Hepatitis B/C

On this page
🦠 The Viral Hepatitis Battlefield: HBV & HCV Mastery
Hepatitis B and C together account for most chronic viral hepatitis worldwide, silently destroying livers while leaving distinct serological fingerprints that reveal infection stage, immunity, and treatment needs. You'll master how to interpret complex antibody and antigen patterns, understand the molecular replication strategies that guide antiviral therapy, recognize acute versus chronic presentations, and build treatment algorithms that prevent cirrhosis and hepatocellular carcinoma. This lesson transforms hepatitis from a confusing alphabet soup into a logical diagnostic and therapeutic framework you'll confidently apply at the bedside.
The hepatitis B virus (HBV) and hepatitis C virus (HCV) represent two of medicine's most sophisticated viral adversaries, affecting over 350 million people worldwide with HBV and 170 million with HCV. These RNA and DNA viruses have evolved distinct strategies for immune evasion, chronic persistence, and hepatocellular destruction.
📌 Remember: HEPATITIS - HBsAg (surface), EBeAg (infectivity), Polymerase (replication), Anti-HBc (core exposure), Total anti-HBs (immunity), IgM anti-HBc (acute), Time window (serology gaps), Interferon response, Serological profiles
- HBV Characteristics
- DNA virus with 3.2 kb genome
- 42 nm Dane particle with surface, core, and polymerase proteins
- Reverse transcriptase mechanism despite being DNA virus
- Surface antigen (HBsAg): 22 nm particles, infectivity marker
- Core antigen (HBcAg): nucleocapsid protein, not detectable in serum
- e-antigen (HBeAg): secreted protein indicating high viral replication
- HCV Characteristics
- RNA virus with 9.6 kb genome
- 6 major genotypes with distinct treatment responses
- High mutation rate: 10^-3 to 10^-5 per nucleotide per replication cycle
- Envelope proteins E1/E2: primary targets for neutralizing antibodies
- NS3/4A protease: essential for viral replication, DAA target
- NS5A protein: replication complex component, resistance hotspot
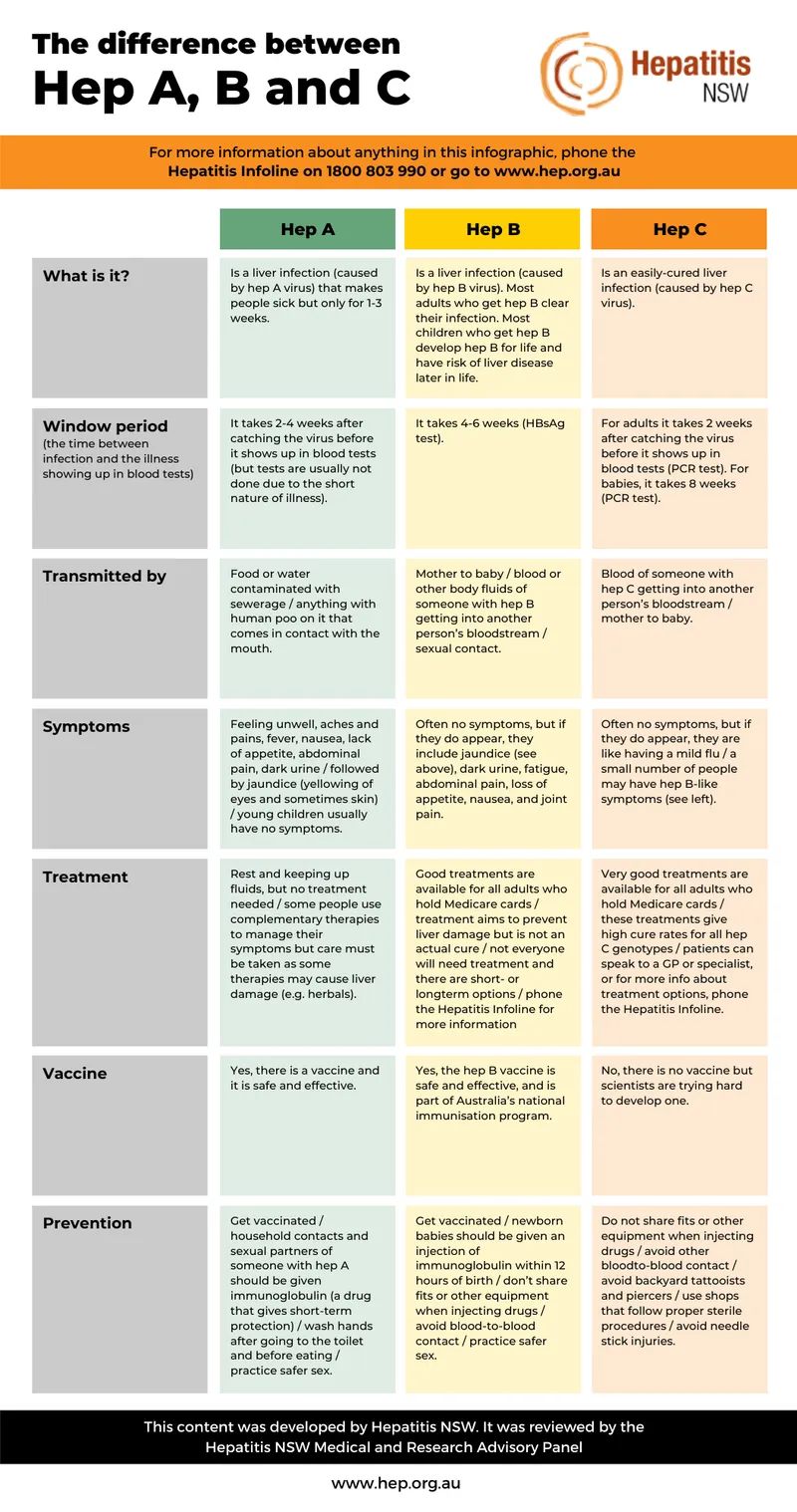
| Parameter | HBV | HCV | Clinical Significance |
|---|---|---|---|
| Genome | DNA, 3.2 kb | RNA, 9.6 kb | Affects mutation rates |
| Chronicity Rate | 90% (neonatal), 5% (adult) | 85% overall | Age-dependent outcomes |
| Genotypes | 8 (A-H) | 6 major types | Treatment selection |
| Vaccine Available | Yes, 95% effective | No | Prevention strategies |
| Cure Rate | <5% with treatment | >95% with DAAs | Treatment expectations |
💡 Master This: The "window period" in HBV occurs when HBsAg disappears but anti-HBs hasn't appeared yet - only anti-HBc IgG remains positive, creating diagnostic confusion lasting weeks to months.
Connect viral structure mastery through serological interpretation to understand diagnostic precision patterns.
🦠 The Viral Hepatitis Battlefield: HBV & HCV Mastery
⚔️ Serological Warfare: Decoding the Immune Response
📌 Remember: WINDOW - Waning HBsAg, Isolated anti-HBc, No anti-HBs yet, Diagnostic gap, Occult infection possible, Weeks to months duration
-
HBV Acute Infection Markers
- HBsAg: First marker positive, appears 2-10 weeks post-exposure
- Anti-HBc IgM: Acute infection marker, positive 1-2 weeks after HBsAg
- HBeAg: High infectivity marker, correlates with >10^5 copies/mL viral load
- ALT elevation: Peaks at 10-20x normal during acute phase
- Anti-HBe seroconversion: Indicates immune control, 90% reduction in viral load
- Anti-HBs appearance: Immunity marker, appears weeks to months after HBsAg clearance
-
HBV Chronic Infection Phases
- Immune tolerant: HBeAg+, normal ALT, viral load >10^7 copies/mL
- Immune active: HBeAg+, elevated ALT >2x normal, active hepatitis
- Inactive carrier: HBeAg-, anti-HBe+, viral load <2000 copies/mL
- Reactivation phase: HBeAg- with elevated ALT and viral load >2000 copies/mL
- Occult HBV: HBsAg negative but HBV DNA detectable, <200 copies/mL
| Serological Pattern | HBsAg | Anti-HBc | Anti-HBs | HBeAg | Anti-HBe | Interpretation |
|---|---|---|---|---|---|---|
| Acute Infection | + | IgM+ | - | +/- | - | Recent exposure |
| Chronic Active | + | IgG+ | - | + | - | High replication |
| Chronic Inactive | + | IgG+ | - | - | + | Low replication |
| Resolved | - | IgG+ | + | - | +/- | Past infection |
| Vaccinated | - | - | + | - | - | Immune protection |
| Window Period | - | IgG+ | - | - | +/- | Diagnostic gap |
💡 Master This: Isolated anti-HBc pattern (HBsAg negative, anti-HBs negative, anti-HBc positive) occurs in 4-20% of patients and requires HBV DNA testing to exclude occult hepatitis B before immunosuppression.
Connect serological mastery through viral replication understanding to predict treatment response patterns.
⚔️ Serological Warfare: Decoding the Immune Response
🎯 Viral Replication Mastery: The Molecular Battlefield
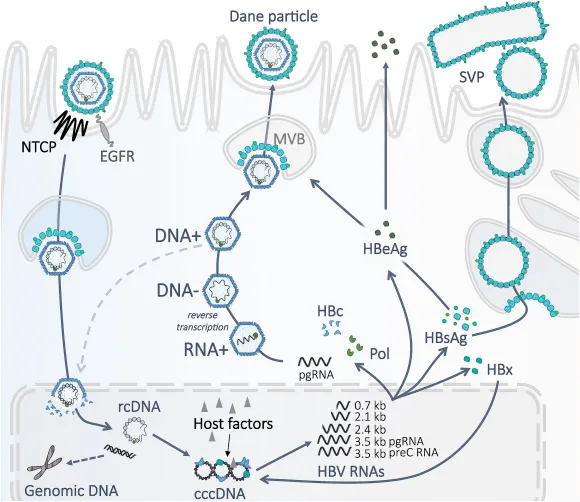
📌 Remember: REPLICATE - Reverse transcriptase (HBV), RNA polymerase (HCV), Error-prone copying, Persistent templates, Low fidelity, Integration (HBV), CccDNA formation, Antiviral targets, Treatment resistance, Escape mutations
-
HBV Replication Strategy
- cccDNA formation: Stable episomal template, 50-100 copies per infected hepatocyte
- Reverse transcriptase: Error rate 10^-4 per nucleotide, lower than HCV
- Integration events: Random chromosomal insertion, 1-10 copies per cell
- Pregenomic RNA: Template for reverse transcription, 3.5 kb transcript
- Surface protein production: Independent of viral replication, 10^6-fold excess
- Polymerase protein: Multifunctional enzyme with RT, RNase H, primer activities
-
HCV Replication Characteristics
- RNA-dependent RNA polymerase: Error rate 10^-3 to 10^-5 per nucleotide
- Quasispecies formation: Viral population diversity, 10^12 particles daily
- Replication complex: ER-associated, NS proteins essential
- NS3/4A protease: Cleaves 4 sites in polyprotein, DAA target
- NS5A protein: Replication and assembly functions, resistance hotspot
- NS5B polymerase: RNA synthesis enzyme, sofosbuvir target
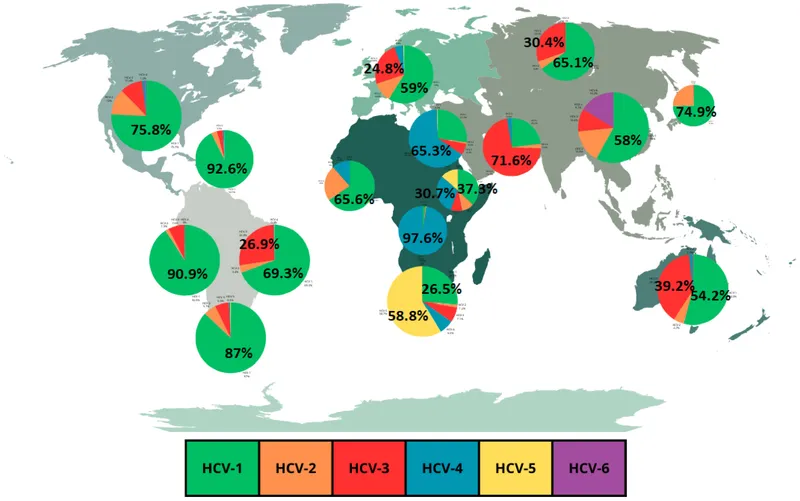
| Viral Target | HBV Mechanism | HCV Mechanism | Clinical Resistance |
|---|---|---|---|
| Polymerase | Reverse transcriptase | RNA-dependent RNA pol | rtM204V/I (HBV), S282T (HCV) |
| Protease | Not applicable | NS3/4A serine protease | R155K, A156T, D168A/E/V |
| NS5A | Not applicable | Replication complex | M28T, Q30R, L31M, Y93H |
| Entry | PreS mutations | E1/E2 envelope | Rare clinical significance |
| Assembly | Core mutations | NS5A domain I | Treatment-emergent variants |
💡 Master This: HCV genotype 3 shows intrinsic NS5A resistance with baseline Y93H variant in 10-15% of patients, requiring extended 24-week treatment duration compared to 12 weeks for genotypes 1, 2, 4-6.
Connect replication understanding through clinical pattern recognition to master diagnostic frameworks.
🎯 Viral Replication Mastery: The Molecular Battlefield
🔍 Clinical Pattern Recognition: The Diagnostic Framework
📌 Remember: PATTERNS - Peak ALT timing, Acute vs chronic markers, Transmission history, Temporal relationships, Extrahepatic features, Risk factors, Normal vs abnormal ranges, Serological evolution
-
Acute Hepatitis Recognition
- ALT elevation: >10x normal suggests acute viral hepatitis
- Temporal pattern: Symptoms 2-8 weeks after exposure (HBV), 6-12 weeks (HCV)
- Serological evolution: Anti-HBc IgM positive in acute HBV
- Jaundice pattern: Bilirubin >3 mg/dL in 70% of acute HBV
- Symptom duration: 4-12 weeks typical for acute hepatitis B
- Fulminant risk: <1% for HBV, rare for HCV
-
Chronic Disease Indicators
- Duration markers: HBsAg positive >6 months defines chronic HBV
- Fibrosis assessment: FIB-4 >3.25 suggests advanced fibrosis
- Viral load thresholds: >2000 IU/mL indicates treatment consideration
- Cirrhosis markers: Platelet count <150,000, albumin <3.5 g/dL
- HCC surveillance: Required when cirrhosis or HBsAg positive >40 years
- Extrahepatic manifestations: 25% of chronic HCV patients
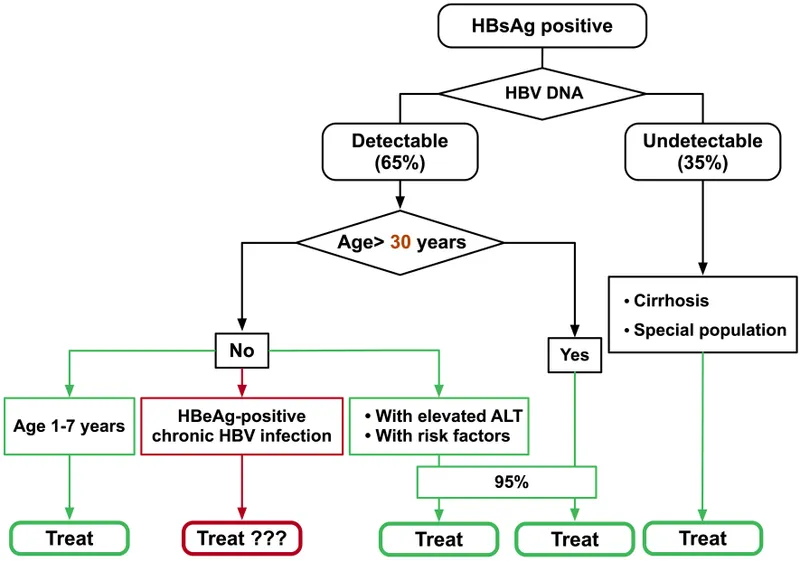
| Clinical Scenario | HBV Pattern | HCV Pattern | Immediate Action |
|---|---|---|---|
| Acute Infection | HBsAg+, anti-HBc IgM+ | HCV RNA+, Ab- | Supportive care, monitor |
| Chronic Active | HBsAg+, elevated ALT | HCV RNA+, elevated ALT | Consider treatment |
| Treatment Candidate | VL >2000, ALT >2x | Any detectable RNA | Initiate antivirals |
| Cured/Resolved | Anti-HBs+, anti-HBc+ | HCV Ab+, RNA- | Routine monitoring |
| Reactivation Risk | Isolated anti-HBc | Past HCV infection | Screen before immunosuppression |
💡 Master This: Acute HCV shows ALT >10x normal in 80% of cases but symptoms in only 20-30%, making high clinical suspicion essential in high-risk populations with unexplained transaminase elevation.
Connect pattern recognition through treatment algorithms to understand therapeutic decision-making.
🔍 Clinical Pattern Recognition: The Diagnostic Framework
⚖️ Treatment Algorithm Mastery: Evidence-Based Therapeutics
📌 Remember: TREATMENT - Target selection, Resistance testing, Efficacy rates, Adverse effects, Timing optimization, Monitoring protocols, End-of-treatment, Non-response management, Tolerance assessment
-
HCV Treatment Principles
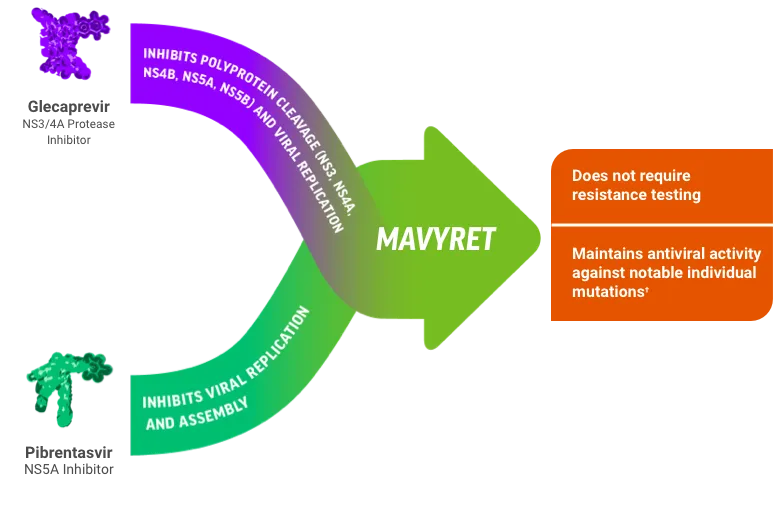
- Pan-genotypic regimens: Sofosbuvir/velpatasvir effective against all genotypes
- Treatment duration: 12 weeks standard, 24 weeks for genotype 3 with cirrhosis
- Cure definition: Undetectable HCV RNA at 12 weeks post-treatment (SVR12)
- Glecaprevir/pibrentasvir: 8 weeks for treatment-naive without cirrhosis
- Drug interactions: Significant with proton pump inhibitors, anticonvulsants
- Resistance testing: Recommended for NS5A failures, genotype 3
-
HBV Treatment Strategy
- First-line agents: Entecavir 0.5 mg or tenofovir 300 mg daily
- Treatment goals: Viral suppression <20 IU/mL, ALT normalization
- Finite therapy: HBeAg seroconversion plus 12 months consolidation
- Indefinite therapy: HBeAg-negative patients, cirrhosis, immunosuppressed
- Resistance monitoring: Annual for lamivudine/adefovir, rare with entecavir/tenofovir
- HBsAg loss: Ultimate goal, achieved in <5% annually
| Treatment Scenario | HBV Approach | HCV Approach | Expected Outcome |
|---|---|---|---|
| Treatment-Naive | Entecavir/tenofovir | SOF/VEL 12 weeks | >90% suppression/cure |
| Cirrhosis | Indefinite therapy | Extended duration | Monitor complications |
| Previous Failure | Switch nucleos(t)ide | Resistance testing | Salvage regimens |
| Renal Disease | Entecavir preferred | Dose adjustments | Modified monitoring |
| Pregnancy | Tenofovir safe | Defer treatment | Vertical transmission prevention |
💡 Master This: HBV treatment stopping rules require HBeAg seroconversion plus undetectable viral load for ≥12 months, but relapse rates reach 50% in HBeAg-negative patients, favoring indefinite therapy.
Connect treatment mastery through multi-system integration to understand complex clinical scenarios.
⚖️ Treatment Algorithm Mastery: Evidence-Based Therapeutics
🔗 Multi-System Integration: Advanced Clinical Synthesis
📌 Remember: INTEGRATE - Immunosuppression risks, Nephrotoxicity monitoring, Transplant considerations, Extrahepatic disease, Genetic factors, Resistance patterns, Adverse interactions, Timing coordination, Emergent complications
-
HBV Reactivation Management
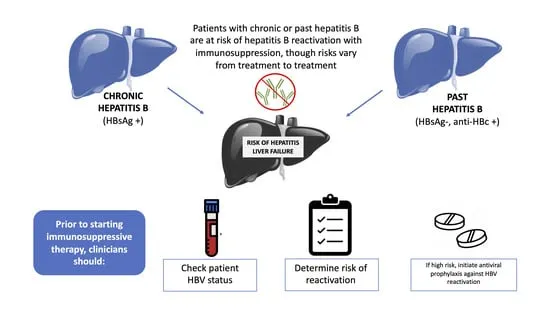
- High-risk immunosuppression: Anti-CD20, anthracyclines, high-dose steroids
- Prophylaxis indications: HBsAg positive or isolated anti-HBc with rituximab
- Monitoring protocol: HBV DNA every 1-3 months during immunosuppression
- Reactivation definition: >1 log increase in viral load or HBsAg reappearance
- Prophylactic duration: 6-12 months post-immunosuppression completion
- Breakthrough risk: <5% with entecavir/tenofovir prophylaxis
-
Co-infection Complexities
- HIV/HBV: Tenofovir/emtricitabine backbone treats both viruses
- HIV/HCV: Drug interactions with protease inhibitors and DAAs
- HBV/HCV: HCV dominance with HBV suppression, treat HCV first
- HBV/HDV: Peginterferon only effective therapy for hepatitis delta
- Transplant setting: HBV immunoglobulin plus antivirals prevent recurrence
- Renal disease: Entecavir preferred over tenofovir for HBV
| Co-morbidity | HBV Interaction | HCV Interaction | Management Strategy |
|---|---|---|---|
| HIV Co-infection | Accelerated progression | Faster fibrosis | Dual antiviral therapy |
| Chronic Kidney Disease | Tenofovir nephrotoxicity | DAA dose adjustment | Modified regimens |
| Diabetes Mellitus | Insulin resistance | Improved with SVR | Metabolic monitoring |
| Autoimmune Disease | Reactivation risk | Cryoglobulinemia | Immunosuppression caution |
| Malignancy | Chemotherapy reactivation | Lymphoma association | Prophylactic antivirals |
💡 Master This: HBV reactivation can occur months to years after immunosuppression, requiring indefinite monitoring in high-risk patients with isolated anti-HBc pattern and history of rituximab or stem cell transplant.
Connect multi-system understanding through rapid mastery tools to create clinical reference frameworks.
🔗 Multi-System Integration: Advanced Clinical Synthesis
🎯 Clinical Command Center: Rapid Mastery Arsenal
📌 Remember: MASTERY - Monitor viral loads, Assess treatment response, Screen for complications, Time interventions, Evaluate resistance, Recognize reactivation, Yield optimal outcomes
- Essential Clinical Thresholds
- HBV treatment threshold: >2000 IU/mL with ALT elevation
- HCV treatment indication: Any detectable RNA regardless of level
- Cirrhosis screening: FIB-4 >3.25 or APRI >2.0
- HCC surveillance: Ultrasound q6 months for cirrhosis or HBsAg >40 years
- Reactivation monitoring: HBV DNA q1-3 months during immunosuppression
- Treatment response: Undetectable at week 12 (HCV) or week 48 (HBV)
| Clinical Scenario | Critical Action | Time Frame | Success Metric |
|---|---|---|---|
| Acute Hepatitis | Supportive care, monitor | Weekly labs x4 | ALT normalization |
| Treatment Initiation | Start antivirals | Within 1-2 weeks | Viral suppression |
| Reactivation Risk | Prophylactic therapy | Before immunosuppression | Prevent flares |
| Treatment Failure | Resistance testing | At confirmed failure | Salvage regimen |
| Post-Treatment | SVR monitoring | 12 weeks post-Rx | Sustained response |
💡 Master This: HBV prophylaxis before high-risk immunosuppression prevents reactivation in >95% of cases, while delayed treatment after reactivation carries 10-30% mortality risk in severe cases.
Connect rapid mastery tools through systematic clinical application to achieve hepatitis expertise excellence.
🎯 Clinical Command Center: Rapid Mastery Arsenal
Practice Questions: Hepatitis B/C
Test your understanding with these related questions
A 29-year-old man comes to the physician for a routine health maintenance examination. He feels well. He works as a nurse at a local hospital in the city. Three days ago, he had a needlestick injury from a patient whose serology is positive for hepatitis B. He completed the 3-dose regimen of the hepatitis B vaccine 2 years ago. His other immunizations are up-to-date. He appears healthy. Physical examination shows no abnormalities. He is concerned about his risk of being infected with hepatitis B following his needlestick injury. Serum studies show negative results for hepatitis B surface antigen, hepatitis B surface antibody, and hepatitis C antibody. Which of the following is the most appropriate next step in management?













