Nephrology (CKD, glomerular diseases)

On this page
🔬 The Glomerular Filtration Fortress: Your Kidney's Command Center
Your kidneys filter 180 liters daily through microscopic barriers that distinguish health from disease with molecular precision. This lesson decodes how glomerular architecture drives filtration, why hemodynamic forces determine function, and how pattern recognition transforms urine findings and clinical syndromes into targeted diagnoses. You'll master the nephritic-nephrotic distinction, build evidence-based treatment frameworks, and integrate renal physiology with multi-system disease to make rapid, confident decisions at the bedside.
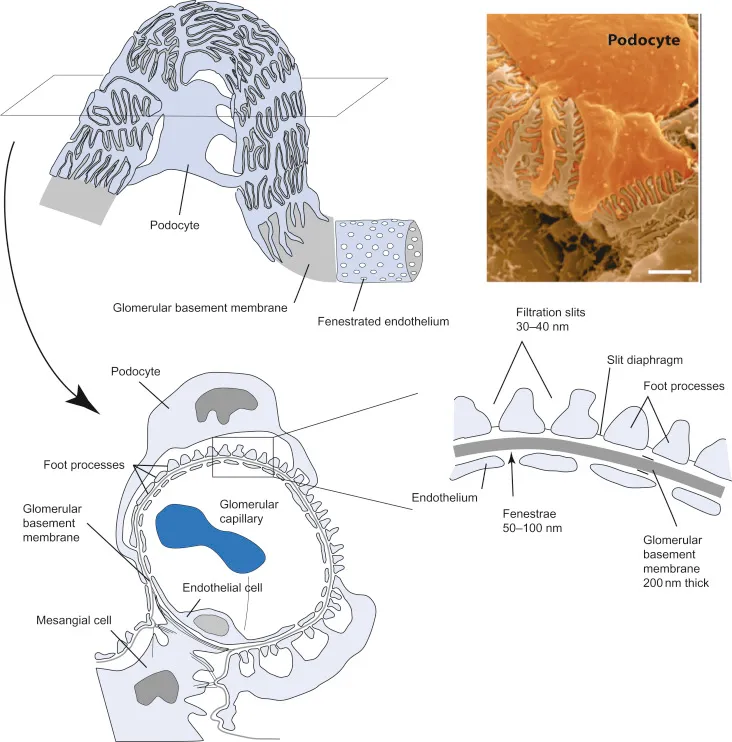
The glomerular filtration apparatus operates as a three-layer security system, each component contributing specific filtration properties:
-
Fenestrated Endothelium (First Barrier)
- Pore size: 70-100 nm diameter
- Blocks cellular elements and large proteins
- Maintains 99% selectivity for molecules >100 kDa
- Albumin retention: >95% in healthy kidneys
- Allows free passage of water, electrolytes, small molecules
-
Glomerular Basement Membrane (Second Barrier)
- Thickness: 300-350 nm in adults
- Negative charge repels anionic proteins
- Type IV collagen provides structural integrity
- Laminin networks create size-selective pores
- Heparan sulfate contributes charge selectivity
-
Podocyte Foot Processes (Final Barrier)
- Slit diaphragm width: 4-14 nm
- Nephrin and podocin proteins form filtration slits
- Dynamic regulation of filtration selectivity
- Foot process effacement indicates podocyte injury
- Proteinuria develops when slit diaphragms fail
📌 Remember: GBM-CHARGE - Glomerular Basement Membrane maintains Charge selectivity, Heparan sulfate Arrangement, Restricts Giant proteins, and Guards Electrolyte balance
| Filtration Component | Pore Size | Primary Function | Failure Pattern | Clinical Marker |
|---|---|---|---|---|
| Fenestrated Endothelium | 70-100 nm | Size exclusion | Hematuria | RBC casts |
| Basement Membrane | Variable | Charge/size barrier | Proteinuria | Albumin loss |
| Podocyte Slits | 4-14 nm | Final selectivity | Nephrotic syndrome | Massive proteinuria |
| Mesangial Matrix | N/A | Structural support | GFR decline | Creatinine rise |
| Juxtaglomerular Apparatus | N/A | Pressure sensing | Hypertension | Renin elevation |
-
Young Adults (20-30 years)
- Males: 120-130 mL/min/1.73m²
- Females: 110-120 mL/min/1.73m²
- Annual decline: 0.5-1 mL/min/1.73m² after age 30
-
Elderly Population (>65 years)
- Expected GFR: 60-90 mL/min/1.73m²
- Accelerated decline: 1-2 mL/min/1.73m² annually
- Functional reserve: 50% reduction from peak values
⭐ Clinical Pearl: GFR must decline by >50% before serum creatinine rises above normal range. A "normal" creatinine of 1.2 mg/dL in an elderly patient may represent stage 3 CKD with GFR <60 mL/min/1.73m².
💡 Master This: The filtration fraction (GFR/renal plasma flow) normally equals 20%. When this ratio increases above 25%, suspect prerenal azotemia with enhanced sodium reabsorption and concentrated urine.
Understanding filtration dynamics reveals why early CKD detection requires calculated GFR rather than serum creatinine alone. This foundation connects directly to CKD staging systems that stratify 26 million Americans with undiagnosed kidney disease.
🔬 The Glomerular Filtration Fortress: Your Kidney's Command Center
⚙️ The Filtration Engine: Hemodynamic Powerhouse
The renin-angiotensin-aldosterone system (RAAS) provides precise efferent arteriole control:
-
Angiotensin II Effects
- Efferent arteriole constriction: 3-5x greater than afferent
- Maintains filtration fraction during hypotension
- Increases intraglomerular pressure by 15-25 mmHg
- Preserves GFR when systemic BP drops
- Becomes maladaptive in chronic kidney disease
-
ACE Inhibitor Mechanisms
- Preferential efferent arteriole dilation
- Reduces intraglomerular pressure by 10-20 mmHg
- Decreases proteinuria by 30-50% in diabetic nephropathy
- Slows CKD progression by 40% over 3-5 years
- Most effective when started at GFR >60 mL/min/1.73m²
📌 Remember: RAAS-EFFECT - Renin Angiotensin Aldosterone System creates Efferent constriction, Filtration maintenance, Electrolyte control, Chronic damage, and Treatment targets
| Hemodynamic Parameter | Normal Range | CKD Changes | Clinical Significance | Therapeutic Target |
|---|---|---|---|---|
| Renal Blood Flow | 1200 mL/min | ↓50-70% | Reduced oxygen delivery | Optimize BP control |
| Glomerular Pressure | 45-60 mmHg | ↑60-80 mmHg | Hyperfiltration injury | ACE-I/ARB therapy |
| Filtration Fraction | 15-25% | ↑25-35% | Enhanced sodium retention | Volume management |
| Autoregulation Range | 80-180 mmHg | Narrowed range | Pressure-dependent GFR | Gentle BP reduction |
| Single Nephron GFR | Variable | ↑150-200% | Compensatory hyperfiltration | Protein restriction |
-
Compensatory Mechanisms
- Remaining nephrons increase GFR by 50-100%
- Glomerular hypertrophy develops within 2-4 weeks
- Enhanced protein reabsorption activates tubular inflammation
-
Maladaptive Consequences
- Podocyte stress leads to foot process effacement
- Mesangial expansion reduces filtration surface area
- Glomerulosclerosis becomes irreversible after 6-12 months
⭐ Clinical Pearl: The "creatinine bump" after ACE inhibitor initiation indicates effective reduction of intraglomerular pressure. Creatinine increases <30% represent appropriate hemodynamic response, not drug toxicity.
💡 Master This: Autoregulation failure occurs when mean arterial pressure drops below 80 mmHg or exceeds 180 mmHg. Within this range, GFR remains stable despite ±40% blood pressure variations through afferent arteriole adjustments.
These hemodynamic principles explain why blood pressure targets differ in CKD patients and connect to the systematic approach for recognizing early filtration dysfunction patterns.
⚙️ The Filtration Engine: Hemodynamic Powerhouse
🎯 The Diagnostic Arsenal: Pattern Recognition Mastery
The KDIGO-RAPID assessment protocol provides systematic evaluation:
- Kidney function baseline (prior GFR values)
- Drug nephrotoxicity review (NSAIDs, contrast, antibiotics)
- Imaging for obstruction (hydronephrosis, stones)
- Glomerular vs tubular patterns (proteinuria type)
- Other systemic diseases (diabetes, lupus, vasculitis)
- Rapid vs gradual decline (acute vs chronic patterns)
- Albumin-to-creatinine ratio quantification
- Pressure control assessment (hypertension management)
- Infection or inflammation markers (urinalysis, CRP)
- Dialysis planning considerations (vascular access)
📌 Remember: RAPID-GFR - Recognize Acute vs Progressive decline, Identify Drug causes, and Plan Dialysis early
| GFR Decline Pattern | Rate of Change | Typical Causes | Diagnostic Clues | Reversibility |
|---|---|---|---|---|
| Acute (<48h) | >50% decrease | Prerenal, ATN, obstruction | Oliguria, casts | 60-80% |
| Subacute (days-weeks) | 25-50% decrease | Glomerulonephritis, drugs | Proteinuria, hematuria | 40-60% |
| Chronic (months-years) | 1-5 mL/min/year | Diabetes, hypertension | Gradual, asymptomatic | 10-20% |
| Acute-on-chronic | Variable | Dehydration, infection | Baseline elevation | 30-50% |
| Rapidly progressive | >50% in weeks | RPGN, vasculitis | Crescents, ANCA+ | 20-40% |
-
Glomerular Proteinuria (Albumin-predominant)
- Albumin-to-creatinine ratio: >300 mg/g
- Indicates glomerular barrier dysfunction
- Associated with hypertension and edema
- Diabetic nephropathy: Progressive increase over years
- Minimal change disease: Sudden onset, massive proteinuria
-
Tubular Proteinuria (Low molecular weight)
- β2-microglobulin, retinol-binding protein elevation
- Normal or minimally elevated albumin
- Suggests tubulointerstitial disease
- Drug nephrotoxicity: NSAIDs, lithium, calcineurin inhibitors
- Chronic interstitial nephritis: Gradual GFR decline
⭐ Clinical Pearl: The protein-to-creatinine ratio correlates with 24-hour urine protein collection (ratio × 1000 = mg protein/day). A ratio of 3.5 equals 3500 mg daily protein loss, defining nephrotic-range proteinuria.
Advanced diagnostic markers enhance pattern recognition:
- Cystatin C - GFR estimation independent of muscle mass
- NGAL (Neutrophil Gelatinase-Associated Lipocalin) - Early AKI detection
- KIM-1 (Kidney Injury Molecule-1) - Tubular injury marker
- ANCA testing - Vasculitis screening in RPGN
- Complement levels - Glomerulonephritis evaluation
💡 Master This: When serum creatinine increases >0.3 mg/dL within 48 hours or >50% within 7 days, suspect AKI. However, non-oliguric AKI occurs in 60% of cases, making urine output an unreliable screening tool.
This diagnostic framework connects to systematic comparison of glomerular disease patterns that distinguish nephritic from nephrotic presentations with >90% accuracy.
🎯 The Diagnostic Arsenal: Pattern Recognition Mastery
🔍 The Syndrome Decoder: Nephritic vs Nephrotic Mastery
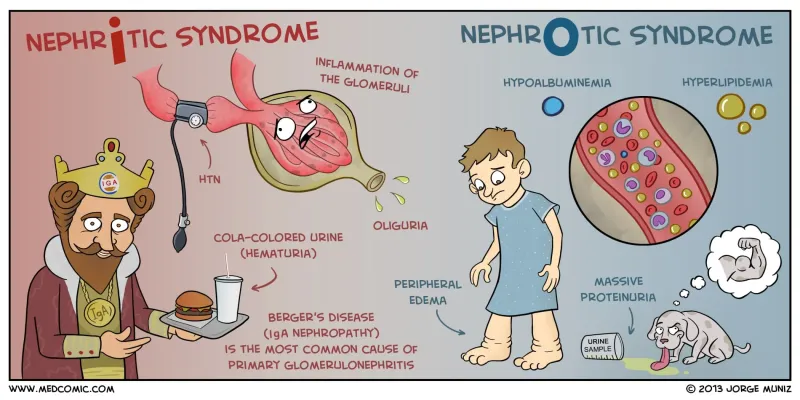
The INFLAME-LEAK differentiation matrix provides systematic syndrome recognition:
NEPHRITIC (INFLAME) Pattern:
- Inflammation (hematuria, RBC casts)
- Normal or mild proteinuria (<3.5 g/day)
- Fluid retention (edema, hypertension)
- Low complement (C3, C4 consumption)
- Acute GFR decline (oliguria common)
- Mild hypoalbuminemia (>2.5 g/dL)
- Elevated inflammatory markers (ESR, CRP)
NEPHROTIC (LEAK) Pattern:
- Large proteinuria (>3.5 g/day)
- Edema (periorbital, dependent)
- Albumin depletion (<2.5 g/dL)
- Kidney function preserved initially
📌 Remember: INFLAME vs LEAK - Nephritic shows INFLAMmation with preserved albumin, Nephrotic shows massive LEAK with low albumin
| Syndrome Feature | Nephritic Pattern | Nephrotic Pattern | Mixed Presentation | Clinical Significance |
|---|---|---|---|---|
| Proteinuria | <3.5 g/day | >3.5 g/day | Variable | Barrier selectivity |
| Hematuria | Gross/microscopic | Rare/microscopic | Common | Inflammatory activity |
| Hypertension | 80-90% | 30-40% | 60-70% | Volume vs oncotic |
| GFR Decline | Acute, severe | Gradual, mild | Variable | Inflammatory damage |
| Complement | Low (60-80%) | Normal (90%) | Variable | Immune complex |
| Edema Pattern | Periorbital, facial | Dependent, ascites | Combined | Pathophysiology |
| Albumin Level | >2.5 g/dL | <2.5 g/dL | 2.0-3.0 g/dL | Synthetic vs loss |
| %%{init: {'flowchart': {'htmlLabels': true}}}%% | ||||
| flowchart TD |
Start["🩺 Glomerular Disease
• Kidney pathology• Renal evaluation"]
ProtCheck["📋 Proteinuria Level
• Check 24h urine• High or low range"]
Nephrotic["🩺 Nephrotic Syndrome
• Massive edema• ⬆️ Lipid levels"]
AgeCheck["📋 Patient Age
• Pediatric vs adult• Threshold 18 yrs"]
MCD["🩺 Minimal Change
• Steroid responsive• Effacement seen"]
FSGS["🩺 FSGS / Membranous
• Focal segmental• Thickened GBM"]
HemCheck["📋 Heme + HTN?
• RBC in sediment• High blood pressure"]
Asymp["🩺 Asymptomatic
• Isolated protein• Monitor function"]
Nephritic["🩺 Nephritic Syndrome
• Inflammatory GN• Active sediment"]
GFRCheck["📋 Rapid GFR Loss?
• Sharp Cr ⬆️• Urgent biopsy"]
RPGN["⚠️ RPGN Workup
• Crescentic GN• Serology tests"]
PostInf["🩺 Post-infectious GN
• Post-strep common• Low C3 levels"]
Start --> ProtCheck ProtCheck -->|>3.5g/day| Nephrotic ProtCheck -->|Low range| HemCheck
Nephrotic --> AgeCheck AgeCheck -->|<18 years| MCD AgeCheck -->|>=18 years| FSGS
HemCheck -->|No| Asymp HemCheck -->|Yes| Nephritic
Nephritic --> GFRCheck GFRCheck -->|Yes| RPGN GFRCheck -->|No| PostInf
style Start fill:#F7F5FD, stroke:#F0EDFA, stroke-width:1.5px, rx:12, ry:12, color:#6B21A8 style ProtCheck fill:#FEF8EC, stroke:#FBECCA, stroke-width:1.5px, rx:12, ry:12, color:#854D0E style Nephrotic fill:#F7F5FD, stroke:#F0EDFA, stroke-width:1.5px, rx:12, ry:12, color:#6B21A8 style AgeCheck fill:#FEF8EC, stroke:#FBECCA, stroke-width:1.5px, rx:12, ry:12, color:#854D0E style MCD fill:#F7F5FD, stroke:#F0EDFA, stroke-width:1.5px, rx:12, ry:12, color:#6B21A8 style FSGS fill:#F7F5FD, stroke:#F0EDFA, stroke-width:1.5px, rx:12, ry:12, color:#6B21A8 style HemCheck fill:#FEF8EC, stroke:#FBECCA, stroke-width:1.5px, rx:12, ry:12, color:#854D0E style Asymp fill:#F7F5FD, stroke:#F0EDFA, stroke-width:1.5px, rx:12, ry:12, color:#6B21A8 style Nephritic fill:#F7F5FD, stroke:#F0EDFA, stroke-width:1.5px, rx:12, ry:12, color:#6B21A8 style GFRCheck fill:#FEF8EC, stroke:#FBECCA, stroke-width:1.5px, rx:12, ry:12, color:#854D0E style RPGN fill:#FDF4F3, stroke:#FCE6E4, stroke-width:1.5px, rx:12, ry:12, color:#B91C1C style PostInf fill:#F7F5FD, stroke:#F0EDFA, stroke-width:1.5px, rx:12, ry:12, color:#6B21A8
**Nephritic Syndrome Subtypes** demonstrate distinct temporal patterns:
* **Acute Post-infectious GN**
- Onset: **1-3 weeks** after streptococcal infection
- Complement recovery: **6-8 weeks**
- Spontaneous resolution: **>90%** in children
+ Gross hematuria: "Coca-Cola" colored urine
+ Hypertension: **80%** of cases, often severe
* **Rapidly Progressive GN (RPGN)**
- GFR decline: **>50%** within **3 months**
- Crescents on biopsy: **>50%** of glomeruli
- Dialysis requirement: **60-80%** without treatment
+ ANCA-associated: **70%** of adult RPGN
+ Anti-GBM disease: **5%** but most aggressive
**Nephrotic Syndrome Subtypes** show age-related distributions:
* **Pediatric Nephrotic Syndrome**
- Minimal change disease: **85%** of cases
- Steroid responsiveness: **>90%** achieve remission
- Relapse rate: **60-70%** within 2 years
+ Complete remission: Proteinuria **<0.2 g/day**
+ Steroid dependence: **30-40%** require maintenance
* **Adult Nephrotic Syndrome**
- Focal segmental glomerulosclerosis: **35%**
- Membranous nephropathy: **30%**
- Diabetic nephropathy: **25%**
+ Primary vs secondary: Biopsy required **>40 years**
+ Spontaneous remission: **<10%** in adults
> ⭐ **Clinical Pearl**: The **"telescoped" urinary sediment** with RBC casts, WBC casts, and granular casts simultaneously suggests **acute glomerulonephritis** with **>90%** specificity for inflammatory glomerular disease.
**Mixed Nephritic-Nephrotic Presentations** occur in **20-30%** of glomerular diseases:
* **Membranoproliferative GN** - Classic mixed pattern
* **Lupus nephritis** - Variable presentation by class
* **IgA nephropathy** - Spectrum from asymptomatic to RPGN
* **Diabetic nephropathy** - Late-stage mixed features
> 💡 **Master This**: Complement consumption patterns distinguish disease categories: **C3 depression alone** suggests alternative pathway activation (C3 glomerulopathy), while **C3 + C4 depression** indicates classical pathway activation (lupus, post-infectious GN).
This syndrome recognition framework connects to evidence-based treatment algorithms that optimize **immunosuppressive protocols** and **renoprotective strategies** for each glomerular disease pattern.
🔍 The Syndrome Decoder: Nephritic vs Nephrotic Mastery
⚖️ The Treatment Command Center: Evidence-Based Intervention Protocols
Renoprotective Therapy Foundation provides universal benefit across glomerular diseases:
-
ACE Inhibitors/ARBs (First-line for all patients)
- Proteinuria reduction: 30-50% within 3-6 months
- CKD progression delay: 40% reduction in ESRD risk
- Target blood pressure: <130/80 mmHg in proteinuric patients
- Maximum tolerated dose regardless of blood pressure
- Combination ACE-I + ARB only in selected cases
-
SGLT2 Inhibitors (Expanding indications)
- GFR preservation: 2-3 mL/min/1.73m² annually
- Cardiovascular protection: 20% reduction in heart failure
- Effective in diabetic and non-diabetic CKD
- Initiate when GFR >25 mL/min/1.73m²
- Continue until dialysis initiation
📌 Remember: RAAS-SGLT2 - Renin Angiotensin blockade plus SGLT2 inhibition provides Synergistic renoprotection, Lowers Tension, and reduces Cardiovascular risk
| Treatment Category | Specific Agent | Target Population | Efficacy Measure | Monitoring Parameter |
|---|---|---|---|---|
| RAAS Blockade | Lisinopril 40mg | All proteinuric CKD | 30-50% proteinuria ↓ | Creatinine, K+ |
| SGLT2 Inhibition | Dapagliflozin 10mg | GFR >25, T2DM/HF | 40% ESRD risk ↓ | GFR, UTI risk |
| Immunosuppression | Prednisone 1mg/kg | Nephrotic syndrome | 80% remission rate | Infection, glucose |
| Plasmapheresis | 7 exchanges | Anti-GBM, severe ANCA | 60% dialysis avoidance | Coagulation, access |
| Rituximab | 375mg/m² × 4 | Steroid-resistant NS | 70% remission rate | B-cell count, PML |
-
Minimal Change Disease
- Initial: Prednisone 1 mg/kg/day (max 80mg) × 8 weeks
- Taper: 50% reduction every 2 weeks after remission
- Relapse management: 75% respond to repeat steroids
- Steroid-dependent: Rituximab 375 mg/m² × 4 doses
- Frequent relapsers: Cyclophosphamide 2 mg/kg/day × 12 weeks
-
FSGS Treatment Hierarchy
- First-line: High-dose steroids × 16-24 weeks
- Second-line: Calcineurin inhibitors (cyclosporine/tacrolimus)
- Third-line: Rituximab or mycophenolate mofetil
- Remission rates: 20-30% with steroids, 40-50% with CNI
- Genetic forms: Poor response to immunosuppression
-
ANCA-Associated Vasculitis
- Induction: Cyclophosphamide 15 mg/kg IV monthly × 6 months
- Alternative: Rituximab 375 mg/m² weekly × 4 doses
- Maintenance: Methotrexate or azathioprine × 18-24 months
- Remission rates: 85-90% with either induction regimen
- Relapse prevention: 60% reduction with maintenance therapy
⭐ Clinical Pearl: The "steroid-sparing" approach uses rituximab for frequent relapsers (≥2 relapses/year) or steroid-dependent patients, reducing cumulative steroid exposure by 70-80% while maintaining remission rates.
Supportive Care Optimization addresses complications systematically:
-
Edema Management
- Loop diuretics: Furosemide 40-80 mg twice daily
- Combination therapy: Add thiazide for synergy
- Albumin infusion: Reserved for severe hypoalbuminemia
- Target: 2-3 kg weight loss weekly maximum
- Monitor: Electrolytes, kidney function daily
-
Thromboembolism Prevention
- Risk factors: Albumin <2.5 g/dL, proteinuria >10 g/day
- Prophylaxis: Consider anticoagulation in high-risk patients
- Treatment duration: Until remission achieved
-
Infection Prevention
- Pneumocystis prophylaxis: TMP-SMX during high-dose steroids
- Vaccination: Complete before immunosuppression
- Monitoring: Regular CBC, immunoglobulin levels
💡 Master This: Treatment response definitions vary by disease: Complete remission requires proteinuria <0.3 g/day with normal albumin, while partial remission accepts 50% proteinuria reduction with stable kidney function.
These evidence-based protocols connect to multi-system integration approaches that address cardiovascular risk, bone disease, and progressive CKD complications in the comprehensive nephrology patient.
⚖️ The Treatment Command Center: Evidence-Based Intervention Protocols
🔗 The Nephrology Network: Multi-System Integration Mastery
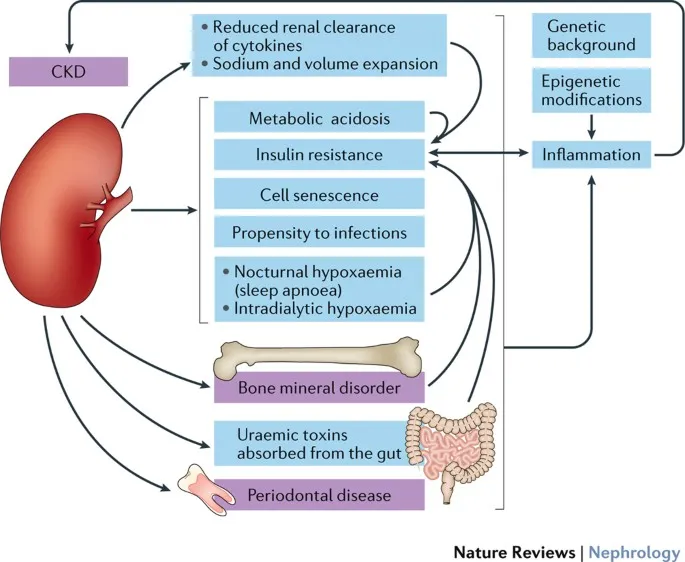
The CKD-CARDIAC-BONE integration matrix reveals interconnected pathophysiology:
-
Cardiovascular-Kidney Integration
- Shared risk factors: Diabetes, hypertension, inflammation
- Accelerated atherosclerosis: 2-3x higher CAD risk per GFR category
- Heart failure prevalence: 40% in stage 4-5 CKD
- Cardiorenal syndrome: Bidirectional organ dysfunction
- Volume overload: Central to both heart and kidney failure
-
Mineral-Bone Disorder (CKD-MBD)
- FGF23 elevation: Begins at GFR <60 mL/min/1.73m²
- Phosphorus retention: Drives secondary hyperparathyroidism
- Vascular calcification: Links bone and cardiovascular disease
- Fracture risk: 4x higher in dialysis patients
- Coronary calcification: Predicts cardiovascular mortality
-
Anemia-Inflammation Network
- EPO deficiency: Develops at GFR <30 mL/min/1.73m²
- Iron deficiency: 60-80% of CKD patients
- Chronic inflammation: Suppresses erythropoiesis
- Hemoglobin targets: 10-11.5 g/dL optimal range
- ESA therapy: Avoid hemoglobin >12 g/dL
📌 Remember: CKD-COMPLICATIONS - Cardiovascular disease, Kidney-bone disorders, Diabetes acceleration, Complications multiply, Outcomes worsen, Management requires Prevention, Life expectancy decreases, Integrated care Improves outcomes, Comprehensive approach, Addresses all Systems
| System Integration | Pathophysiology | Clinical Manifestation | Therapeutic Target | Outcome Benefit |
|---|---|---|---|---|
| Cardio-Renal | Volume/pressure overload | Heart failure, HTN | RAAS blockade, SGLT2-I | 20-40% event reduction |
| Bone-Mineral | FGF23, PTH elevation | Fractures, calcification | Phosphate binders, vitamin D | 30% fracture reduction |
| Anemia-Inflammation | EPO deficiency, iron loss | Fatigue, LV hypertrophy | Iron, ESA therapy | QOL improvement |
| Metabolic-Endocrine | Insulin resistance, acidosis | Diabetes progression | Bicarbonate, metformin | Slower GFR decline |
| Immune-Inflammatory | Uremic toxins, oxidative stress | Infection susceptibility | Vaccination, nutrition | Reduced hospitalizations |
| %%{init: {'flowchart': {'htmlLabels': true}}}%% | ||||
| flowchart TD |
CKD["🩺 CKD Progression
• Kidney decline• Chronic disease"]
FGF["🔬 FGF23 Elevation
• ⬆️ FGF23 levels• Mineral imbalance"]
CVD["🩺 CV Disease
• Cardiac damage• Vessel disease"]
AGE["👵 Accelerated Aging
• Cellular senescence• Rapid decline"]
VOL["📋 Volume Retention
• Fluid overload• Sodium balance"]
HF["🩺 Heart Failure
• Pump dysfunction• Reduced CO"]
EX["📋 Reduced Exercise
• Physical fatigue• low work capacity"]
TOX["🔬 Uremic Toxins
• Metabolic waste• Nitrogenous buildup"]
INF["🩺 Inflammation
• Systemic response• Cytokine release"]
PEW["📋 Protein-Energy
• Nutritive wasting• Muscle loss"]
MSF["⚠️ Multi-System Failure
• Organ collapse• Final pathway"]
CKD --> FGF CKD --> VOL CKD --> TOX
FGF --> CVD CVD --> AGE AGE --> MSF
VOL --> HF HF --> EX EX --> MSF
TOX --> INF INF --> PEW PEW --> MSF
style CKD fill:#F7F5FD, stroke:#F0EDFA, stroke-width:1.5px, rx:12, ry:12, color:#6B21A8 style FGF fill:#FFF7ED, stroke:#FFEED5, stroke-width:1.5px, rx:12, ry:12, color:#C2410C style CVD fill:#F7F5FD, stroke:#F0EDFA, stroke-width:1.5px, rx:12, ry:12, color:#6B21A8 style AGE fill:#F6F5F5, stroke:#E7E6E6, stroke-width:1.5px, rx:12, ry:12, color:#525252 style VOL fill:#FEF8EC, stroke:#FBECCA, stroke-width:1.5px, rx:12, ry:12, color:#854D0E style HF fill:#F7F5FD, stroke:#F0EDFA, stroke-width:1.5px, rx:12, ry:12, color:#6B21A8 style EX fill:#FEF8EC, stroke:#FBECCA, stroke-width:1.5px, rx:12, ry:12, color:#854D0E style TOX fill:#FFF7ED, stroke:#FFEED5, stroke-width:1.5px, rx:12, ry:12, color:#C2410C style INF fill:#F7F5FD, stroke:#F0EDFA, stroke-width:1.5px, rx:12, ry:12, color:#6B21A8 style PEW fill:#FEF8EC, stroke:#FBECCA, stroke-width:1.5px, rx:12, ry:12, color:#854D0E style MSF fill:#FDF4F3, stroke:#FCE6E4, stroke-width:1.5px, rx:12, ry:12, color:#B91C1C
**Cutting-Edge Integration Strategies** optimize multi-system outcomes:
* **SGLT2 Inhibitor Pleiotropy**
- Renal protection: **40%** ESRD risk reduction
- Heart failure prevention: **30%** hospitalization reduction
- Weight loss: **2-4 kg** average benefit
+ Mechanism: Glucose-independent effects on inflammation
+ Expanding use: Non-diabetic CKD, heart failure with preserved EF
* **Comprehensive Mineral Management**
- Early phosphate control: Prevents FGF23 elevation
- Calcimimetic therapy: Reduces PTH without calcium loading
- Vitamin D analogs: Selective VDRA minimize hypercalcemia
+ Target PTH: **2-9x** upper normal limit by CKD stage
+ Avoid calcium-based binders when possible
* **Precision Anemia Management**
- Iron studies: Transferrin saturation **>30%**, ferritin **>500 ng/mL**
- Hypoxia-inducible factor stabilizers: Novel EPO alternative
- Inflammation control: Address underlying CKD-related inflammation
+ Iron deficiency: **Absolute** vs **functional** patterns
+ ESA hyporesponsiveness: Often iron or inflammation-related
> ⭐ **Clinical Pearl**: The **"uremic cardiomyopathy"** phenotype includes LV hypertrophy, diastolic dysfunction, and accelerated coronary disease, developing **5-10 years earlier** than in age-matched controls without CKD.
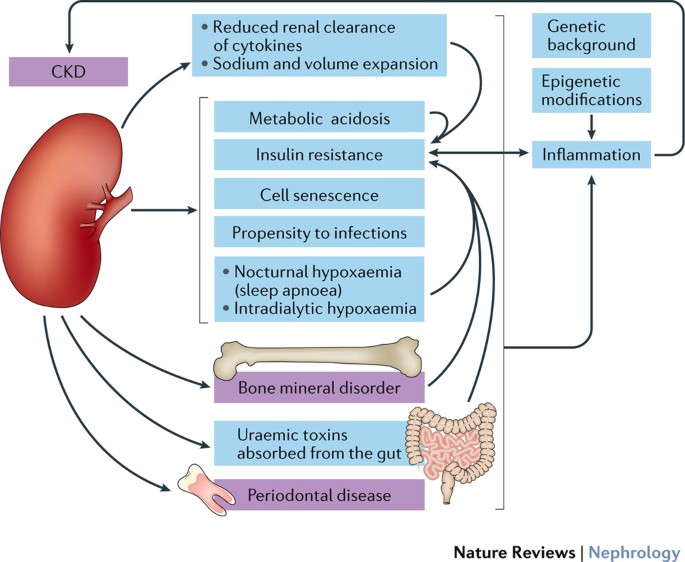
**Advanced Risk Stratification** guides intensive interventions:
* **Cardiovascular Risk Calculators**
- CKD-specific equations: Account for **2-3x** baseline risk elevation
- Coronary calcium scoring: Predicts events in CKD patients
- Biomarker integration: Troponin, BNP, FGF23 levels
+ Risk reclassification: **30-40%** of patients change categories
+ Intensive statin therapy: Target LDL **<70 mg/dL**
* **Bone Disease Monitoring**
- DEXA scanning: **Every 2 years** in CKD stages 4-5
- Biochemical markers: PTH, 25(OH)D, FGF23, alkaline phosphatase
- Fracture risk assessment: FRAX calculator with CKD adjustments
+ Bisphosphonate use: Controversial in advanced CKD
+ Denosumab alternative: Safer in low GFR patients
> 💡 **Master This**: The **"CKD-MBD"** paradigm recognizes that abnormal mineral metabolism begins early in CKD and drives both skeletal and cardiovascular complications, requiring integrated management rather than isolated parameter correction.
This multi-system approach connects to rapid mastery frameworks that synthesize **complex CKD management** into **practical clinical tools** for immediate patient care optimization.
🔗 The Nephrology Network: Multi-System Integration Mastery
🎯 The Clinical Mastery Toolkit: Rapid Assessment and Decision Frameworks
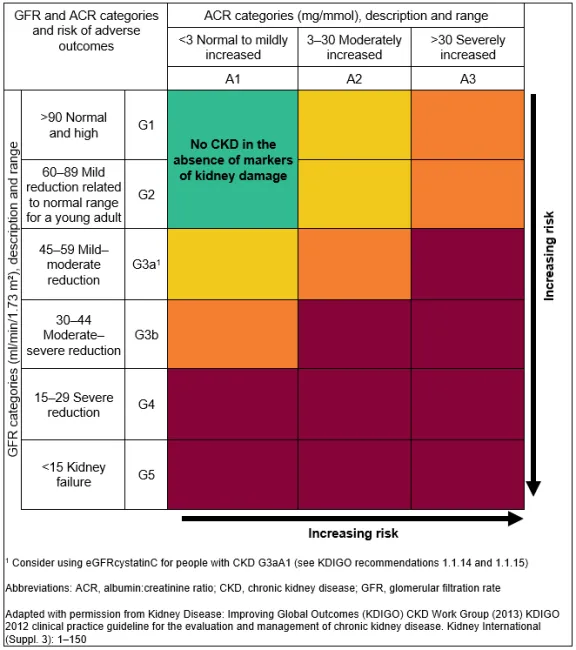
The KIDNEY-MASTER Assessment Protocol:
- Kidney function trajectory (GFR slope calculation)
- Identify reversible factors (drugs, obstruction, volume)
- Determine proteinuria significance (ACR quantification)
- Navigate complications (anemia, bone disease, acidosis)
- Evaluate cardiovascular risk (integrated assessment)
- Yield treatment priorities (evidence-based hierarchy)
- Monitor response markers (specific targets)
- Advance care planning (dialysis, transplant timing)
- Systemic disease screening (diabetes, lupus, vasculitis)
- Time-sensitive interventions (urgent vs routine)
- Education and lifestyle (patient engagement)
- Referral coordination (multidisciplinary care)
📌 Remember: KIDNEY-MASTER - Systematic assessment Keeps Interventions Directed, Nephrology Excellence Yields Mastery, Achieves Superior Treatment, Ensures Results
| Clinical Scenario | Rapid Assessment | Key Decision Point | Immediate Action | Follow-up Interval |
|---|---|---|---|---|
| New CKD diagnosis | GFR, ACR, imaging | Reversible causes? | RAAS blockade, education | 3 months |
| AKI presentation | Timeline, volume status | Prerenal vs intrinsic? | Fluid management, stop nephrotoxins | 24-48 hours |
| Nephrotic syndrome | Age, proteinuria level | Steroid trial vs biopsy? | Supportive care, specialist referral | 2-4 weeks |
| RPGN suspected | Urinalysis, complement | Immunosuppression urgency? | Pulse steroids, nephrology consult | 24 hours |
| CKD progression | GFR slope, complications | Dialysis planning? | Vascular access, transplant evaluation | 6 months |
-
GFR Categories (Automatic Actions)
- >90: Screen for proteinuria, optimize BP
- 60-89: Annual monitoring, cardiovascular risk assessment
- 45-59: Semi-annual visits, bone/mineral labs
- 30-44: Quarterly monitoring, anemia screening, dialysis education
- 15-29: Monthly visits, access planning, transplant evaluation
- <15: Dialysis initiation, urgent nephrology management
-
Proteinuria Thresholds (Treatment Intensity)
- <30 mg/g: Normal, routine monitoring
- 30-300 mg/g: Microalbuminuria, RAAS blockade
- >300 mg/g: Macroalbuminuria, intensive management
- >3500 mg/g: Nephrotic range, specialist referral
-
Acute Change Triggers (Immediate Evaluation)
- Creatinine increase >0.3 mg/dL in 48 hours
- GFR decline >25% from baseline
- New proteinuria >1 g/day
- Oliguria <400 mL/day
⭐ Clinical Pearl: The "Rule of 4s" for CKD staging: Stage 4 CKD (GFR 15-29) requires 4 key actions - 4-monthly visits, 4 complications screening (anemia, bone disease, acidosis, volume), 4-way medication review, and 4-option renal replacement therapy discussion.
Rapid Complication Screening Matrix:
-
Anemia Workup (GFR <30)
- Target hemoglobin: 10-11.5 g/dL
- Iron studies: TSAT >30%, ferritin >500 ng/mL
- ESA initiation: Consider if iron-replete and symptomatic
-
Bone Disease Prevention (GFR <45)
- Phosphorus target: 2.7-4.6 mg/dL
- PTH target: 2-9x upper normal limit
- Vitamin D repletion: 25(OH)D >30 ng/mL
-
Cardiovascular Protection (All stages)
- Blood pressure: <130/80 mmHg if proteinuric
- Statin therapy: Target LDL <100 mg/dL (or <70 if high risk)
- SGLT2 inhibitor: Consider if GFR >25 and appropriate
💡 Master This: The "CKD Acceleration Points" occur at GFR 60, 45, and 30 mL/min/1.73m² - these thresholds trigger intensified monitoring, complication screening, and specialist involvement with evidence-based protocols that improve long-term outcomes.
This clinical mastery framework provides the systematic foundation for expert nephrology practice, enabling rapid assessment, evidence-based decisions, and optimal patient outcomes across the complete spectrum of kidney disease presentations.
🎯 The Clinical Mastery Toolkit: Rapid Assessment and Decision Frameworks
Practice Questions: Nephrology (CKD, glomerular diseases)
Test your understanding with these related questions
A 45-year-old woman comes to the physician because of a 3-month history of worsening fatigue, loss of appetite, itching of the skin, and progressive leg swelling. Although she has been drinking 2–3 L of water daily, she has been passing only small amounts of urine. She has type 1 diabetes mellitus, chronic kidney disease, hypertension, and diabetic polyneuropathy. Her current medications include insulin, torasemide, lisinopril, and synthetic erythropoietin. Her temperature is 36.7°C (98°F), pulse is 87/min, and blood pressure is 138/89 mm Hg. She appears pale. There is 2+ pitting edema in the lower extremities. Sensation to pinprick and light touch is decreased over the feet and legs bilaterally. Laboratory studies show: Hemoglobin 11.4 g/dL Leukocyte count 6000/mm3 Platelet count 280,000/mm3 Serum Na+ 137 mEq/L K+ 5.3 mEq/L Cl− 100 mEq/L HCO3− 20 mEq/L Urea nitrogen 85 mg/dL Creatinine 8 mg/dL pH 7.25 Which of the following long-term treatments would best improve quality of life and maximize survival in this patient?













