Novel anti-biofilm therapies US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Novel anti-biofilm therapies. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Novel anti-biofilm therapies US Medical PG Question 1: A 42-year-old woman with a history of multiple sclerosis and recurrent urinary tract infections comes to the emergency department because of flank pain and fever. Her temperature is 38.8°C (101.8°F). Examination shows left-sided costovertebral angle tenderness. She is admitted to the hospital and started on intravenous vancomycin. Three days later, her symptoms have not improved. Urine culture shows growth of Enterococcus faecalis. Which of the following best describes the most likely mechanism of antibiotic resistance in this patient?
- A. Increased efflux across bacterial cell membranes
- B. Production of beta-lactamase
- C. Alteration of penicillin-binding proteins
- D. Alteration of peptidoglycan synthesis (Correct Answer)
- E. Alteration of ribosomal targets
Novel anti-biofilm therapies Explanation: ***Alteration of peptidoglycan synthesis***
- **Vancomycin** targets the **D-Ala-D-Ala terminus** on the peptidoglycan precursor, preventing cross-linking during bacterial cell wall synthesis.
- **Vancomycin resistance in Enterococcus faecalis** occurs through acquisition of resistance genes (vanA, vanB) that encode enzymes modifying the peptidoglycan precursor from **D-Ala-D-Ala to D-Ala-D-Lac**.
- This structural change reduces vancomycin's binding affinity by approximately 1000-fold, rendering the antibiotic ineffective.
- The mechanism directly involves **alteration of the peptidoglycan synthesis pathway**, specifically the terminal amino acid residues of the pentapeptide precursor.
*Increased efflux across bacterial cell membranes*
- This mechanism involves **efflux pumps that actively transport antibiotics out of the bacterial cell**, reducing intracellular concentration.
- While efflux pumps contribute to resistance for antibiotics like **tetracyclines, fluoroquinolones, and macrolides**, this is not the primary mechanism of vancomycin resistance in Enterococcus.
*Production of beta-lactamase*
- **Beta-lactamase enzymes** hydrolyze the **beta-lactam ring** of antibiotics like **penicillins and cephalosporins**, rendering them inactive.
- **Vancomycin is a glycopeptide antibiotic, not a beta-lactam**, so its efficacy is not affected by beta-lactamase production.
*Alteration of ribosomal targets*
- This mechanism confers resistance to antibiotics that target **bacterial ribosomes** to inhibit protein synthesis, such as **macrolides, aminoglycosides, and tetracyclines**.
- **Vancomycin acts on cell wall synthesis**, not protein synthesis, so alteration of ribosomal targets is not relevant to vancomycin resistance.
*Alteration of penicillin-binding proteins*
- **Penicillin-binding proteins (PBPs)** are the targets of **beta-lactam antibiotics** (penicillins, cephalosporins, carbapenems).
- Alterations in PBPs cause resistance to beta-lactams, not to vancomycin.
- **Vancomycin does not interact with PBPs**; it binds directly to the D-Ala-D-Ala terminus of peptidoglycan precursors in the cell wall.
Novel anti-biofilm therapies US Medical PG Question 2: A 45-year-old man comes to the physician because of a 1-month history of fever and poor appetite. Five weeks ago, he underwent molar extraction for dental caries. His temperature is 38°C (100.4°F). Cardiac examination shows a grade 2/6 holosystolic murmur heard best at the apex. A blood culture shows gram-positive, catalase-negative cocci. Transesophageal echocardiography shows a small vegetation on the mitral valve with mild regurgitation. The causal organism most likely has which of the following characteristics?
- A. Production of dextrans (Correct Answer)
- B. Production of CAMP factor
- C. Conversion of fibrinogen to fibrin
- D. Formation of germ tubes at body temperature
- E. Replication in host macrophages
Novel anti-biofilm therapies Explanation: **Production of dextrans**
- The clinical picture of **fever**, **poor appetite**, a **holosystolic murmur**, and **mitral valve vegetation** following a dental procedure (molar extraction) strongly points to **infective endocarditis** caused by **Viridans streptococci**.
- **Viridans streptococci**, commonly found in the oral cavity, produce **dextrans**, which allow them to adhere to damaged heart valves and fibrin-platelet aggregates, initiating vegetation formation.
*Production of CAMP factor*
- **CAMP factor** is a characteristic of **Group B Streptococcus (Streptococcus agalactiae)**, which primarily causes infections in neonates and immunocompromised adults, not typically infective endocarditis post-dental procedure.
- *Streptococcus agalactiae* is also catalase-negative and gram-positive but is rarely associated with endocarditis arising from oral flora.
*Conversion of fibrinogen to fibrin*
- The ability to convert **fibrinogen to fibrin** is characteristic of **coagulase-positive organisms**, such as *Staphylococcus aureus*, which is a catalase-positive organism.
- The blood culture in this case specifically states **catalase-negative cocci**, ruling out *Staphylococcus aureus* as the causative agent.
*Formation of germ tubes at body temperature*
- **Germ tube formation** at body temperature is a distinguishing characteristic of *Candida albicans*, a **fungus**, not a gram-positive, catalase-negative coccus.
- While *Candida* can cause endocarditis, the microbiological findings described do not align with a fungal infection.
*Replication in host macrophages*
- **Intracellular replication in host macrophages** is characteristic of certain bacteria like *Mycobacterium tuberculosis*, *Listeria monocytogenes*, or *Salmonella typhi*, which typically cause systemic infections
- This characteristic is not associated with the gram-positive, catalase-negative cocci responsible for subacute bacterial endocarditis following dental procedures.
Novel anti-biofilm therapies US Medical PG Question 3: An investigator is studying the growth of an organism in different media. The organism is inoculated on a petri dish that contains heated sheep blood, vancomycin, nystatin, trimethoprim, and colistin. The resulting growth medium is incubated at 37°C. Numerous small, white colonies are seen after incubation for 48 hours. This organism is most likely to cause which of the following conditions?
- A. Pontiac fever
- B. Pseudomembranous colitis
- C. Hemolytic uremic syndrome
- D. Oral thrush
- E. Gonorrhea (Correct Answer)
Novel anti-biofilm therapies Explanation: ***Gonorrhea***
- The growth medium described is **Thayer-Martin agar**, a selective medium containing **heated sheep blood** (supplies NAD+), **vancomycin** (inhibits Gram-positives), **colistin** (inhibits Gram-negatives), **nystatin** (inhibits fungi), and **trimethoprim** (inhibits Proteus). This medium is specifically designed for the isolation of *Neisseria gonorrhoeae* from polymicrobial samples.
- *Neisseria gonorrhoeae* typically grows as **small, translucent-to-white colonies** on selective media like Thayer-Martin agar, and incubation at 37°C in CO2 (not explicitly mentioned but often required) for 24-48 hours yields visible growth, causing **gonorrhea**.
*Pontiac fever*
- Pontiac fever is a mild, self-limiting form of **legionellosis**, caused by *Legionella pneumophila*.
- *Legionella* requires a specialized medium such as **buffered charcoal yeast extract (BCYE) agar** for growth, not Thayer-Martin agar.
*Pseudomembranous colitis*
- This condition is caused by **toxin-producing *Clostridioides difficile***, often after antibiotic use.
- *C. difficile* is an obligate anaerobe and requires **anaerobic conditions** and specific selective media (e.g., CCFA agar) for isolation, not Thayer-Martin agar under aerobic conditions.
*Hemolytic uremic syndrome*
- Hemolytic uremic syndrome (HUS) is often caused by **Shiga toxin-producing *Escherichia coli* (STEC)**, particularly O157:H7.
- STEC can be isolated on media like **sorbitol MacConkey agar (SMAC)**, where O157:H7 appears as non-sorbitol fermenting colonies, distinct from the growth seen on Thayer-Martin.
*Oral thrush*
- Oral thrush is caused by *Candida albicans*, a yeast.
- *Candida* would be inhibited by **nystatin** in the Thayer-Martin medium, which is an antifungal agent.
Novel anti-biofilm therapies US Medical PG Question 4: A 64-year-old female with type 2 diabetes mellitus comes to the physician because of a 1-week history of painful red swelling on her left thigh. Examination shows a 3- x 4-cm, tender, fluctuant mass. Incision and drainage of the abscess are performed. Culture of the abscess fluid grows gram-positive, coagulase-positive cocci that are resistant to oxacillin. Which of the following best describes the mechanism of resistance of the causal organism to oxacillin?
- A. Degradation of the antibiotic
- B. Decreased uptake of the antibiotic
- C. Decreased activation of the antibiotic
- D. Altered target of the antibiotic (Correct Answer)
- E. Acetylation of the antibiotic
Novel anti-biofilm therapies Explanation: ***Altered target of the antibiotic***
- The organism described (gram-positive, coagulase-positive cocci, oxacillin-resistant) is **methicillin-resistant *Staphylococcus aureus* (MRSA)**.
- MRSA achieves oxacillin (and other beta-lactam) resistance by acquiring the ***mecA* gene**, which encodes for a **modified penicillin-binding protein (PBP2a)** with reduced affinity for beta-lactam antibiotics.
*Degradation of the antibiotic*
- This mechanism, primarily through the production of **beta-lactamase enzymes**, can degrade beta-lactam antibiotics.
- While *Staphylococcus aureus* can produce beta-lactamases, oxacillin (a **penicillinase-resistant penicillin**) is specifically engineered to be stable against these enzymes.
*Decreased uptake of the antibiotic*
- Reduced permeability of the bacterial cell wall can lead to decreased uptake, a mechanism more commonly associated with **gram-negative bacteria** due to their outer membrane.
- This is not the primary mechanism of resistance for MRSA to oxacillin.
*Decreased activation of the antibiotic*
- Some antibiotics are prodrugs that require activation by bacterial enzymes, and resistance can arise from mutations affecting this activation.
- Oxacillin is active in its administered form and does not require bacterial activation.
*Acetylation of the antibiotic*
- **Enzymatic modification**, such as acetylation, adenylylation, or phosphorylation, is a common mechanism of resistance, particularly against **aminoglycoside antibiotics**.
- This specific mechanism is not responsible for oxacillin resistance in MRSA.
Novel anti-biofilm therapies US Medical PG Question 5: A researcher is studying a new antituberculosis drug. In the laboratory, the drug has been shown to be effective against mycobacteria located within phagolysosomes of macrophages, but it is also significantly less effective against extracellular tuberculoid bacteria. The characteristics of this drug are most similar to which of the following agents?
- A. Isoniazid
- B. Pyrazinamide (Correct Answer)
- C. Ethambutol
- D. Streptomycin
- E. Rifampin
Novel anti-biofilm therapies Explanation: ***Pyrazinamide***
- Pyrazinamide is unique among antituberculosis drugs for its efficacy in the **acidic environment of phagolysosomes**, where dormant mycobacteria reside.
- It is **less effective against actively replicating extracellular bacteria** at neutral pH, aligning with the drug's described characteristics.
*Isoniazid*
- Isoniazid is primarily effective against **rapidly dividing, extracellular *M. tuberculosis*** by inhibiting mycolic acid synthesis.
- While it can penetrate macrophages, its activity is not specifically enhanced or limited by the acidic phagolysosomal environment as described.
*Ethambutol*
- Ethambutol primarily inhibits **arabinogalactan synthesis**, affecting the cell wall of growing mycobacteria, both intracellular and extracellular.
- Its efficacy is not selectively focused on the acidic intracellular environment.
*Streptomycin*
- Streptomycin is an **aminoglycoside antibiotic** that inhibits protein synthesis and is active against extracellular mycobacteria.
- It has limited penetration into cells and is not particularly effective against intracellular organisms, nor is its activity pH-dependent.
*Rifampin*
- Rifampin is highly effective against both **extracellular and intracellular mycobacteria** by inhibiting DNA-dependent RNA polymerase.
- It exhibits strong sterilizing activity across various environments, which contradicts the described drug's selective efficacy.
Novel anti-biofilm therapies US Medical PG Question 6: A group of medical students is studying bacteria and their pathogenesis. They have identified that a substantial number of bacteria cause human disease by producing exotoxins. Exotoxins are typically proteins, but they have different mechanisms of action and act at different sites. The following is a list of exotoxins together with mechanisms of action. Which of the following pairs is correctly matched?
- A. Tetanospasmin - binds 60S ribosome subunit and inhibits protein synthesis
- B. Cholera toxin - ADP-ribosylates Gs, keeping adenylate cyclase active and ↑ [cAMP] (Correct Answer)
- C. Diphtheria toxin - cleaves synaptobrevin, blocking vesicle formation and the release of acetylcholine
- D. Botulinum toxin - cleaves synaptobrevin, blocking vesicle formation and the release of the inhibitory neurotransmitters GABA and glycine
- E. Anthrax toxin - ADP-ribosylates elongation factor - 2 (EF-2) and inhibits protein synthesis
Novel anti-biofilm therapies Explanation: ***Cholera toxin - ADP-ribosylates Gs, keeping adenylate cyclase active and ↑ [cAMP]***
- **Cholera toxin** works by irrevocably activating **adenylate cyclase** via **ADP-ribosylation** of the **alpha subunit of Gs protein**.
- This leads to a persistent increase in intracellular **cyclic AMP (cAMP)**, resulting in excessive secretion of water and electrolytes into the intestinal lumen, causing characteristic **rice-water diarrhea**.
*Tetanospasmin - binds 60S ribosome subunit and inhibits protein synthesis*
- **Tetanospasmin (tetanus toxin)** acts by cleaving **synaptobrevin**, a SNARE protein, which inhibits the release of **inhibitory neurotransmitters (GABA and glycine)** from Renhaw cells in the spinal cord.
- This blockade of inhibitory signals leads to uncontrolled muscle contractions and **spastic paralysis**.
*Diphtheria toxin - cleaves synaptobrevin, blocking vesicle formation and the release of acetylcholine*
- **Diphtheria toxin** works by **ADP-ribosylating elongation factor-2 (EF-2)**, which is crucial for protein synthesis.
- The inactivation of **EF-2** leads to the arrest of protein synthesis and ultimately **cell death**.
*Botulinum toxin - cleaves synaptobrevin, blocking vesicle formation and the release of the inhibitory neurotransmitters GABA and glycine*
- **Botulinum toxin** cleaves **SNARE proteins** (including synaptobrevin) at the **neuromuscular junction**, specifically blocking the release of **acetylcholine**.
- This inhibition of neurotransmitter release at the presynaptic terminal leads to **flaccid paralysis**.
*Anthrax toxin - ADP-ribosylates elongation factor - 2 (EF-2) and inhibits protein synthesis*
- **Anthrax toxin** consists of three proteins: Protective Antigen (PA), Edema Factor (EF), and Lethal Factor (LF). The **Edema Factor (EF)** is a **calmodulin-dependent adenylate cyclase** that increases intracellular **cAMP**, and the **Lethal Factor (LF)** is a **metalloprotease** that targets MAPK pathways.
- **Anthrax toxin** does not work by ADP-ribosylating EF-2; that mechanism is characteristic of **diphtheria toxin**.
Novel anti-biofilm therapies US Medical PG Question 7: A drug that inhibits mRNA synthesis has the well-documented side effect of red-orange body fluids. For which of the following is this drug used as monotherapy?
- A. Brucellosis
- B. Tuberculosis
- C. Methicillin-resistant staphylococcus aureus infection
- D. Mycobacterium avium intracellulare infection
- E. Neisseria meningitidis prophylaxis (Correct Answer)
Novel anti-biofilm therapies Explanation: ***Neisseria meningitidis prophylaxis***
- The drug described is **rifampin**, which inhibits bacterial **DNA-dependent RNA polymerase**, thereby blocking **mRNA synthesis** and causes characteristic **red-orange discoloration of body fluids** (tears, urine, sweat).
- Rifampin is used as **monotherapy** for **prophylaxis** against **Neisseria meningitidis** infection in close contacts of infected patients.
- This is the **only indication** where rifampin monotherapy is appropriate, as prophylaxis requires short-term use where resistance development is not a concern.
*Tuberculosis*
- Rifampin is a **first-line agent** for tuberculosis treatment and a cornerstone of all TB regimens.
- However, it is **never used as monotherapy** for TB due to rapid development of resistance.
- Standard TB treatment requires **multidrug therapy** with rifampin, isoniazid, pyrazinamide, and ethambutol (RIPE) for initial phase.
*Methicillin-resistant Staphylococcus aureus infection*
- Rifampin is sometimes used in **combination** with other antibiotics (e.g., vancomycin, daptomycin) to treat **MRSA infections**, especially those involving **prosthetic devices** or **biofilms**.
- It is **not used as monotherapy** for active MRSA infections due to extremely high rates of spontaneous resistance.
*Mycobacterium avium intracellulare infection*
- **Mycobacterium avium complex (MAC)** infections require a multidrug regimen, typically including **macrolides (azithromycin or clarithromycin)**, **ethambutol**, and sometimes **rifabutin** (a rifamycin derivative preferred over rifampin).
- **Monotherapy is never appropriate** for MAC infections due to resistance concerns and treatment failure.
*Brucellosis*
- **Brucellosis** treatment requires **combination therapy**, typically **doxycycline plus rifampin** for 6 weeks or longer.
- **Rifampin monotherapy** is inadequate for eradicating Brucella infection and leads to treatment failure and resistance development.
Novel anti-biofilm therapies US Medical PG Question 8: An investigator is studying a strain of bacteria that retains a blue color after crystal violet dye and acetone are applied. The bacteria are inoculated in a petri dish containing hypotonic saline. After the addition of an antibiotic, the bacteria swell and rupture. This antibiotic most likely belongs to which of the following classes?
- A. Macrolide
- B. Cephalosporin (Correct Answer)
- C. Sulfonamide
- D. Fluoroquinolone
- E. Tetracycline
Novel anti-biofilm therapies Explanation: ***Cephalosporin***
- This scenario describes a **Gram-positive bacterium** (retains blue color) which, after antibiotic treatment, swells and lyses in a hypotonic solution. This indicates a defect in the **peptidoglycan cell wall**.
- **Cephalosporins** are **β-lactam antibiotics** that inhibit bacterial cell wall synthesis by interfering with **peptidoglycan cross-linking**, leading to osmotic lysis in hypotonic environments.
*Macrolide*
- Macrolides like **azithromycin** and **erythromycin** inhibit bacterial **protein synthesis** by binding to the 50S ribosomal subunit.
- They do not directly target the cell wall, so they would not cause immediate osmotic lysis in this manner.
*Sulfonamide*
- Sulfonamides inhibit bacterial **folic acid synthesis** by acting as a competitive inhibitor of dihydropteroate synthase, disrupting DNA and RNA production.
- Their mechanism of action does not involve direct cell wall disruption or osmotic lysis.
*Fluoroquinolone*
- Fluoroquinolones interfere with bacterial **DNA replication and transcription** by inhibiting **DNA gyrase** and **topoisomerase IV**.
- This class of antibiotics does not primarily target the cell wall, and therefore would not lead to prompt osmotic swelling and rupture.
*Tetracycline*
- Tetracyclines inhibit bacterial **protein synthesis** by binding to the 30S ribosomal subunit, preventing the attachment of aminoacyl-tRNA.
- They do not affect the cell wall, so they would not cause the observed osmotic lysis.
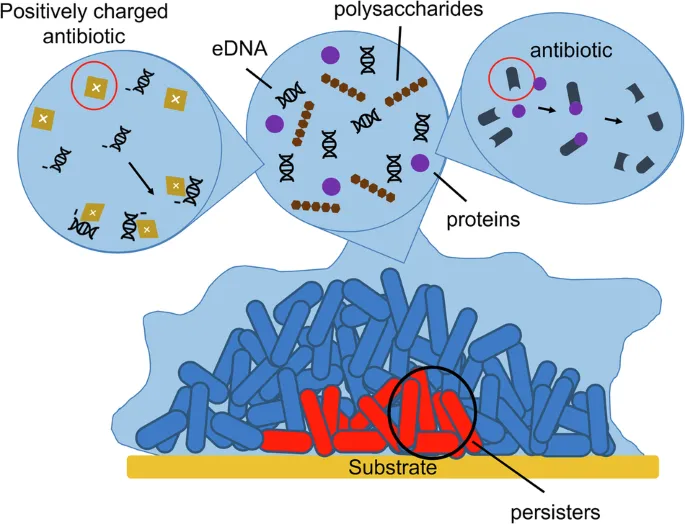
Novel anti-biofilm therapies US Medical PG Question 9: A hospital implements silver-coated central venous catheters to reduce catheter-related bloodstream infections. Initial results show 60% reduction in infections at 1 week, but this benefit decreases to 20% reduction by 4 weeks. Electron microscopy of explanted catheters shows biofilm formation with embedded bacteria despite the silver coating. What mechanism best explains the loss of antimicrobial efficacy over time?
- A. Depletion of silver ions from the catheter surface through diffusion
- B. Matrix proteins binding silver ions and reducing bioavailability
- C. Development of silver-tolerant persister cell populations
- D. Bacterial mutation conferring genetic resistance to silver ions
- E. Host protein deposition creating a conditioning film blocking silver release (Correct Answer)
Novel anti-biofilm therapies Explanation: ***Host protein deposition creating a conditioning film blocking silver release***
- Rapid adsorption of host proteins like **fibrinogen, fibronectin, and albumin** creates a **conditioning film** that physically masks the antimicrobial surface.
- This protein layer acts as a barrier to **ion release** and provides a scaffold for **bacterial adhesion**, facilitating the transition to a long-term **biofilm** state.
*Depletion of silver ions from the catheter surface through diffusion*
- Modern antimicrobial catheters are designed for **sustained release**, and the presence of silver on explanted microscopy suggests the reservoir is not yet empty.
- If diffusion were the only factor, efficacy would decline linearly rather than being linked to the physical observation of **biofilm formation** over the coating.
*Matrix proteins binding silver ions and reducing bioavailability*
- While some binding may occur, this is not the primary mechanism of clinical failure; the principal issue is the physical **obstruction of the surface**.
- This theory does not account for how bacteria are able to initially colonize and survive in **close physical contact** with the coated surface.
*Development of silver-tolerant persister cell populations*
- **Persister cells** are phenotypically dormant and survive antibiotics, but they do not typically cause the gradual, large-scale reduction in antimicrobial device efficacy seen here.
- The microscopy findings emphasize **structural biofilm layers** rather than a specific metabolic state of individual bacteria.
*Bacterial mutation conferring genetic resistance to silver ions*
- True **genetic resistance** to silver (via sil operons) is clinically rare and usually occurs through **efflux pumps**, not biofilm-mediated shielding.
- The scenario describes a loss of efficacy common across multiple hospital settings, whereas **mutational resistance** would be more sporadic or localized.
Novel anti-biofilm therapies US Medical PG Question 10: A 28-year-old woman with cystic fibrosis undergoes lung transplantation. Pre-transplant sputum cultures show mucoid Pseudomonas aeruginosa. Post-transplant, she receives immunosuppression and antibiotic prophylaxis. Six months later, she develops pneumonia, and cultures grow non-mucoid P. aeruginosa with identical genetic fingerprint to pre-transplant isolates. What evolutionary adaptation most likely explains this phenotypic reversion?
- A. Horizontal gene transfer from colonizing respiratory flora
- B. Decreased selective pressure for biofilm formation in absence of mucus obstruction (Correct Answer)
- C. Selection pressure favoring planktonic phenotype in immunosuppressed state
- D. Loss of mucA mutations due to genetic reversion in new host environment
- E. Antibiotic prophylaxis eliminating mucoid variants selectively
Novel anti-biofilm therapies Explanation: ***Decreased selective pressure for biofilm formation in absence of mucus obstruction***
- In the **Cystic Fibrosis (CF)** lung, the presence of thick **mucus plugs** and chronic inflammation exerts selective pressure that favors the **mucoid phenotype** (alginate production) for survival.
- Following **lung transplantation**, the new lungs lack the original CF environment, causing the bacteria to revert to a **non-mucoid** state which is more energetically efficient for **planktonic growth** and rapid replication.
*Horizontal gene transfer from colonizing respiratory flora*
- Genetic identity via **fingerprinting** confirms the post-transplant isolate is a direct descendant of the original strain, not a result of **recombination** with other flora.
- The change in phenotype is an **adaptive response** to environmental shifts rather than the acquisition of new genetic material from the host microbiome.
*Selection pressure favoring planktonic phenotype in immunosuppressed state*
- While **immunosuppression** affects the host's ability to clear infections, it is the **structural change** (removal of mucus) that primarily influences the bacterial transition from biofilm to planktonic form.
- Biofilms are generally more resistant to the host immune system; thus, a lack of immunity would not logically drive the bacteria *away* from a protective **biofilm phenotype**.
*Loss of mucA mutations due to genetic reversion in new host environment*
- The **mucoid phenotype** in CF is often caused by **mucA mutations**, but spontaneous **back-mutations** (genetic reversion) are extremely rare in large bacterial populations.
- Phenotypic changes are more likely due to **compensatory mutations** or changes in **gene expression** rather than a literal restoration of the wild-type DNA sequence.
*Antibiotic prophylaxis eliminating mucoid variants selectively*
- **Mucoid variants** and their associated **biofilms** typically show *increased* resistance to antibiotics compared to non-mucoid forms.
- Therefore, **antibiotic prophylaxis** would be expected to select *for* mucoid variants rather than eliminating them to favor non-mucoid ones.
More Novel anti-biofilm therapies US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.