they are not involved in mediating viral entry.
*rev*
- The **rev (regulator of virion expression) gene** encodes a regulatory protein that facilitates the transport of unspliced and partially spliced viral RNAs from the nucleus to the cytoplasm.
- This transport is crucial for the synthesis of structural and enzymatic proteins and for packaging viral RNA into new virions, but it does not directly participate in viral fusion and entry.
More HCV structure and genotypes US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.