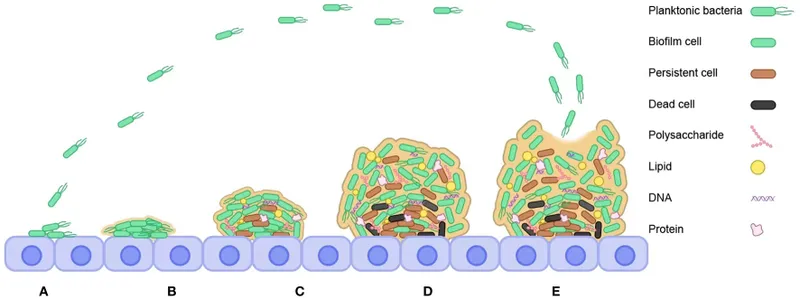
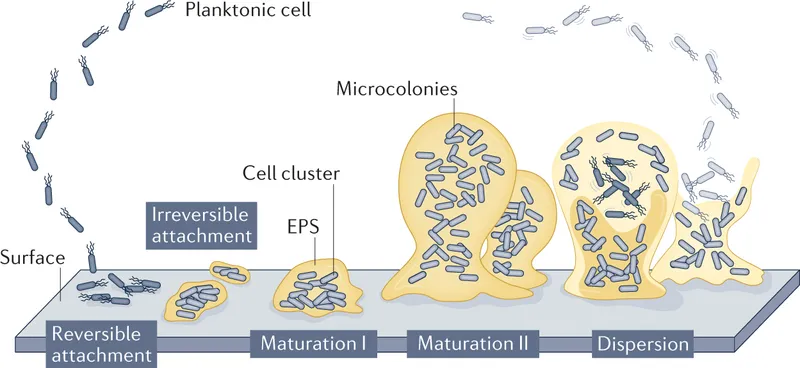
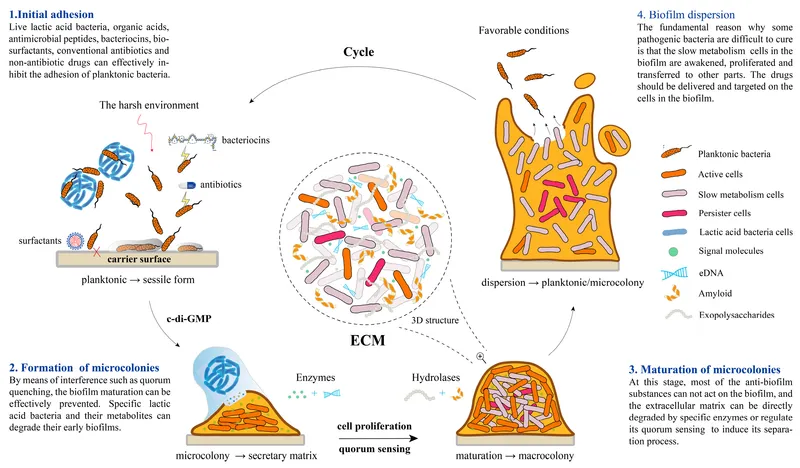
Biofilm formation stages US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Biofilm formation stages. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Biofilm formation stages US Medical PG Question 1: A pharmaceutical company has modified one of its existing antibiotics to have an improved toxicity profile. The new antibiotic blocks protein synthesis by first entering the cell and then binding to active ribosomes. The antibiotic mimics the structure of aminoacyl-tRNA. The drug is covalently bonded to the existing growing peptide chain via peptidyl transferase, thereby impairing the rest of protein synthesis and leading to early polypeptide truncation. Where is the most likely site that this process occurs?
- A. E site
- B. 30S small subunit
- C. A site (Correct Answer)
- D. 40S small subunit
- E. P site
Biofilm formation stages Explanation: ***A site***
- The **A (aminoacyl) site** is where incoming aminoacyl-tRNAs bind during translation, bringing new amino acids to the ribosome. Since the antibiotic mimics **aminoacyl-tRNA** and is covalently bonded to the peptide chain by **peptidyl transferase**, its action must occur at the A site.
- Binding at the A site and subsequent peptide bond formation with the antibiotic would lead to premature polypeptide truncation, as no further amino acids can be added.
*E site*
- The **E (exit) site** is where deacylated tRNAs are released from the ribosome after having delivered their amino acid to the growing peptide chain in the P site.
- The antibiotic's mechanism of action, involving binding and covalent incorporation into the peptide, does not align with the function of the E site.
*30S small subunit*
- The **30S small ribosomal subunit** in prokaryotes is primarily involved in mRNA binding and decoding, ensuring the correct aminoacyl-tRNA binds to the mRNA codon.
- While the antibiotic binds to active ribosomes, its key action described as mimicking aminoacyl-tRNA and being incorporated by peptidyl transferase points to a specific binding site within the ribosome rather than the entire subunit's general function.
*40S small subunit*
- The **40S small ribosomal subunit** is found in **eukaryotic ribosomes**, not prokaryotic ones, and is involved in mRNA binding during initiation.
- The question implies an antibiotic targeting bacterial protein synthesis (given its discussion of modifying an existing antibiotic), making eukaryotic ribosomal subunits an unlikely target.
*P site*
- The **P (peptidyl) site** holds the tRNA carrying the growing polypeptide chain. Peptidyl transferase activity forms a peptide bond between the amino acid in the A site and the peptide in the P site.
- While peptidyl transferase is involved, the antibiotic *mimics* aminoacyl-tRNA, which is delivered to the A site for peptide bond formation, rather than the P site which already holds the growing chain.
Biofilm formation stages US Medical PG Question 2: A stool sample was taken from a 19-year-old male who presented with profuse watery diarrhea. He recently returned from a trip to Central America. A microbiologist identified the causative agent as a gram-negative, oxidase-positive, comma-shaped bacteria that is able to grow well in a pH > 8. Which of the following is a mechanism of action of the toxin produced by this bacteria?
- A. Overactivation of adenylate cyclase by inhibition of Gi subunit by ADP-ribosylation
- B. Inactivation of the 60S ribosomal subunit by cleaving an adenine from the 28S rRNA
- C. Overactivation of guanylate cyclase
- D. Overactivation of adenylate cyclase by activation of Gs subunit by ADP-ribosylation (Correct Answer)
- E. Degradation of cell membranes by hydrolysis of the phospholipids
Biofilm formation stages Explanation: ***Overactivation of adenylate cyclase by activation of Gs subunit by ADP-ribosylation***
- The description of the bacterium as **gram-negative, oxidase-positive, comma-shaped, growing well in pH > 8**, and causing **profuse watery diarrhea** after travel to Central America points to *Vibrio cholerae*.
- **Cholera toxin** (CTX) produced by *V. cholerae* is an A-B toxin that **ADP-ribosylates the Gs α-subunit**, permanently activating **adenylate cyclase**. This leads to increased cAMP levels, causing secretion of water and electrolytes into the intestinal lumen.
*Overactivation of adenylate cyclase by inhibition of Gi subunit by ADP-ribosylation*
- This mechanism describes the action of **pertussis toxin** from *Bordetella pertussis*, which ADP-ribosylates and **inhibits the Gi subunit**, preventing adenylate cyclase inhibition.
- While both ultimately increase cAMP, the specific target and mechanism (inhibition of Gi vs. activation of Gs) differ from cholera toxin.
*Inactivation of the 60S ribosomal subunit by cleaving an adenine from the 28S rRNA*
- This mechanism is characteristic of **Shiga toxin** produced by *Shigella dysenteriae* and Shiga-like toxins (verotoxins) produced by **enterohemorrhagic *E. coli*** (EHEC).
- These toxins inhibit protein synthesis, leading to cell death and often bloody diarrhea and hemolytic uremic syndrome, which is not described here.
*Overactivation of guanylate cyclase*
- **Heat-stable enterotoxins (ST)** produced by **enterotoxigenic *E. coli*** (ETEC) activate **guanylate cyclase**, leading to increased cGMP and subsequent fluid secretion.
- While ETEC can cause watery diarrhea, the bacterial characteristics provided (oxidase-positive, comma-shaped) do not fit *E. coli*.
*Degradation of cell membranes by hydrolysis of the phospholipids*
- This mechanism is associated with toxins like **phospholipases** or **lecithinases** (e.g., alpha-toxin of *Clostridium perfringens*).
- These toxins cause direct cell lysis and tissue damage, which is not the primary mechanism of action for the watery diarrhea seen in cholera.
Biofilm formation stages US Medical PG Question 3: An investigator is studying collagen synthesis in human fibroblast cells. Using a fluorescent tag, α-collagen chains are identified and then monitored as they travel through the rough endoplasmic reticulum, the Golgi apparatus, and eventually into the extracellular space. Which of the following steps in collagen synthesis occurs extracellularly?
- A. Triple-helix formation
- B. Translation of pro-α chains
- C. Cleavage of procollagen C- and N-terminals (Correct Answer)
- D. Glycosylation of pro-α chains
- E. Hydroxylation of proline and lysine
Biofilm formation stages Explanation: ***Cleavage of procollagen C- and N-terminals***
- After procollagen is secreted into the extracellular space, specific **proteolytic enzymes** (procollagen peptidases) cleave the bulky N- and C-terminal propeptides.
- This cleavage transforms procollagen into insoluble **tropocollagen** molecules, which then spontaneously self-assemble into collagen fibrils.
*Triple-helix formation*
- This crucial step occurs within the **rough endoplasmic reticulum (RER)**, after hydroxylation and glycosylation of pro-α chains.
- The three pro-α chains intertwine to form a stable, rod-like **procollagen molecule**.
*Translation of pro-α chains*
- The synthesis of pro-α chains (polypeptide chains) takes place on **ribosomes** attached to the **rough endoplasmic reticulum (RER)**.
- This process is initiated in the cytosol and completed *into* the lumen of the RER.
*Glycosylation of pro-α chains*
- The addition of specific **oligosaccharide units** to hydroxylysine residues occurs in the **rough endoplasmic reticulum (RER)** and **Golgi apparatus**.
- This modification is important for the stability of the collagen triple helix and for interactions with other extracellular matrix components.
*Hydroxylation of proline and lysine*
- This post-translational modification, essential for the stability of the collagen triple helix, occurs in the **rough endoplasmic reticulum (RER)**.
- Enzymes like **prolyl hydroxylase** and **lysyl hydroxylase** require **vitamin C** as a cofactor for this reaction.
Biofilm formation stages US Medical PG Question 4: A 25-day-old male infant presents to the emergency department because his mother states that he has been acting irritable for the past 2 days and has now developed a fever. On exam, the infant appears uncomfortable and has a temperature of 39.1 C. IV access is immediately obtained and a complete blood count and blood cultures are drawn. Lumbar puncture demonstrates an elevated opening pressure, elevated polymorphonuclear neutrophil, elevated protein, and decreased glucose. Ampicillin and cefotaxime are immediately initiated and CSF culture eventually demonstrates infection with a Gram-negative rod. Which of the following properties of this organism was necessary for the infection of this infant?
- A. K capsule (Correct Answer)
- B. M protein
- C. Fimbriae
- D. IgA protease
- E. LPS endotoxin
Biofilm formation stages Explanation: ***K capsule***
- The K capsule (specifically **K1 antigen**) is a specific virulence factor found in **E. coli** strains, which are a common cause of neonatal meningitis.
- This capsule is **antiphagocytic** and helps the bacteria evade the immune system, allowing it to cross the **blood-brain barrier** and cause meningitis in neonates.
*M protein*
- **M protein** is a major virulence factor associated with **Streptococcus pyogenes** (Group A Strep), playing a role in attachment and immune evasion.
- While *S. pyogenes* can cause infections, it is not typically the Gram-negative rod responsible for **neonatal meningitis** and its M protein is not relevant here.
*Fimbriae*
- **Fimbriae** (pili) are important for bacterial **adhesion** to host cells, often in the initial stages of infection, particularly in urinary tract infections (UTIs).
- While gram-negative rods possess fimbriae, the specific virulence factor critical for **meningitis** caused by *E. coli* in neonates is the K1 capsule, not fimbriae which are more for initial colonization.
*IgA protease*
- **IgA protease** is an enzyme produced by some bacteria (e.g., *N. meningitidis, H. influenzae, S. pneumoniae*) that cleaves **IgA antibodies**, helping them colonize mucous membranes.
- This enzyme is not a primary virulence factor for the **Gram-negative rod** causing neonatal meningitis, where capsule formation is more critical for invasion.
*LPS endotoxin*
- **Lipopolysaccharide (LPS) endotoxin** is a component of the outer membrane of Gram-negative bacteria and is responsible for many symptoms of sepsis and **systemic inflammation**.
- While LPS contributes to the overall disease severity, it primarily mediates **inflammation and fever**, and is not the specific factor necessary for **invasion and survival within the central nervous system**, which is facilitated by the K capsule.
Biofilm formation stages US Medical PG Question 5: You are treating a neonate with meningitis using ampicillin and a second antibiotic, X, that is known to cause ototoxicity. What is the mechanism of antibiotic X?
- A. It binds the 50S ribosomal subunit and inhibits formation of the initiation complex
- B. It binds the 30S ribosomal subunit and inhibits formation of the initiation complex (Correct Answer)
- C. It binds the 30S ribosomal subunit and reversibly inhibits translocation
- D. It binds the 50S ribosomal subunit and inhibits peptidyltransferase
- E. It binds the 50S ribosomal subunit and reversibly inhibits translocation
Biofilm formation stages Explanation: ***It binds the 30s ribosomal subunit and inhibits formation of the initiation complex***
- The second antibiotic, X, is likely an **aminoglycoside**, such as **gentamicin** or **amikacin**, which are commonly used in combination with ampicillin for neonatal meningitis and are known to cause ototoxicity.
- Aminoglycosides exert their bactericidal effect by **irreversibly binding to the 30S ribosomal subunit**, thereby **inhibiting the formation of the initiation complex** and leading to misreading of mRNA.
*It binds the 50S ribosomal subunit and inhibits formation of the initiation complex*
- This mechanism is characteristic of **linezolid**, which targets the 50S ribosomal subunit to prevent the formation of the initiation complex.
- While linezolid can cause side effects, **ototoxicity** is less commonly associated with it compared to aminoglycosides, and it is not a primary drug for neonatal meningitis alongside ampicillin.
*It binds the 50S ribosomal subunit and inhibits peptidyltransferase*
- This is the mechanism of action for **chloramphenicol**, which inhibits **peptidyltransferase** activity on the 50S ribosomal subunit, preventing peptide bond formation.
- Although chloramphenicol can cause **ototoxicity** and **aplastic anemia**, its use in neonates is limited due to the risk of **Gray Baby Syndrome**.
*It binds the 30s ribosomal subunit and reversibly inhibits translocation*
- This describes the mechanism of action of **tetracyclines**, which reversibly bind to the 30S ribosomal subunit and prevent the attachment of aminoacyl-tRNA, thereby inhibiting protein synthesis.
- Tetracyclines are **contraindicated in neonates** due to their potential to cause **tooth discoloration** and **bone growth inhibition**, and ototoxicity is not their primary adverse effect.
*It binds the 50s ribosomal subunit and reversibly inhibits translocation*
- This mechanism of reversibly inhibiting translocation by binding to the 50S ribosomal subunit is characteristic of **macrolides** (e.g., erythromycin, azithromycin) and **clindamycin**.
- While some macrolides can cause **transient ototoxicity**, they are not typically the second antibiotic of choice for neonatal meningitis in combination with ampicillin, and clindamycin's side effect profile is different.
Biofilm formation stages US Medical PG Question 6: A 63-year-old female recovering from a total shoulder arthroplasty completed 6 days ago presents complaining of joint pain in her repaired shoulder. Temperature is 39 degrees Celsius. Physical examination demonstrates erythema and significant tenderness around the incision site. Wound cultures reveal Gram-positive cocci that are resistant to nafcillin. Which of the following organisms is the most likely cause of this patient's condition?
- A. Streptococcus pyogenes
- B. Escherichia coli
- C. Streptococcus viridans
- D. Staphylococcus epidermidis
- E. Staphylococcus aureus (Correct Answer)
Biofilm formation stages Explanation: ***Staphylococcus aureus***
- The combination of **post-surgical infection**, **erythema**, and fever with **Gram-positive cocci** that are **nafcillin-resistant** is highly indicative of **Methicillin-Resistant Staphylococcus aureus (MRSA)**.
- *S. aureus* is a common cause of **surgical site infections**, and its resistance to nafcillin implies it is MRSA, a significant clinical concern for its difficulty in treatment.
*Streptococcus pyogenes*
- While *S. pyogenes* is a Gram-positive coccus that can cause skin and soft tissue infections, it is typically **susceptible to penicillin** and related antibiotics like nafcillin, unlike the organism described.
- It is more commonly associated with **streptococcal pharyngitis** or **cellulitis**, and while it can cause severe disease, its resistance profile doesn't match the clinical picture.
*Escherichia coli*
- *E. coli* is a **Gram-negative rod**, not a Gram-positive coccus.
- It is a common cause of **urinary tract infections** and **gastrointestinal infections**, making it an unlikely pathogen for a post-surgical joint infection unless contaminated from a visceral source.
*Streptococcus viridans*
- **Viridans streptococci** are Gram-positive cocci but are typically associated with **endocarditis** or dental infections, especially after poor dental hygiene or procedures.
- They are usually **susceptible to penicillin** and do not typically exhibit nafcillin resistance as the primary feature in a post-arthroplasty infection.
*Staphylococcus epidermidis*
- *S. epidermidis* is a **coagulase-negative Staphylococcus** known for forming **biofilms on prosthetic devices**, leading to chronic, low-grade infections.
- While it can be nafcillin-resistant, the **acute presentation** with fever and significant inflammation suggests a more virulent pathogen like *S. aureus*, as *S. epidermidis* infections are typically indolent.
Biofilm formation stages US Medical PG Question 7: Blood cultures are sent to the laboratory. Intravenous antibiotic therapy is started. Transesophageal echocardiography shows a large, oscillating vegetation attached to the tricuspid valve. There are multiple small vegetations attached to tips of the tricuspid valve leaflets. There is moderate tricuspid regurgitation. The left side of the heart and the ejection fraction are normal. Which of the following is the most likely causal organism of this patient's condition?
- A. Streptococcus sanguinis
- B. Staphylococcus aureus (Correct Answer)
- C. Enterococcus faecalis
- D. Neisseria gonorrhoeae
- E. Staphylococcus epidermidis
Biofilm formation stages Explanation: ***Staphylococcus aureus***
- **_Staphylococcus aureus_** is the most common cause of **acute infective endocarditis**, particularly in intravenous drug users, which often affects the **tricuspid valve**.
- The presence of large, oscillating vegetations and **multiple small vegetations** on the tricuspid valve strongly suggests an aggressive infection, typical of _S. aureus_.
*Streptococcus sanguinis*
- _Streptococcus sanguinis_ is a common cause of **subacute infective endocarditis** in patients with pre-existing valvular disease but rarely causes acute, aggressive right-sided endocarditis.
- It's typically associated with **dental procedures** and usually affects the left side of the heart.
*Enterococcus faecalis*
- _Enterococcus faecalis_ can cause endocarditis, often associated with **genitourinary or gastrointestinal procedures**, and typically affects older men.
- While it can cause virulent endocarditis, it is less commonly associated with acute right-sided disease in this demographic compared to _S. aureus_.
*Neisseria gonorrhoeae*
- **_Neisseria gonorrhoeae_** is a rare cause of endocarditis, usually seen in younger, sexually active individuals, and often involves the aortic valve.
- While it can be acute, it is an extremely uncommon cause of **tricuspid valve endocarditis**.
*Staphylococcus epidermidis*
- **_Staphylococcus epidermidis_** is primarily associated with **prosthetic valve endocarditis** or foreign bodies, often presenting as a subacute infection.
- It rarely causes natural valve endocarditis, especially acute right-sided disease in this context.
Biofilm formation stages US Medical PG Question 8: A 35-year-old female presents to the emergency room complaining of diarrhea and dehydration. She has been experiencing severe watery diarrhea for the past 3 days. She reports that she has been unable to leave the bathroom for more than a few minutes at a time. The diarrhea is profuse and watery without visible blood or mucus. She recently returned from a volunteer trip to Yemen where she worked at an orphanage. Her past medical history is notable for psoriasis for which she takes sulfasalazine. The patient drinks socially and does not smoke. Her temperature is 99°F (37.2°C), blood pressure is 100/55 mmHg, pulse is 130/min, and respirations are 20/min. Mucous membranes are dry. Her eyes appear sunken. Capillary refill is 4 seconds. The patient is started on intravenous fluid resuscitation. Which of the following processes is capable of transmitting the genetic material for the toxin responsible for this patient's condition?
- A. Transposition
- B. Conjugation
- C. Endospore formation
- D. Transduction (Correct Answer)
- E. Transformation
Biofilm formation stages Explanation: ***Transduction***
- The patient's symptoms are highly suggestive of **cholera**, caused by *Vibrio cholerae*, which produces **cholera toxin**.
- The genes for cholera toxin are carried on a **bacteriophage (CTXφ)**, and their transfer between bacteria occurs via **transduction**.
*Transposition*
- **Transposition** involves the movement of **transposons ("jumping genes")** within a genome or between DNA molecules.
- While transposons can carry antimicrobial resistance genes or virulence factors, this mechanism is not typically associated with the transfer of the primary cholera toxin genes.
*Conjugation*
- **Conjugation** is the transfer of genetic material between bacteria through direct cell-to-cell contact, often involving a **pilus** and the transfer of **plasmids**.
- While *Vibrio cholerae* can engage in conjugation, the cholera toxin genes are predominantly acquired via specialized transduction with the CTXφ phage, not typically plasmid-mediated conjugation.
*Endospore formation*
- **Endospore formation** is a survival mechanism used by certain bacteria (e.g., *Clostridium*, *Bacillus*) to withstand harsh environmental conditions.
- It is not a mechanism for **horizontal gene transfer** or the transmission of toxin-encoding genetic material between bacteria.
*Transformation*
- **Transformation** is the uptake of **naked DNA** from the environment by a bacterial cell.
- While *Vibrio cholerae* can be naturally competent for transformation, the cholera toxin genes are primarily acquired through **phage-mediated transduction**, not free DNA uptake.
Biofilm formation stages US Medical PG Question 9: A hospital implements silver-coated central venous catheters to reduce catheter-related bloodstream infections. Initial results show 60% reduction in infections at 1 week, but this benefit decreases to 20% reduction by 4 weeks. Electron microscopy of explanted catheters shows biofilm formation with embedded bacteria despite the silver coating. What mechanism best explains the loss of antimicrobial efficacy over time?
- A. Depletion of silver ions from the catheter surface through diffusion
- B. Matrix proteins binding silver ions and reducing bioavailability
- C. Development of silver-tolerant persister cell populations
- D. Bacterial mutation conferring genetic resistance to silver ions
- E. Host protein deposition creating a conditioning film blocking silver release (Correct Answer)
Biofilm formation stages Explanation: ***Host protein deposition creating a conditioning film blocking silver release***
- Rapid adsorption of host proteins like **fibrinogen, fibronectin, and albumin** creates a **conditioning film** that physically masks the antimicrobial surface.
- This protein layer acts as a barrier to **ion release** and provides a scaffold for **bacterial adhesion**, facilitating the transition to a long-term **biofilm** state.
*Depletion of silver ions from the catheter surface through diffusion*
- Modern antimicrobial catheters are designed for **sustained release**, and the presence of silver on explanted microscopy suggests the reservoir is not yet empty.
- If diffusion were the only factor, efficacy would decline linearly rather than being linked to the physical observation of **biofilm formation** over the coating.
*Matrix proteins binding silver ions and reducing bioavailability*
- While some binding may occur, this is not the primary mechanism of clinical failure; the principal issue is the physical **obstruction of the surface**.
- This theory does not account for how bacteria are able to initially colonize and survive in **close physical contact** with the coated surface.
*Development of silver-tolerant persister cell populations*
- **Persister cells** are phenotypically dormant and survive antibiotics, but they do not typically cause the gradual, large-scale reduction in antimicrobial device efficacy seen here.
- The microscopy findings emphasize **structural biofilm layers** rather than a specific metabolic state of individual bacteria.
*Bacterial mutation conferring genetic resistance to silver ions*
- True **genetic resistance** to silver (via sil operons) is clinically rare and usually occurs through **efflux pumps**, not biofilm-mediated shielding.
- The scenario describes a loss of efficacy common across multiple hospital settings, whereas **mutational resistance** would be more sporadic or localized.
Biofilm formation stages US Medical PG Question 10: A 28-year-old woman with cystic fibrosis undergoes lung transplantation. Pre-transplant sputum cultures show mucoid Pseudomonas aeruginosa. Post-transplant, she receives immunosuppression and antibiotic prophylaxis. Six months later, she develops pneumonia, and cultures grow non-mucoid P. aeruginosa with identical genetic fingerprint to pre-transplant isolates. What evolutionary adaptation most likely explains this phenotypic reversion?
- A. Horizontal gene transfer from colonizing respiratory flora
- B. Decreased selective pressure for biofilm formation in absence of mucus obstruction (Correct Answer)
- C. Selection pressure favoring planktonic phenotype in immunosuppressed state
- D. Loss of mucA mutations due to genetic reversion in new host environment
- E. Antibiotic prophylaxis eliminating mucoid variants selectively
Biofilm formation stages Explanation: ***Decreased selective pressure for biofilm formation in absence of mucus obstruction***
- In the **Cystic Fibrosis (CF)** lung, the presence of thick **mucus plugs** and chronic inflammation exerts selective pressure that favors the **mucoid phenotype** (alginate production) for survival.
- Following **lung transplantation**, the new lungs lack the original CF environment, causing the bacteria to revert to a **non-mucoid** state which is more energetically efficient for **planktonic growth** and rapid replication.
*Horizontal gene transfer from colonizing respiratory flora*
- Genetic identity via **fingerprinting** confirms the post-transplant isolate is a direct descendant of the original strain, not a result of **recombination** with other flora.
- The change in phenotype is an **adaptive response** to environmental shifts rather than the acquisition of new genetic material from the host microbiome.
*Selection pressure favoring planktonic phenotype in immunosuppressed state*
- While **immunosuppression** affects the host's ability to clear infections, it is the **structural change** (removal of mucus) that primarily influences the bacterial transition from biofilm to planktonic form.
- Biofilms are generally more resistant to the host immune system; thus, a lack of immunity would not logically drive the bacteria *away* from a protective **biofilm phenotype**.
*Loss of mucA mutations due to genetic reversion in new host environment*
- The **mucoid phenotype** in CF is often caused by **mucA mutations**, but spontaneous **back-mutations** (genetic reversion) are extremely rare in large bacterial populations.
- Phenotypic changes are more likely due to **compensatory mutations** or changes in **gene expression** rather than a literal restoration of the wild-type DNA sequence.
*Antibiotic prophylaxis eliminating mucoid variants selectively*
- **Mucoid variants** and their associated **biofilms** typically show *increased* resistance to antibiotics compared to non-mucoid forms.
- Therefore, **antibiotic prophylaxis** would be expected to select *for* mucoid variants rather than eliminating them to favor non-mucoid ones.
More Biofilm formation stages US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.