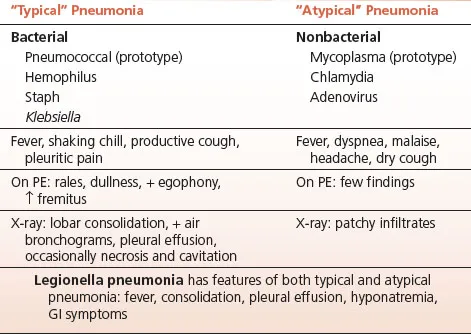
Atypical

On this page
🦠 Atypical Pathogens: The Stealth Squadron
Atypical pneumonia earns its name not from rarity but from its deceptive clinical presentation-pathogens like Mycoplasma, Chlamydia, and Legionella bypass traditional defenses, hijack cellular machinery without triggering classic lobar consolidation, and challenge even experienced clinicians to distinguish them from viral syndromes and typical bacterial pneumonia. You'll master the unique biology driving these infections, build a systematic framework for clinical recognition and differential diagnosis, and command evidence-based treatment algorithms that account for resistance patterns and special populations. This lesson transforms atypical pneumonia from a diagnostic puzzle into a condition you'll confidently identify, differentiate, and manage across diverse clinical scenarios.
📌 Remember: ATYPICAL - Alternate culture requirements, Transmission vectors, Yield poor Gram stains, Peculiar cell walls, Intracellular lifestyle, Chronic presentations, Antibiotic resistance patterns, Laboratory challenges
The defining characteristics of atypical pathogens create a constellation of clinical challenges. These organisms typically lack conventional peptidoglycan cell walls, making them invisible to Gram staining and resistant to beta-lactam antibiotics. Their fastidious growth requirements mean standard bacterial cultures fail in 60-80% of cases, necessitating specialized media, prolonged incubation periods, or molecular diagnostic methods.
| Pathogen | Cell Wall | Culture Time | Primary Transmission | Antibiotic Class | Diagnostic Method |
|---|---|---|---|---|---|
| Mycoplasma | None | 7-21 days | Respiratory droplets | Macrolides | PCR, Cold agglutinins |
| Chlamydia | Modified | Cannot culture | Sexual, respiratory | Tetracyclines | NAAT, Antigen |
| Rickettsia | Typical | Rarely attempted | Arthropod vectors | Doxycycline | Serology, PCR |
| Coxiella | Typical | BSL-3 required | Aerosol inhalation | Fluoroquinolones | Phase I/II serology |
| Legionella | Typical | 3-5 days BCYE | Water aerosols | Fluoroquinolones | Urinary antigen |
- Intracellular Lifestyle Patterns
- Obligate intracellular: Chlamydia, Rickettsia, Coxiella (100% host-dependent)
- Facultative intracellular: Legionella (survives in macrophages)
- Extracellular with adhesion: Mycoplasma (binds respiratory epithelium)
- Energy parasitism through ATP depletion
- Immune evasion via antigenic variation
- Chronic persistence through biofilm formation
💡 Master This: Atypical pathogens exploit three key vulnerabilities: energy metabolism (Mycoplasma), intracellular trafficking (Chlamydia/Rickettsia), and immune recognition (all species). Understanding these mechanisms predicts both clinical presentation patterns and therapeutic targets.
The epidemiological patterns of atypical infections reflect their unique transmission mechanisms and environmental reservoirs. Vector-borne transmission (Rickettsia, Ehrlichia) shows seasonal clustering during arthropod activity periods, while aerosol transmission (Legionella, Coxiella) correlates with environmental exposure to contaminated water systems or animal products.
Understanding atypical pathogen biology establishes the foundation for recognizing their distinctive clinical presentations and diagnostic challenges, setting the stage for mastering their complex pathophysiological mechanisms.
🦠 Atypical Pathogens: The Stealth Squadron
⚙️ Pathophysiological Mastery: The Cellular Hijacking Network
Atypical pathogens have evolved sophisticated strategies to survive and replicate within hostile host environments. Their pathophysiological mechanisms center on three core strategies: cellular invasion, immune evasion, and metabolic exploitation. Each strategy represents millions of years of evolutionary refinement, creating organisms perfectly adapted to their specific ecological niches.
📌 Remember: INVASION - Internalization mechanisms, Nucleotide scavenging, Vacuole modification, Actin manipulation, Signal transduction, Immune suppression, Organelle hijacking, Nutrient acquisition
The cellular invasion process begins with highly specific adhesion mechanisms. Chlamydia species utilize heparan sulfate receptors and PDGF receptor binding, achieving >95% attachment efficiency to susceptible epithelial cells. Rickettsia species employ OmpA and OmpB outer membrane proteins to bind α2β1 integrins, enabling invasion of endothelial cells within 15-30 minutes of contact.
- Membrane Invasion Strategies
- Trigger mechanism: Chlamydia induces receptor-mediated endocytosis
- Elementary body → reticulate body transformation
- ATP-dependent process requiring 2-6 hours
- Inclusion body formation within 12-24 hours
- Zipper mechanism: Rickettsia promotes actin rearrangement
- Direct cytoplasmic entry within 30 minutes
- Phospholipase A and hemolysin activity
- Immediate access to cytosolic nutrients
- Novel entry: Coxiella survives phagolysosomal fusion
- pH 4.5-5.0 tolerance (unique among bacteria)
- Acid shock protein expression
- Parasitophorous vacuole establishment
- Trigger mechanism: Chlamydia induces receptor-mediated endocytosis
⭐ Clinical Pearl: Rickettsia's ability to spread cell-to-cell without extracellular exposure explains why Rocky Mountain Spotted Fever can progress rapidly despite appropriate antibiotic therapy-established intracellular populations require 48-72 hours for complete clearance.
| Pathogen | Invasion Time | Replication Site | Generation Time | Host Cell Damage | Spread Mechanism |
|---|---|---|---|---|---|
| Chlamydia | 2-6 hours | Inclusion body | 24-48 hours | Lysis at 48-72h | Cell death/release |
| Rickettsia | 15-30 minutes | Cytoplasm | 8-12 hours | Membrane damage | Actin propulsion |
| Coxiella | 1-2 hours | Phagolysosome | 12-24 hours | Minimal | Chronic persistence |
| Mycoplasma | Surface only | Extracellular | 6-24 hours | Membrane fusion | Direct contact |
| Legionella | 30 minutes | Phagosome | 2-4 hours | Macrophage lysis | Aerosol release |
💡 Master This: The obligate intracellular lifestyle of Chlamydia and Rickettsia reflects their genome reduction-Chlamydia retains only ~1,000 genes compared to ~4,000 in free-living bacteria. This genetic streamlining eliminates biosynthetic pathways while preserving virulence factors and host manipulation systems.
Immune evasion mechanisms demonstrate the evolutionary arms race between pathogens and host defenses. Rickettsia species express variable outer membrane proteins that undergo antigenic variation at frequencies of 10^-3 to 10^-4 per generation, creating immune escape variants faster than adaptive immune responses can develop. Coxiella exhibits phase variation between Phase I (virulent, smooth LPS) and Phase II (avirulent, rough LPS) forms, with Phase I organisms showing >1000-fold greater resistance to complement-mediated killing.
These sophisticated pathophysiological mechanisms create the foundation for understanding why atypical pathogens produce distinctive clinical syndromes and require specialized therapeutic approaches, leading directly into pattern recognition frameworks for clinical diagnosis.
⚙️ Pathophysiological Mastery: The Cellular Hijacking Network
🎯 Clinical Recognition Arsenal: The Diagnostic Detective Framework
Atypical pathogen recognition requires mastering syndrome-specific patterns rather than memorizing individual case presentations. These organisms produce characteristic clinical signatures that reflect their unique pathophysiological mechanisms and tissue tropisms. The key lies in recognizing constellation patterns that combine epidemiological clues, clinical presentations, and laboratory findings.
📌 Remember: ATYPICAL CLUES - Afebrile or low-grade fever, Time course (gradual onset), Young adults affected, Poor response to beta-lactams, Insidious progression, Cough without purulent sputum, Abnormal labs (low WBC), Lack of typical consolidation
- Respiratory Syndrome Patterns
- "Walking Pneumonia" Profile: Mycoplasma pneumoniae
- Age group: 5-40 years (peak 15-25 years)
- Gradual onset over 1-3 weeks
- Dry, hacking cough persisting >2 weeks
- Normal or low WBC count (<10,000/μL)
- Bilateral lower lobe infiltrates on CXR
- "Legionnaire's Disease" Profile: Legionella pneumophila
- Hyponatremia in 60-80% of cases (Na+ <130 mEq/L)
- High fever (>39°C) with relative bradycardia
- GI symptoms in 50% (diarrhea, nausea)
- Elevated LDH (>500 U/L) and CK levels
- Rapid progression to respiratory failure
- "Walking Pneumonia" Profile: Mycoplasma pneumoniae
⭐ Clinical Pearl: The "Atypical Pneumonia Triad" consists of gradual onset, extrapulmonary symptoms, and poor response to beta-lactams. When all three are present, atypical pathogens account for >80% of cases, compared to <20% when none are present.
| Clinical Feature | Mycoplasma | Legionella | Chlamydia | Q Fever | Typical Bacteria |
|---|---|---|---|---|---|
| Onset | Gradual (weeks) | Rapid (days) | Gradual (weeks) | Variable | Acute (hours) |
| Fever | Low-grade | High + bradycardia | Low-grade | High | High |
| Cough | Dry, persistent | Productive | Dry | Dry | Purulent |
| WBC Count | Normal/low | Variable | Normal/low | Normal/low | Elevated |
| Hyponatremia | Rare | 60-80% | Rare | 20-30% | Rare |
| CXR Pattern | Bilateral lower | Unilateral/bilateral | Bilateral | Variable | Lobar consolidation |
- Rickettsial Disease Recognition
- Rocky Mountain Spotted Fever (R. rickettsii)
- Triad: fever, headache, rash (only 60% have all three initially)
- Rash progression: wrists/ankles → centripetal spread
- Appears day 3-5 of illness (delayed presentation)
- Thrombocytopenia (<100,000/μL) in 70-80%
- Epidemic Typhus (R. prowazekii)
- Body louse transmission (poor sanitation)
- Centrifugal rash (trunk → extremities)
- CNS involvement (stupor, delirium)
- Mortality 10-40% if untreated
- Rocky Mountain Spotted Fever (R. rickettsii)
💡 Master This: The "Doxycycline Decision Rule" states that any febrile illness with tick exposure in endemic areas during tick season warrants empirical doxycycline therapy. Waiting for rash development or laboratory confirmation increases mortality risk from <5% to >20% in RMSF.
Q Fever Recognition requires understanding occupational and geographic risk factors. Coxiella burnetii infections show strong associations with livestock exposure, veterinary occupations, and rural residence. Acute Q fever presents as flu-like illness in 60% of cases, pneumonia in 20%, and hepatitis in 20%, while chronic Q fever develops endocarditis in patients with pre-existing valvular disease.
The "See This, Think That" approach transforms pattern recognition into rapid clinical decision-making, establishing the foundation for systematic differential diagnosis frameworks that distinguish between closely related atypical syndromes.
🎯 Clinical Recognition Arsenal: The Diagnostic Detective Framework
🔬 Differential Diagnosis Mastery: The Systematic Discrimination Matrix
Systematic differentiation of atypical pathogens requires multi-dimensional analysis combining temporal patterns, epidemiological factors, clinical presentations, and laboratory discriminators. The challenge lies not in recognizing atypical infections, but in distinguishing specific pathogens within this category to guide targeted therapy and predict clinical outcomes.
📌 Remember: DISCRIMINATE - Demographics matter, Incubation periods, Seasonal patterns, Clinical severity, Rash characteristics, Immune status, Mortality risk, Infectious dose, Natural reservoirs, Antibiotic responses, Transmission routes, Endemic regions
- Temporal Discrimination Framework
- Incubation Period Patterns
- Mycoplasma pneumoniae: 1-3 weeks (longest among respiratory pathogens)
- Legionella pneumophila: 2-10 days (average 5-6 days)
- Rickettsia rickettsii: 3-12 days (average 7 days)
- Coxiella burnetii: 9-40 days (highly variable, average 20 days)
- Chlamydia pneumoniae: 7-21 days (similar to Mycoplasma)
- Disease Duration Without Treatment
- Self-limiting: Mycoplasma (4-6 weeks), Chlamydia pneumoniae
- Progressive: Legionella (mortality 15-20%), RMSF (mortality >20%)
- Chronic potential: Q fever (1-2% develop chronic disease)
- Incubation Period Patterns
Laboratory Discriminator Matrix provides quantitative criteria for pathogen differentiation. These discriminators achieve >85% accuracy when used systematically, compared to <60% accuracy with clinical criteria alone.
| Discriminator | Mycoplasma | Legionella | Chlamydia | Q Fever | Rickettsia |
|---|---|---|---|---|---|
| Cold Agglutinins | Positive 70% | Negative | Negative | Negative | Negative |
| Hyponatremia | Rare (<10%) | Common 60-80% | Rare | 20-30% | Rare |
| Thrombocytopenia | Rare | 25-50% | Rare | 25-40% | Common 70-80% |
| Elevated LDH | Mild elevation | Marked >500 U/L | Mild | Moderate | Variable |
| Hepatitis Pattern | Rare | 10-20% | Rare | Common 60% | 10-30% |
| Rash Presence | Never | Rare | Never | Rare | Classic 60-90% |
Severity Stratification enables risk-based management decisions and predicts clinical outcomes. Legionella pneumophila demonstrates the highest mortality risk (15-20% overall, >40% in immunocompromised patients), while Mycoplasma pneumoniae rarely causes life-threatening disease (<1% mortality) except in patients with sickle cell disease or immunodeficiency.
- High-Risk Presentations Requiring Immediate Recognition
- Legionella pneumophila
- Rapid progression to respiratory failure (<48 hours)
- Multi-organ involvement (renal, hepatic, CNS)
- ICU admission required in 25-30% of cases
- Delayed diagnosis increases mortality 3-fold
- Rocky Mountain Spotted Fever
- Fulminant course possible (death within 5 days)
- Vascular involvement (DIC, gangrene)
- CNS complications (encephalitis, seizures)
- Treatment delay >5 days increases mortality to >20%
- Legionella pneumophila
💡 Master This: The "72-Hour Rule" states that empirical therapy for suspected atypical pneumonia should begin within 72 hours of symptom onset for Legionella and within 5 days for RMSF to prevent irreversible complications. Laboratory confirmation should never delay treatment initiation.
Geographic and Seasonal Discriminators provide powerful epidemiological tools for pathogen prediction. Rickettsia rickettsii shows >95% geographic clustering in southeastern and south-central United States, with peak incidence during May-August corresponding to Dermacentor tick activity. Coxiella burnetii demonstrates occupational clustering in veterinarians, farmers, and abattoir workers with >10-fold increased risk compared to general population.
The systematic application of these discriminatory frameworks transforms clinical uncertainty into evidence-based diagnostic confidence, establishing the foundation for targeted therapeutic interventions and optimal patient outcomes.
🔬 Differential Diagnosis Mastery: The Systematic Discrimination Matrix
⚕️ Therapeutic Command Center: Evidence-Based Treatment Algorithms
📌 Remember: THERAPY RULES - Tissue penetration essential, High intracellular levels, Empiricism often required, Resistance patterns matter, Adjust for severity, Protein synthesis inhibitors, Yield better outcomes with early treatment
First-Line Therapeutic Agents demonstrate superior intracellular accumulation and proven clinical efficacy. Azithromycin achieves intracellular concentrations 10-100 times higher than serum levels, with tissue half-life of 68 hours enabling once-daily dosing and excellent patient compliance.
| Antibiotic Class | Mechanism | Intracellular Ratio | Half-life | Typical Dosing | Clinical Efficacy |
|---|---|---|---|---|---|
| Macrolides | 50S ribosome | 10-100:1 | 68h (azithromycin) | 500mg x1, 250mg x4d | 90-95% |
| Tetracyclines | 30S ribosome | 5-10:1 | 18-22h | 100mg BID | 85-90% |
| Fluoroquinolones | DNA gyrase | 2-5:1 | 6-8h | 500-750mg daily | 90-95% |
| Chloramphenicol | 50S ribosome | 3-5:1 | 2-3h | 500mg QID | 80-85% |
- Pathogen-Specific Therapeutic Optimization
- Mycoplasma pneumoniae
- First-line: Azithromycin 500mg day 1, then 250mg days 2-5
- Alternative: Doxycycline 100mg BID x 10-14 days
- Pediatric: Azithromycin 10mg/kg day 1, then 5mg/kg days 2-5
- Resistance: <5% to macrolides, <1% to tetracyclines
- Legionella pneumophila
- Severe disease: Levofloxacin 750mg IV daily x 7-10 days
- Mild-moderate: Azithromycin 500mg daily x 5-7 days
- Combination therapy: Consider azithromycin + levofloxacin for ICU patients
- Duration: Minimum 10-14 days for immunocompromised patients
- Mycoplasma pneumoniae
Rickettsial Disease Therapy represents a medical emergency where treatment delay directly correlates with mortality risk. Doxycycline remains the gold standard with >95% efficacy when initiated within 5 days of symptom onset.
- Rickettsia Treatment Protocols
- Adults: Doxycycline 100mg BID x 7-10 days
- Children: Doxycycline 2.2mg/kg BID (regardless of age)
- Pregnancy: Doxycycline preferred despite pregnancy category D
- Severe disease: Consider IV doxycycline 100mg BID
- Alternative: Chloramphenicol 500mg QID (higher relapse rate)
💡 Master This: The "Doxycycline Dogma" for rickettsial diseases: Never withhold doxycycline from children or pregnant women with suspected RMSF. The mortality risk from untreated disease (>20%) far exceeds the theoretical risks of doxycycline in these populations.
Q Fever Therapeutic Complexity requires differentiation between acute and chronic disease forms. Acute Q fever responds well to standard therapy, while chronic Q fever demands prolonged treatment with combination antibiotics for 18-24 months.
- Q Fever Treatment Stratification
- Acute Q fever: Doxycycline 100mg BID x 14-21 days
- Q fever pneumonia: Doxycycline + fluoroquinolone x 14-21 days
- Chronic Q fever: Doxycycline + hydroxychloroquine x 18-24 months
- Endocarditis: Surgical valve replacement often required
- Monitoring: Phase I/II serology every 3-6 months
Resistance Surveillance reveals emerging threats requiring therapeutic adaptation. Macrolide resistance in Mycoplasma pneumoniae has increased from <1% to >20% in some regions, particularly Asia, necessitating fluoroquinolone or tetracycline alternatives in treatment failures.
These evidence-based therapeutic algorithms provide the foundation for optimal patient outcomes while minimizing resistance development and adverse effects, leading to advanced integration strategies for complex clinical scenarios.
⚕️ Therapeutic Command Center: Evidence-Based Treatment Algorithms
🌐 Advanced Integration Hub: Multi-System Clinical Mastery
Advanced atypical pathogen management requires understanding systemic complications, immune-mediated sequelae, and co-infection synergies that extend beyond primary infectious syndromes. These organisms can trigger autoimmune phenomena, vascular complications, and chronic inflammatory states that persist long after pathogen clearance.
📌 Remember: COMPLICATIONS - Cardiac involvement, Organ failure, Multi-system disease, Post-infectious sequelae, Long-term effects, Immune dysregulation, Chronic inflammation, Autoimmune triggers, Thrombotic events, Immunocompromised risks, Outbreak potential, Neurologic manifestations, Secondary infections
Cardiovascular Complications represent life-threatening sequelae that require immediate recognition and specialized management. Mycoplasma pneumoniae can trigger autoimmune myocarditis in 1-10% of cases through molecular mimicry mechanisms, while Chlamydia pneumoniae shows strong epidemiological associations with atherosclerotic cardiovascular disease.
- Cardiac Manifestation Patterns
- Mycoplasma-Associated Carditis
- Incidence: 1-10% of pneumonia cases
- Mechanism: Molecular mimicry with cardiac myosin
- Presentation: Chest pain, arrhythmias, heart failure
- Diagnosis: Elevated troponins, ECG changes, echocardiography
- Treatment: Supportive care + anti-inflammatory therapy
- Chlamydia-Atherosclerosis Link
- Seroprevalence: >50% in CAD patients vs <30% controls
- Mechanism: Chronic inflammation, endothelial dysfunction
- Evidence: C. pneumoniae DNA in atherosclerotic plaques
- Trials: Antibiotic therapy shows mixed results
- Mycoplasma-Associated Carditis
⭐ Clinical Pearl: Post-infectious glomerulonephritis occurs in <1% of Mycoplasma infections but carries significant morbidity. Urinalysis should be performed in all patients with severe Mycoplasma pneumonia, especially those with prolonged fever or systemic symptoms.
| Complication | Pathogen | Incidence | Mechanism | Clinical Features | Management |
|---|---|---|---|---|---|
| Myocarditis | Mycoplasma | 1-10% | Molecular mimicry | Chest pain, arrhythmias | Supportive + anti-inflammatory |
| Encephalitis | Mycoplasma | 0.1-1% | Direct invasion | Altered mental status | Doxycycline + steroids |
| Endocarditis | Q fever | 1-2% chronic | Valve seeding | New murmur, emboli | Surgery + antibiotics |
| Vasculitis | Rickettsia | 5-15% | Endothelial infection | Rash, organ dysfunction | Doxycycline + supportive |
| Pneumonitis | Chlamydia | 10-20% | Immune complex | Bilateral infiltrates | Macrolides + steroids |
- CNS Involvement Recognition
- Mycoplasma Encephalitis
- Onset: 1-2 weeks after respiratory symptoms
- Presentation: Altered mental status, seizures, focal deficits
- CSF findings: Lymphocytic pleocytosis, elevated protein
- Imaging: Bilateral thalamic or brainstem lesions
- Treatment: Doxycycline + corticosteroids
- Rickettsial CNS Disease
- Incidence: 20-30% of RMSF cases
- Manifestations: Confusion, stupor, focal neurologic signs
- Pathophysiology: Endothelial infection → cerebral edema
- Prognosis: Permanent sequelae in 10-20% of survivors
- Mycoplasma Encephalitis
💡 Master This: Immune-mediated complications often occur after successful pathogen clearance, requiring prolonged monitoring for 2-4 weeks post-treatment. Autoimmune phenomena may require immunosuppressive therapy independent of antimicrobial treatment.
Immunocompromised Host Considerations reveal dramatically altered disease patterns with increased severity, atypical presentations, and prolonged courses. HIV patients with CD4 counts <200 show 10-fold higher rates of disseminated Mycoplasma infection, while transplant recipients demonstrate increased mortality from Legionella pneumonia (>40% vs 15-20% in immunocompetent hosts).
Co-infection Synergies create complex clinical scenarios where multiple atypical pathogens or atypical-typical combinations produce synergistic pathogenicity. Mycoplasma-viral co-infections occur in 20-30% of cases, potentially explaining severe presentations in otherwise healthy young adults.
These advanced integration concepts establish the foundation for developing rapid mastery frameworks and clinical reference tools that enable expert-level decision-making in complex atypical pathogen scenarios.
🌐 Advanced Integration Hub: Multi-System Clinical Mastery
🎯 Clinical Mastery Arsenal: The Expert's Rapid Reference
Clinical mastery requires instant access to critical decision points, quantitative thresholds, and evidence-based protocols that enable rapid, accurate management of atypical pathogen infections. These essential tools transform complex clinical scenarios into systematic, manageable decisions.
📌 Remember: MASTERY TOOLS - Memorize key numbers, Algorithms for decisions, Systematic approaches, Thresholds for action, Emergency protocols, Risk stratification, Yield optimization
Essential Numbers Arsenal provides instant clinical reference for critical thresholds and decision points:
-
Diagnostic Thresholds
- Cold agglutinins: ≥1:64 suggests Mycoplasma (70% sensitivity)
- Hyponatremia: Na+ <130 mEq/L in 60-80% of Legionella cases
- Thrombocytopenia: Platelets <100,000 in 70-80% of RMSF
- LDH elevation: >500 U/L strongly suggests Legionella
- Q fever serology: Phase I:Phase II ratio >1 indicates chronic disease
-
Treatment Timing Thresholds
- RMSF mortality: <5% if treated <5 days, >20% if >5 days
- Legionella ICU admission: 25-30% of cases, mortality 15-20%
- Mycoplasma complications: <1% mortality, 1-10% cardiac involvement
- Q fever chronicity: 1-2% develop chronic disease (mainly valve disease)
⭐ Clinical Pearl: The "Rule of 5s" for RMSF: 5-day incubation average, 5-day treatment window for <5% mortality, day 5 typical rash onset. Remember: Don't wait for the rash-empirical doxycycline saves lives.
| Clinical Scenario | Immediate Action | Key Threshold | Monitoring Parameter | Duration |
|---|---|---|---|---|
| Suspected RMSF | Doxycycline 100mg BID | <5 days onset | Platelet count | 7-10 days |
| Severe Legionella | Levofloxacin 750mg IV | ICU if hypoxic | Sodium levels | 10-14 days |
| Mycoplasma pneumonia | Azithromycin 500mg | Outpatient if stable | Cardiac enzymes | 5 days |
| Q fever acute | Doxycycline 100mg BID | 14-21 day course | Phase I/II serology | Monitor 6 months |
| Atypical pneumonia | Empirical macrolide | Beta-lactam failure | Clinical response | 5-10 days |
-
30-Second Assessment
- Age group: <40 years → Mycoplasma likely
- Severity: ICU criteria → Legionella/RMSF concern
- Rash present: Tick exposure → RMSF until proven otherwise
- Hyponatremia: Water exposure → Legionella suspected
-
2-Minute History
- Exposure history: Animals, ticks, water, travel
- Symptom timeline: Gradual vs acute onset
- Response to antibiotics: Beta-lactam failure
- Comorbidities: Immunosuppression, valve disease
💡 Master This: The "Atypical Pneumonia Decision Tree" uses three key branch points: Age (<40 vs >40), Severity (outpatient vs ICU), and Epidemiology (exposure history). These three decisions correctly classify >85% of atypical pneumonia cases.
Emergency Protocols for life-threatening presentations:
-
RMSF Emergency Protocol
- Any fever + tick exposure in endemic area during tick season
- Immediate doxycycline 100mg BID (regardless of age/pregnancy)
- No waiting for rash, laboratory confirmation, or specialist consultation
- Admission criteria: Altered mental status, severe headache, hypotension
-
Legionella Severe Disease Protocol
- ICU criteria: Hypoxemia, hypotension, altered mental status
- Combination therapy: Levofloxacin 750mg IV + azithromycin 500mg IV
- Supportive care: Aggressive fluid resuscitation, respiratory support
- Monitoring: Sodium, renal function, respiratory status
Quality Metrics for clinical excellence:
- Diagnostic accuracy: >90% with systematic approach
- Treatment initiation: <4 hours for suspected RMSF
- Appropriate antibiotics: >95% non-beta-lactam selection
- Complication recognition: <24 hours for cardiac/neurologic involvement
- Follow-up compliance: >90% post-treatment monitoring
These mastery tools enable expert-level clinical performance through systematic approaches, memorized protocols, and evidence-based decision-making that optimize patient outcomes while minimizing diagnostic delays and therapeutic errors.
🎯 Clinical Mastery Arsenal: The Expert's Rapid Reference
Practice Questions: Atypical
Test your understanding with these related questions
An 18-year-old male in his first year of college presents to the emergency room with a fever and a severe headache. He reports having unprotected sex with several partners over the past few weeks. Upon examination, the male demonstrates nuchal rigidity and photophobia. His past medical history is notable for a lack of vaccinations beginning from infancy due to his parents' belief that vaccinations may cause autism. The bacteria causing these symptoms would most likely demonstrate which of the following?













