Dimorphic fungi

On this page
🔬 The Shape-Shifting Pathogens: Dimorphic Fungi Mastery
Dimorphic fungi are master shape-shifters that transform between mold and yeast forms in response to temperature, enabling them to thrive in soil and then invade human tissue with devastating precision. You'll discover how this thermal switch operates at the molecular level, recognize the distinct clinical patterns each pathogen creates across organ systems, and build systematic approaches to diagnosis and evidence-based treatment. Understanding these organisms means connecting environmental exposure to geographic distribution, linking morphology to pathogenesis, and translating laboratory findings into life-saving therapeutic decisions for patients facing these often-missed infections.
📌 Remember: BASH-PT - Blastomyces, Aspergillus (not dimorphic), Sporothrix, Histoplasma, Paracoccidioides, Talaromyces. (Note: Aspergillus is the impostor - not dimorphic!)
The fundamental principle governing dimorphic fungi involves thermal dimorphism - a genetically programmed response to temperature changes. At 25°C (environmental temperature), these fungi exist as filamentous molds producing infectious conidia. Upon inhalation or inoculation into human hosts at 37°C, they undergo morphological transformation into yeast forms or specialized structures like spherules.
| Organism | Environmental Form (25°C) | Host Form (37°C) | Geographic Distribution | Primary Route |
|---|---|---|---|---|
| Histoplasma capsulatum | Septate hyphae with microconidia | Small budding yeasts (2-4 μm) | Mississippi/Ohio River valleys | Inhalation |
| Blastomyces dermatitidis | Septate hyphae with conidia | Large yeasts with broad-based buds (8-15 μm) | Great Lakes, Southeastern US | Inhalation |
| Coccidioides immitis | Septate hyphae with arthroconidia | Spherules with endospores (20-200 μm) | Southwestern US, Mexico | Inhalation |
| Paracoccidioides brasiliensis | Septate hyphae with conidia | Multiple budding yeasts ("pilot's wheel") | Central/South America | Inhalation |
| Sporothrix schenckii | Septate hyphae with conidia | Cigar-shaped yeasts (2-6 μm) | Worldwide (tropical/subtropical) | Cutaneous inoculation |
The pathogenesis involves biphasic lifecycle adaptation where environmental molds produce infectious propagules (conidia, arthroconidia) that become airborne. Upon inhalation, these structures encounter the 37°C host environment, triggering genetic expression changes that promote yeast morphology. This transformation typically occurs within 24-72 hours and represents a critical virulence mechanism.
- Primary Infection Patterns:
- Pulmonary involvement: >90% of cases begin with respiratory exposure
- Asymptomatic infection: 80-95% of immunocompetent individuals
- Progressive disease: <5% in healthy hosts, >50% in immunocompromised
- HIV patients with CD4+ <200 cells/μL show 10-fold increased risk
- Organ transplant recipients demonstrate 15-20% incidence rates
💡 Master This: Dimorphic fungi exploit the 37°C thermal switch as their primary virulence mechanism. Understanding this temperature-dependent transformation predicts both diagnostic approaches and therapeutic targets.
The endemic nature of dimorphic fungi creates distinct epidemiological patterns. Histoplasma dominates the Mississippi and Ohio River valleys where >80% of residents show positive skin tests. Blastomyces concentrates around the Great Lakes region with 1-2 cases per 100,000 annual incidence. Coccidioides remains confined to southwestern deserts where >60% of residents demonstrate serological evidence of exposure.
Connect these foundational concepts through morphological recognition patterns to understand how temperature-dependent dimorphism drives both pathogenesis and diagnostic strategies.
🔬 The Shape-Shifting Pathogens: Dimorphic Fungi Mastery
⚙️ The Thermal Switch: Molecular Transformation Machinery
📌 Remember: TEMP-37 - Thermal trigger, Enzyme activation, Morphogenesis genes, Protein synthesis at 37°C creates the transformation cascade.
The heat shock response initiates within 30 minutes of temperature elevation, with HSP90 and HSP70 proteins serving as primary molecular chaperones. These proteins facilitate proper folding of morphogenesis-specific enzymes and transcription factors. Calcineurin signaling plays a crucial role, with >75% of dimorphic fungi requiring functional calcineurin for successful yeast-phase maintenance.
- Transformation Timeline:
- 0-2 hours: Heat shock protein upregulation (5-10 fold increase)
- 2-8 hours: Morphogenesis gene activation (>100 genes involved)
- 8-24 hours: Cell wall restructuring (chitin/glucan ratio changes)
- Mold form: 60% chitin, 40% glucan
- Yeast form: 40% chitin, 60% glucan
- 24-72 hours: Complete morphological conversion (>95% cells transformed)
The cell wall remodeling process involves dramatic changes in polysaccharide composition and surface protein expression. β-1,3-glucan content increases 2-3 fold in yeast forms, while chitin distribution shifts from diffuse to concentrated at budding sites. These changes alter antifungal susceptibility patterns and immune recognition.
⭐ Clinical Pearl: Azole resistance often increases 2-4 fold in yeast forms compared to mold forms due to altered cell wall permeability and efflux pump expression.
| Organism | Transformation Time | Key Molecular Markers | Clinical Significance |
|---|---|---|---|
| Histoplasma | 24-48 hours | YPS3 protein, CBP1 gene | Rapid conversion enables quick dissemination |
| Blastomyces | 48-72 hours | BAD1 adhesin, WI-1 antigen | Slower conversion, localized initial infection |
| Coccidioides | 72-96 hours | SOWgp spherule protein | Longest conversion, spherule maturation |
| Paracoccidioides | 36-60 hours | GP43 glycoprotein | Intermediate timing, multiple budding |
| Sporothrix | 24-36 hours | SsCP1 protein | Fastest conversion, cutaneous adaptation |
💡 Master This: The 37°C thermal switch triggers coordinated molecular reprogramming affecting cell wall structure, metabolism, and virulence factor expression. This transformation determines both pathogenic potential and therapeutic vulnerability.
Virulence factor expression shows phase-specific patterns with many adhesins and immune evasion molecules upregulated exclusively in yeast forms. BAD1 (Blastomyces adhesin) increases >10-fold in yeast phase, while GP43 (Paracoccidioides glycoprotein) shows 8-fold elevation. These phase-specific antigens serve as both diagnostic targets and vaccine candidates.
Connect this molecular transformation machinery through pattern recognition frameworks to understand how morphological changes translate into distinct clinical presentations and diagnostic challenges.
⚙️ The Thermal Switch: Molecular Transformation Machinery
🎯 The Recognition Matrix: Clinical Pattern Mastery
📌 Remember: GEO-MORPH-CLIN - GEOgraphic exposure + MORPHological findings + CLINical presentation = Diagnostic direction
- Geographic Recognition Patterns:
- Mississippi/Ohio River valleys + pulmonary symptoms = Histoplasma (>80% probability)
- Great Lakes region + skin lesions = Blastomyces (>70% probability)
- Southwestern US + erythema nodosum = Coccidioides (>90% probability)
- Arizona/California residents show >60% lifetime exposure rates
- "Valley Fever" affects >10,000 cases annually in endemic areas
- Rose gardening + lymphocutaneous lesions = Sporothrix (>95% probability)
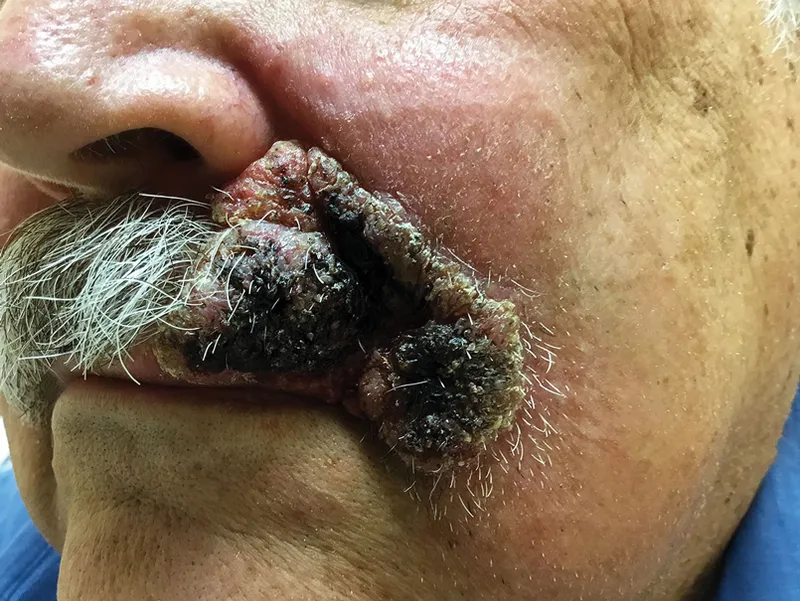
The morphological recognition matrix provides rapid identification clues when organisms are visualized in clinical specimens. Size relationships offer immediate differentiation, with Histoplasma yeasts (2-4 μm) appearing 4-fold smaller than Blastomyces yeasts (8-15 μm). Coccidioides spherules (20-200 μm) dwarf all other forms by 10-50 fold.
| Clinical Scenario | Geographic Clue | Morphological Finding | Diagnostic Probability |
|---|---|---|---|
| Cave explorer, Ohio | Mississippi Valley | Small intracellular yeasts | Histoplasma >90% |
| Fisherman, Wisconsin | Great Lakes | Broad-based budding yeasts | Blastomyces >85% |
| Construction worker, Arizona | Southwest desert | Large spherules with endospores | Coccidioides >95% |
| Gardener, Brazil | South America | Multiple budding "pilot's wheel" | Paracoccidioides >90% |
| Florist, worldwide | Any region | Cigar-shaped yeasts | Sporothrix >80% |
Clinical presentation patterns follow organ system involvement with pulmonary manifestations dominating >90% of initial presentations for inhaled organisms. Skin involvement suggests either primary cutaneous inoculation (Sporothrix) or disseminated disease (Blastomyces, Histoplasma).
- Pulmonary Pattern Recognition:
- Acute pneumonia + hilar lymphadenopathy = Histoplasma (>70% of acute cases)
- Chronic pneumonia + upper lobe cavitation = Blastomyces (>60% of chronic cases)
- Pneumonia + erythema nodosum = Coccidioides (>80% association)
- Erythema nodosum occurs in >25% of symptomatic coccidioidomycosis
- Arthralgias accompany >60% of acute infections
Dissemination risk factors create predictable patterns with immunocompromised states increasing risk 10-100 fold. HIV patients with CD4+ <200 cells/μL show >50% dissemination rates for Histoplasma, while organ transplant recipients demonstrate >30% progression to severe disease.
💡 Master This: Combine geographic exposure + morphological size + clinical syndrome to achieve >85% diagnostic accuracy before culture confirmation. This triad approach enables early targeted therapy.
Timing patterns provide additional diagnostic clues with acute presentations (<4 weeks) suggesting high inoculum exposure or immunocompromise, while chronic presentations (>3 months) indicate reactivation or low-grade progressive infection. Seasonal clustering occurs with Coccidioides showing 2-3 fold increased incidence during dust storm seasons.
Connect these recognition patterns through systematic discrimination frameworks to understand how clinical presentations guide specific diagnostic approaches and treatment decisions.
🎯 The Recognition Matrix: Clinical Pattern Mastery
🔍 The Diagnostic Decoder: Systematic Identification Strategies
📌 Remember: MICRO-CULT-SERO-PCR - MICROscopy (rapid), CULTure (gold standard), SEROlogy (supportive), PCR (confirmatory) create the diagnostic arsenal.
Direct microscopy provides immediate results within 30-60 minutes but requires >10⁴ organisms/mL for reliable detection. KOH preparations show 60-80% sensitivity for most dimorphic fungi, while calcofluor white increases sensitivity to 75-90% by enhancing cell wall visualization.
Culture methodology remains the gold standard despite prolonged incubation requirements. Sabouraud dextrose agar at 25°C promotes mold growth, while brain-heart infusion agar at 37°C encourages yeast development. Conversion studies demonstrating mold-to-yeast transformation provide definitive proof of dimorphism.
| Diagnostic Method | Sensitivity | Specificity | Time to Result | Clinical Utility |
|---|---|---|---|---|
| Direct Microscopy | 60-80% | >95% | 30-60 minutes | Rapid screening |
| Fungal Culture | >95% | >99% | 2-8 weeks | Gold standard |
| Antigen Detection | 70-90% | >95% | 2-4 hours | Acute diagnosis |
| Serology (CF/ID) | 80-95% | >90% | 24-48 hours | Supportive evidence |
| PCR/Sequencing | >95% | >99% | 4-24 hours | Rapid confirmation |
Serological testing provides supportive evidence with complement fixation (CF) and immunodiffusion (ID) remaining standard methods. Rising titers (4-fold increase) indicate active infection, while stable high titers (>1:32) suggest chronic disease. Cross-reactivity occurs in <5% of cases but requires careful interpretation.
- Serology Interpretation Guidelines:
- Histoplasma CF >1:32 or any ID bands = >90% probability active infection
- Blastomyces CF >1:8 or A-band ID = >85% probability active infection
- Coccidioides CF >1:16 or TP/CF bands = >95% probability active infection
- IgM detection indicates recent infection (<8 weeks)
- IgG persistence suggests chronic infection (>6 months)
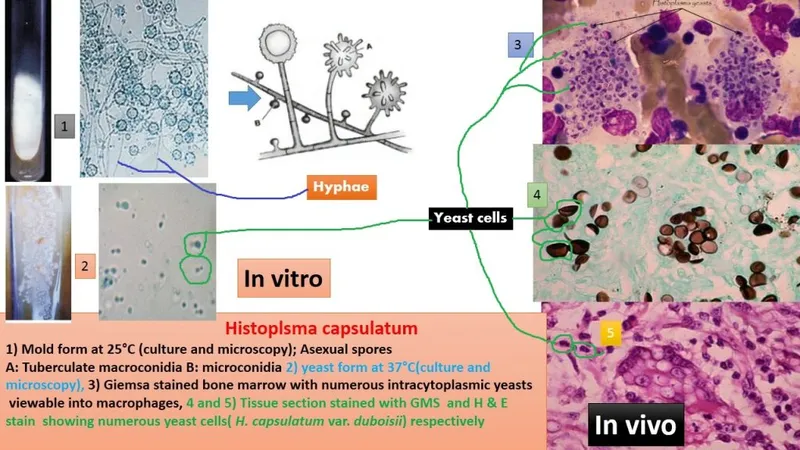
Molecular diagnostics revolutionize rapid identification with PCR-based methods achieving >95% sensitivity and >99% specificity within 4-24 hours. Real-time PCR enables quantitative assessment of fungal burden, while sequencing provides definitive species identification.
💡 Master This: Combine immediate microscopy + rapid antigen/PCR + culture confirmation to achieve both early diagnosis (within 24 hours) and definitive identification (within 2-8 weeks). This parallel approach optimizes both clinical care and diagnostic accuracy.
Histopathological examination reveals characteristic tissue reactions with granulomatous inflammation present in >80% of cases. Special stains (GMS, PAS) enhance organism visualization, while tissue morphology provides species-specific clues even when cultures remain negative.
Connect these diagnostic strategies through evidence-based treatment algorithms to understand how identification methods guide therapeutic decisions and monitoring approaches.
🔍 The Diagnostic Decoder: Systematic Identification Strategies
⚖️ The Therapeutic Arsenal: Evidence-Based Treatment Algorithms
📌 Remember: MILD-ORAL, SEVERE-IV - MILD disease gets ORAL azoles, SEVERE disease gets IV amphotericin B, then ORAL step-down therapy.
Itraconazole serves as the first-line oral agent for Histoplasma and Blastomyces with >90% efficacy in mild-moderate disease. Therapeutic drug monitoring ensures adequate levels (>1.0 μg/mL) as bioavailability varies 10-fold between patients. Food administration increases absorption 3-4 fold compared to fasting states.
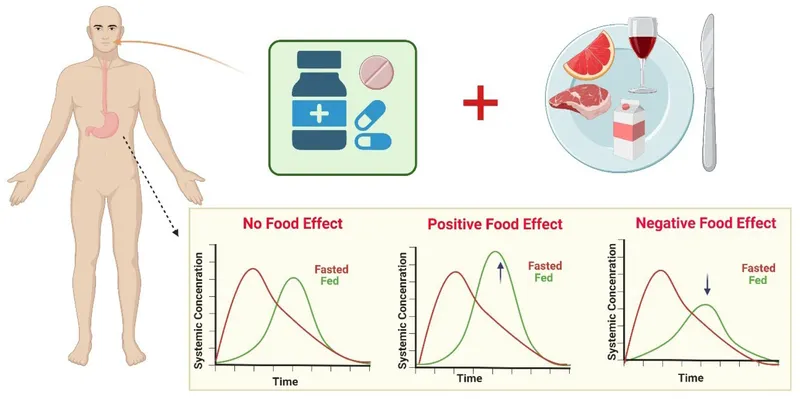
- Itraconazole Dosing Strategies:
- Loading dose: 200 mg TID × 3 days, then 200 mg BID
- Therapeutic levels: >1.0 μg/mL (check after 2 weeks)
- Duration: 6-12 months for pulmonary, 12-24 months for disseminated
- Histoplasma: >95% cure rate with 6-month courses
- Blastomyces: >90% cure rate with 6-month courses
- Treatment failure: <5% with adequate levels and duration
Fluconazole dominates Coccidioides treatment with superior CNS penetration (>70% CSF levels) and excellent bioavailability (>90% oral absorption). High-dose therapy (800-1200 mg daily) shows >85% efficacy in meningeal disease, while standard doses (400 mg daily) suffice for pulmonary infections.
| Organism | Mild-Moderate Disease | Severe-Disseminated | CNS Involvement | Treatment Duration |
|---|---|---|---|---|
| Histoplasma | Itraconazole 200mg BID | AmB → Itraconazole | AmB → Itraconazole | 6-12 months |
| Blastomyces | Itraconazole 200mg BID | AmB → Itraconazole | AmB → Itraconazole | 6-12 months |
| Coccidioides | Fluconazole 400mg daily | AmB → Fluconazole | Fluconazole 800-1200mg | 12-24 months |
| Paracoccidioides | Itraconazole 200mg BID | AmB → Itraconazole | AmB → Itraconazole | 12-24 months |
| Sporothrix | Itraconazole 200mg BID | AmB → Itraconazole | AmB → Itraconazole | 3-6 months |
Treatment monitoring requires systematic assessment of both therapeutic response and drug toxicity. Clinical improvement typically occurs within 2-4 weeks of appropriate therapy, while radiological improvement may lag 4-8 weeks behind clinical response.
- Monitoring Parameters:
- Clinical response: Fever resolution within 1-2 weeks
- Laboratory monitoring: CBC, LFTs every 2-4 weeks
- Drug levels: Itraconazole after 2 weeks, target >1.0 μg/mL
- Imaging: Repeat chest CT at 3-6 months
- >50% improvement indicates adequate response
- <25% improvement suggests treatment failure
Special populations require modified approaches with immunocompromised patients needing longer treatment courses and higher drug doses. HIV patients with CD4+ <200 cells/μL require lifelong suppressive therapy until immune reconstitution occurs (CD4+ >200 cells/μL × 6 months).
💡 Master This: Severity stratification drives initial drug selection (oral vs IV), while organism identification determines specific agent choice (itraconazole vs fluconazole). Therapeutic drug monitoring ensures adequate exposure and optimal outcomes.
Resistance patterns remain uncommon (<5% overall) but show geographic clustering in certain regions. Azole resistance correlates with prior antifungal exposure and prolonged treatment courses. Alternative agents (posaconazole, voriconazole) provide salvage options for resistant infections.
Connect these treatment algorithms through multi-system integration frameworks to understand how antifungal therapy interacts with host immune responses and other therapeutic interventions.
⚖️ The Therapeutic Arsenal: Evidence-Based Treatment Algorithms
🔗 The Integration Network: Multi-System Clinical Mastery
📌 Remember: IMMUNE-ORGAN-DRUG-DISEASE - IMMUNE status + ORGAN involvement + DRUG interactions + DISEASE comorbidities = Integrated management approach
Immune system interactions create bidirectional relationships where dimorphic fungi both trigger immune responses and exploit immune deficiencies. Th1-mediated immunity provides primary protection with interferon-γ and TNF-α playing crucial roles. >90% of immunocompetent individuals** mount effective granulomatous responses that contain infection.
- Immune Integration Patterns:
- CD4+ T-cells: Primary defense mechanism (>500 cells/μL protective)
- Macrophage activation: IFN-γ dependent fungicidal activity
- Granuloma formation: >80% of cases show organized immune response
- Epithelioid cells: Central component of effective containment
- Giant cells: Present in >70% of chronic infections
- Immunosuppression effects: >10-fold increased dissemination risk
Organ system involvement follows predictable patterns based on portal of entry and hematogenous dissemination. Pulmonary manifestations dominate initial presentations (>90% of cases), while extrapulmonary spread indicates either high inoculum exposure or compromised host defenses.
| System Involvement | Frequency | Clinical Manifestations | Diagnostic Clues | Prognostic Significance |
|---|---|---|---|---|
| Pulmonary | >90% | Pneumonia, cavitation, nodules | Chest imaging, sputum | Good prognosis if localized |
| Cutaneous | 20-40% | Ulcers, nodules, plaques | Skin biopsy, culture | Suggests dissemination |
| CNS | 5-15% | Meningitis, brain abscess | CSF analysis, MRI | Poor prognosis |
| Gastrointestinal | 10-30% | Ulceration, bleeding | Endoscopy, biopsy | Advanced disease |
| Bone/Joint | 5-20% | Osteomyelitis, arthritis | Imaging, synovial fluid | Chronic infection |
Drug interaction networks create complex pharmacological challenges particularly with azole antifungals that inhibit CYP3A4 enzymes. Itraconazole increases levels of >50 medications including warfarin (2-3 fold), cyclosporine (2-4 fold), and statins (3-5 fold).
Comorbidity interactions significantly impact treatment outcomes with diabetes mellitus increasing infection severity by 2-3 fold and COPD predisposing to chronic pulmonary forms. Solid organ transplant recipients show >30% incidence of severe disease requiring modified immunosuppression.
- Advanced Integration Concepts:
- Immune reconstitution syndrome: 10-25% of HIV patients starting HAART
- Paradoxical worsening: Initial deterioration despite appropriate therapy
- Chronic suppressive therapy: Required until CD4+ >200 × 6 months
- Histoplasma: Itraconazole 200 mg daily for suppression
- Coccidioides: Fluconazole 400 mg daily for suppression
💡 Master This: Multi-system dimorphic fungi infections require integrated assessment of immune status, organ involvement, drug interactions, and comorbidities to optimize therapeutic outcomes and minimize complications.
Emerging resistance patterns and novel therapeutic approaches represent cutting-edge developments in dimorphic fungi management. Combination therapy with azoles plus echinocandins shows promise in refractory cases, while immunomodulatory approaches using interferon-γ demonstrate benefit in selected patients.
Connect this multi-system integration through rapid mastery frameworks to develop comprehensive clinical tools for immediate application in complex dimorphic fungi cases.
🔗 The Integration Network: Multi-System Clinical Mastery
🎯 The Clinical Command Center: Rapid Mastery Arsenal
📌 Remember: RAPID-MASTER - Recognition patterns, Assessment tools, Practical algorithms, Immediate decisions, Definitive management, Monitoring protocols, Advanced strategies, Systemic thinking, Therapeutic excellence, Evidence-based, Results-oriented
Essential Numbers Arsenal - Critical thresholds for immediate clinical decisions:
- Temperature Threshold: 37°C = Mold-to-yeast conversion trigger
- Geographic Risk: >80% exposure rates in endemic areas
- Diagnostic Sensitivity: Direct microscopy 60-80%, Culture >95%, PCR >95%
- Treatment Efficacy: Itraconazole >90% mild disease, AmB >95% severe disease
- Monitoring Timeline: Clinical response 2-4 weeks, Radiologic response 4-8 weeks
| RAPID RECOGNITION MATRIX | Geographic Clue | Morphology | Clinical Pattern | First-Line Therapy |
|---|---|---|---|---|
| Histoplasma | Mississippi Valley | 2-4 μm intracellular | Hilar lymphadenopathy | Itraconazole 200mg BID |
| Blastomyces | Great Lakes | 8-15 μm broad buds | Skin + lung lesions | Itraconazole 200mg BID |
| Coccidioides | Southwest US | 20-200 μm spherules | Erythema nodosum | Fluconazole 400mg daily |
| Paracoccidioides | South America | Multiple budding | Oral/pulmonary | Itraconazole 200mg BID |
| Sporothrix | Worldwide | Cigar-shaped | Lymphocutaneous | Itraconazole 200mg BID |
Severity Stratification Protocol - 30-second assessment for treatment intensity:
- Severity Indicators (Any 1 = Severe):
- Respiratory failure: O₂ sat <90% or mechanical ventilation
- CNS involvement: Altered mental status or focal deficits
- Disseminated disease: >2 organ systems involved
- Immunocompromise: CD4+ <200 or neutropenia <500
- Hemodynamic instability: Shock or vasopressor requirement
💡 Master This: Rapid severity assessment + geographic exposure history + morphological clues = Immediate treatment decision within <5 minutes of patient encounter. Speed saves lives in severe dimorphic fungi infections.
Treatment Decision Tree - Evidence-based protocols for immediate implementation:
- Mild Disease Protocol: Oral azoles × 6-12 months
- Severe Disease Protocol: IV AmB 3-5 mg/kg/day → Oral step-down
- CNS Disease Protocol: High-dose fluconazole or AmB + azole combination
- Immunocompromised Protocol: Aggressive therapy + prolonged duration + suppressive therapy
Monitoring Excellence Framework - Systematic follow-up ensuring optimal outcomes:
- Week 2: Drug levels (itraconazole >1.0 μg/mL), LFTs, CBC
- Month 1: Clinical response assessment, repeat imaging if severe
- Month 3: Radiological improvement evaluation, treatment duration planning
- Month 6-12: Cure assessment, relapse monitoring, suppression decisions
This Clinical Command Center transforms dimorphic fungi complexity into manageable clinical protocols that ensure rapid recognition, appropriate treatment, and optimal patient outcomes through systematic excellence.
🎯 The Clinical Command Center: Rapid Mastery Arsenal
Practice Questions: Dimorphic fungi
Test your understanding with these related questions
A 34-year-old woman presents with multiple round, scaly patches on her chest and back. The lesions are hypopigmented and slightly pruritic. KOH preparation of skin scrapings shows short, angular hyphae. Which of the following best describes the causative organism's morphology in culture?












