Liver disease

On this page
🏭 The Hepatic Powerhouse: Understanding Liver Disease Fundamentals
The liver orchestrates over 500 biochemical reactions daily, yet its disease often whispers before it shouts-making pattern recognition essential for early intervention. You'll learn how hepatocellular injury cascades into clinical syndromes, master the diagnostic frameworks that distinguish cholestatic from hepatitic patterns, and build treatment algorithms grounded in evidence. By integrating laboratory analysis with multi-system effects, you'll develop the clinical judgment to recognize liver disease across its spectrum and intervene before irreversible damage occurs.
📌 Remember: LIVER - Lipid metabolism, Immune function, Vitamin storage, Enzyme production, RBC breakdown - each function measured in grams per day, not milligrams
- Metabolic Powerhouse Functions
- Protein synthesis: 10-15g albumin daily, half-life 20 days
- Glucose regulation: stores 100-120g glycogen, maintains 80-100mg/dL glucose
- Detoxification: processes 95% of ingested toxins via Phase I/II reactions
- Phase I: CYP450 oxidation, 50+ enzymes
- Phase II: conjugation reactions, 6 major pathways
- Bile production: 600-1000mL daily, 95% reabsorbed in terminal ileum
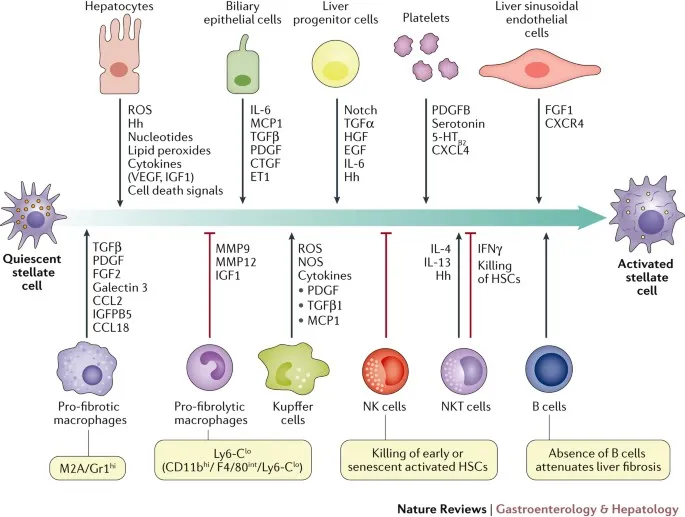
⭐ Clinical Pearl: Normal liver regenerates 75% of its mass within 6-8 weeks after injury, but chronic disease destroys this regenerative capacity through fibrosis progression and stellate cell activation
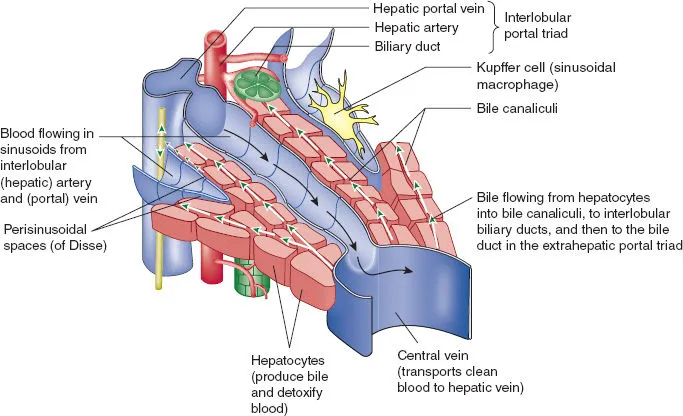
The hepatic lobule represents the functional unit where portal triads (hepatic artery, portal vein, bile duct) create zones of metabolic activity. Zone 1 (periportal) handles oxidative metabolism and gluconeogenesis, while Zone 3 (pericentral) manages detoxification and lipid synthesis. This zonal architecture explains why different toxins create distinct injury patterns.
| Zone | Blood Supply | Primary Functions | Vulnerability | O₂ Tension |
|---|---|---|---|---|
| Zone 1 | Portal triad | Gluconeogenesis, β-oxidation | Viral hepatitis | High (9-13 kPa) |
| Zone 2 | Mixed | Intermediate metabolism | Drug reactions | Medium (4-8 kPa) |
| Zone 3 | Central vein | Detoxification, lipogenesis | Ischemia, acetaminophen | Low (2-4 kPa) |
| Sinusoids | Dual supply | Exchange, filtration | Congestion | Variable |
| Space of Disse | Lymphatic | Stellate cell residence | Fibrosis initiation | Minimal |
Understanding hepatocyte injury mechanisms unlocks the logic behind every liver disease presentation, from acute hepatitis with ALT >1000 U/L to chronic cirrhosis with portal pressures >12 mmHg.
🏭 The Hepatic Powerhouse: Understanding Liver Disease Fundamentals
⚡ The Injury Cascade: Hepatocellular Damage Mechanisms
📌 Remember: CRASH - Cytotoxic (direct cell death), Reactive metabolites (oxidative stress), Apoptosis (programmed death), Stellate activation (fibrosis), Hypoxia (ischemic injury)
- Direct Hepatotoxicity Mechanisms
- Mitochondrial dysfunction: ATP depletion, <70% normal levels trigger cell death
- Oxidative stress: ROS production exceeds antioxidant capacity by >300%
- Membrane disruption: phospholipase activation destroys cellular integrity
- ALT release: cytoplasmic enzyme, half-life 47 hours
- AST release: mitochondrial enzyme, half-life 17 hours
- ALT/AST ratio >2: suggests alcoholic hepatitis
- ALT/AST ratio <1: indicates chronic liver disease
⭐ Clinical Pearl: Acetaminophen toxicity creates Zone 3 necrosis because CYP2E1 concentration is highest pericentrally, producing NAPQI metabolite that depletes glutathione stores when >150mg/kg ingested
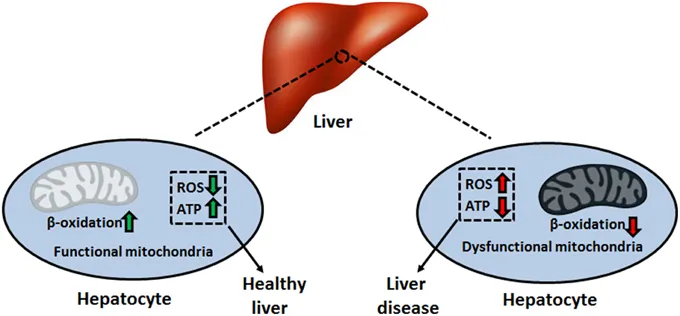
Chronic injury patterns activate hepatic stellate cells in the Space of Disse, transforming from vitamin A storage cells into myofibroblasts that produce excessive collagen. This fibrogenic response progresses through stages: inflammation → fibrosis → cirrhosis → decompensation.
| Injury Type | Timeline | Laboratory Pattern | Histologic Changes | Reversibility |
|---|---|---|---|---|
| Acute Hepatitis | Hours-days | ALT/AST >500 U/L | Hepatocyte necrosis | Complete |
| Chronic Hepatitis | Months-years | ALT/AST <300 U/L | Portal inflammation | Partial |
| Fibrosis | Years | Normal-mild elevation | Collagen deposition | Limited |
| Cirrhosis | Decades | Variable elevation | Nodular regeneration | Irreversible |
| Decompensation | Acute on chronic | Mixed pattern | End-stage changes | Transplant only |
The dual blood supply creates unique vulnerability patterns where portal vein compromise affects 75% of hepatic blood flow, while hepatic artery occlusion impacts 25% but carries higher oxygen content. This explains why portal hypertension dominates chronic liver disease complications.
⚡ The Injury Cascade: Hepatocellular Damage Mechanisms
🎯 Pattern Recognition: Clinical Presentation Frameworks
📌 Remember: JAUNDICE - Jaundice onset, ALT/AST pattern, Urinalysis findings, Nausea/vomiting, Duration of symptoms, Icteric progression, Coagulopathy presence, Encephalopathy signs
- Acute Hepatitis Recognition Patterns
- Viral pattern: ALT >AST, gradual onset, prodromal symptoms
- Toxic pattern: ALT >>AST, rapid onset, exposure history
- Ischemic pattern: ALT >3000 U/L, shock history, rapid recovery
- Acetaminophen: ALT >1000, delayed presentation, metabolic acidosis
- Viral hepatitis: ALT 500-1500, IgM positive, lymphocytosis
- Autoimmune: ALT variable, ANA/ASMA positive, hypergammaglobulinemia
⭐ Clinical Pearl: Acute liver failure defined as INR >1.5 plus encephalopathy in patients without pre-existing liver disease - 20-week rule distinguishes hyperacute (<7 days), acute (7-21 days), and subacute (21-26 weeks) presentations
Chronic liver disease creates compensated versus decompensated states with distinct clinical profiles. Compensated cirrhosis may remain asymptomatic for years, while decompensation triggers life-threatening complications requiring immediate management.
- Decompensation Event Recognition
- Ascites development: first decompensation in 50% of patients
- Variceal bleeding: mortality 15-20% per episode
- Hepatic encephalopathy: precipitant identification crucial
- Infection: 40% of HE episodes, SBP most common
- GI bleeding: 25% of episodes, protein load mechanism
- Medications: 15% of episodes, sedatives/opioids primary
- Electrolyte imbalance: 10% of episodes, hyponatremia key
| Presentation | Timeline | Key Features | Laboratory Pattern | Mortality Risk |
|---|---|---|---|---|
| Hyperacute | <7 days | Encephalopathy, coagulopathy | ALT >3000, INR >2.0 | 80-90% |
| Acute | 7-21 days | Jaundice, nausea | ALT >1000, Bili >10 | 60-70% |
| Subacute | 21-26 weeks | Ascites, bleeding | ALT variable, INR >1.5 | 40-50% |
| Compensated | Years | Asymptomatic | Normal-mild elevation | <5%/year |
| Decompensated | Acute on chronic | Ascites, HE, bleeding | Mixed abnormalities | 15-20%/year |
Recognition of specific syndrome patterns enables targeted therapy: Wilson disease in young patients with neuropsychiatric symptoms, hemochromatosis in middle-aged men with diabetes, autoimmune hepatitis in young women with extrahepatic manifestations.
🎯 Pattern Recognition: Clinical Presentation Frameworks
🔬 Diagnostic Discrimination: Laboratory and Imaging Analysis
📌 Remember: LABS - Liver enzymes (ALT/AST pattern), Alkaline phosphatase (cholestatic vs hepatocellular), Bilirubin fractionation (direct vs indirect), Synthetic function (albumin, INR, factor V)
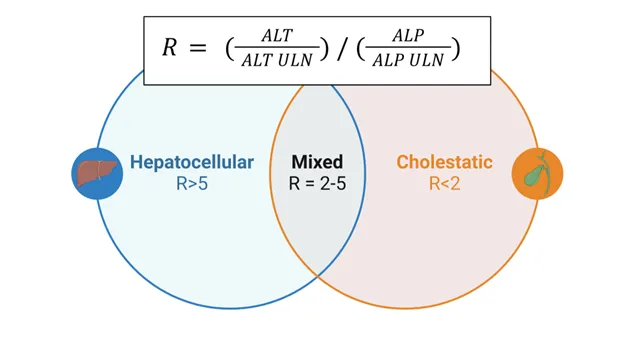
- Hepatocellular vs Cholestatic Patterns
- Hepatocellular injury: ALT/AST >5x normal, ALP <3x normal
- Cholestatic injury: ALP >3x normal, ALT/AST <5x normal
- Mixed pattern: both elevated >3x normal
- R-ratio calculation: (ALT/ULN) ÷ (ALP/ULN)
- R >5: hepatocellular pattern
- R 2-5: mixed pattern
- R <2: cholestatic pattern
⭐ Clinical Pearl: Alkaline phosphatase elevation requires fractionation - liver-specific ALP confirmed by elevated GGT or 5'-nucleotidase, while isolated ALP suggests bone disease or pregnancy
Advanced diagnostic testing targets specific etiologies based on clinical presentation and initial laboratory patterns. Viral serologies, autoimmune markers, and metabolic studies provide definitive diagnosis in >90% of cases.
- Etiology-Specific Testing Algorithms
- Viral hepatitis: HAV IgM, HBsAg/anti-HBc, HCV antibody/RNA
- Autoimmune: ANA, ASMA, anti-LKM, anti-SLA
- Metabolic: ceruloplasmin, transferrin saturation, α1-antitrypsin
- Wilson disease: ceruloplasmin <20mg/dL, 24h urine copper >100μg
- Hemochromatosis: transferrin saturation >45%, ferritin >300μg/L
- α1-antitrypsin deficiency: level <11μM, PiZZ phenotype
| Test Category | Specific Markers | Diagnostic Threshold | Clinical Significance |
|---|---|---|---|
| Viral | HBsAg, HCV RNA | Detectable levels | Active infection |
| Autoimmune | ANA, ASMA | Titer >1:80 | AIH diagnosis |
| Metabolic | Ceruloplasmin | <20 mg/dL | Wilson disease |
| Genetic | α1-AT level | <11 μM | Deficiency state |
| Synthetic | INR, albumin | INR >1.5, Alb <3.5 | Liver dysfunction |
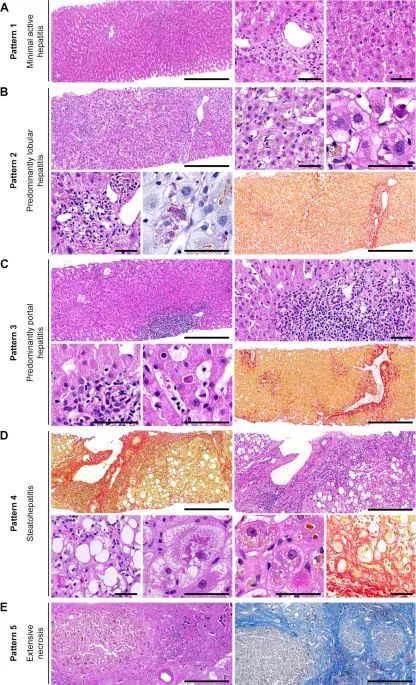
Imaging modalities provide structural assessment and complication detection. Ultrasound offers initial screening, CT/MRI enable detailed characterization, and elastography quantifies liver stiffness as fibrosis surrogate.
Advanced imaging techniques like hepatobiliary scintigraphy and MR elastography provide functional assessment complementing structural evaluation. Portal pressure measurement via hepatic venous pressure gradient remains gold standard for portal hypertension assessment.
🔬 Diagnostic Discrimination: Laboratory and Imaging Analysis
💊 Treatment Algorithms: Evidence-Based Management Strategies
📌 Remember: TREAT - Target etiology, Reduce progression, Evaluate complications, Assess transplant candidacy, Time-sensitive interventions
- Etiology-Specific Treatment Protocols
- Viral hepatitis: Direct-acting antivirals achieve >95% SVR
- Autoimmune hepatitis: Prednisone + azathioprine induces remission in 80%
- Alcoholic liver disease: Abstinence improves survival by 60%
- HCV treatment: sofosbuvir/velpatasvir for 12 weeks
- HBV treatment: tenofovir/entecavir for indefinite duration
- AIH treatment: prednisone 1mg/kg tapered over 6 months
⭐ Clinical Pearl: Alcohol cessation provides greatest survival benefit in alcoholic liver disease - 6-month abstinence required before transplant evaluation, naltrexone reduces relapse risk by 50%
Complication management follows evidence-based protocols with specific targets and monitoring parameters. Portal hypertension complications require immediate intervention with established algorithms for optimal outcomes.
- Decompensation Management Protocols
- Ascites: sodium restriction <2g/day, diuretics (spironolactone 100-400mg)
- Varices: propranolol reduces bleeding risk by 40%
- Hepatic encephalopathy: lactulose 30mL TID, rifaximin 550mg BID
- Large-volume paracentesis: >5L requires albumin replacement
- TIPS procedure: reduces rebleeding but increases HE risk
- Precipitant management: treat infections, stop sedatives
| Complication | First-Line Treatment | Target Parameter | Success Rate |
|---|---|---|---|
| Ascites | Spironolactone + furosemide | Weight loss 0.5-1 kg/day | 70-80% |
| Varices | Propranolol | HR reduction 25% | 60-70% |
| HE | Lactulose + rifaximin | 2-3 BM/day | 80-90% |
| SBP | Ceftriaxone 2g daily | PMN <250/μL | >95% |
| HRS | Albumin + vasoconstrictors | Creatinine improvement | 40-50% |
Emerging therapies target fibrosis reversal and regenerative medicine. Antifibrotic agents like simtuzumab and cenicriviroc show promise in clinical trials, while stem cell therapy and bioartificial liver devices offer future therapeutic options.
Monitoring protocols ensure treatment efficacy and early complication detection. Regular surveillance includes laboratory monitoring, imaging studies, and endoscopic screening based on disease stage and risk stratification.
💊 Treatment Algorithms: Evidence-Based Management Strategies
🌐 Multi-System Integration: The Hepatic Network
📌 Remember: SYSTEMS - Splanchnic circulation, Yellow (bilirubin) metabolism, Synthetic proteins, Toxin clearance, Endocrine functions, Metabolic regulation, Storage functions
- Cardiovascular System Integration
- Hyperdynamic circulation: cardiac output ↑40%, SVR ↓50%
- Portal hypertension: pressure >12 mmHg creates collateral circulation
- Cardiomyopathy: diastolic dysfunction in 70% of cirrhotic patients
- Splanchnic vasodilation: NO overproduction, endotoxemia
- Portosystemic shunting: bypasses hepatic clearance
- QT prolongation: >440ms in 60% of patients
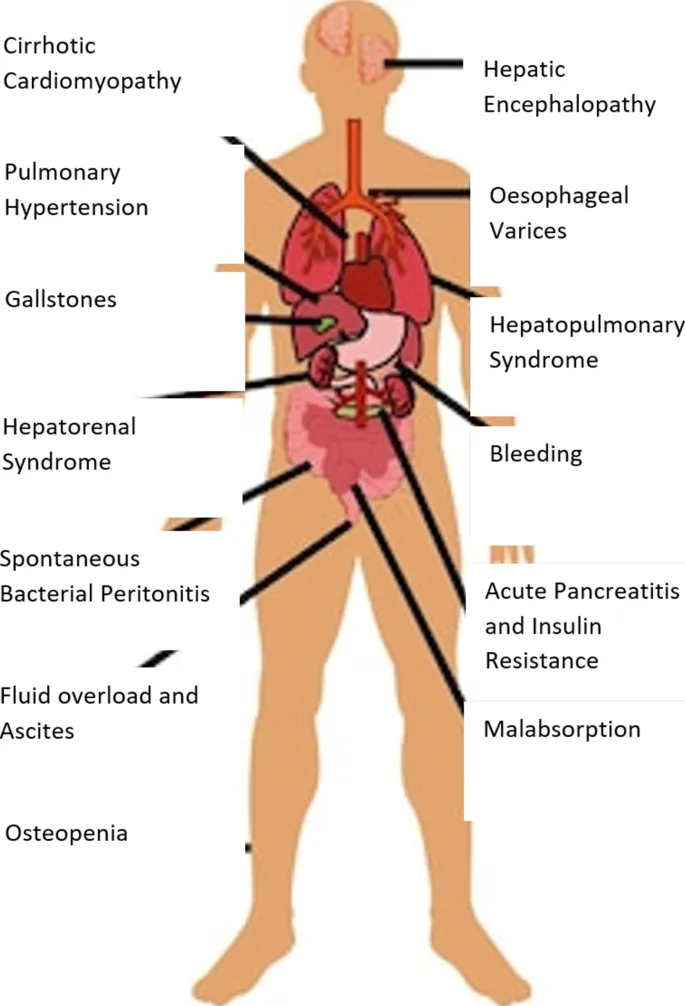
⭐ Clinical Pearl: Hepatorenal syndrome represents functional kidney failure in advanced liver disease - Type 1 HRS progresses rapidly with doubling creatinine in <2 weeks, Type 2 HRS shows gradual decline over months
Renal system interactions create complex fluid and electrolyte disorders that require specialized management. Hepatorenal syndrome represents the most severe manifestation with poor prognosis without liver transplantation.
- Neurologic System Complications
- Hepatic encephalopathy: ammonia accumulation affects >50% of cirrhotic patients
- Cerebral edema: occurs in 80% of acute liver failure cases
- Osmotic demyelination: rapid sodium correction risk
- Minimal HE: subclinical cognitive impairment
- Overt HE: Grade 1-4 classification system
- Precipitants: infection 40%, GI bleeding 25%, medications 15%
| System | Primary Effect | Mechanism | Clinical Manifestation | Prevalence |
|---|---|---|---|---|
| Cardiovascular | Hyperdynamic state | Vasodilation | High CO, low SVR | >90% |
| Renal | Functional failure | Vasoconstriction | HRS, electrolyte disorders | 40-50% |
| Neurologic | Encephalopathy | Ammonia toxicity | Confusion, coma | 50-80% |
| Pulmonary | Hepatopulmonary syndrome | Intrapulmonary shunting | Hypoxemia | 15-30% |
| Hematologic | Coagulopathy | Synthetic dysfunction | Bleeding, thrombosis | >95% |
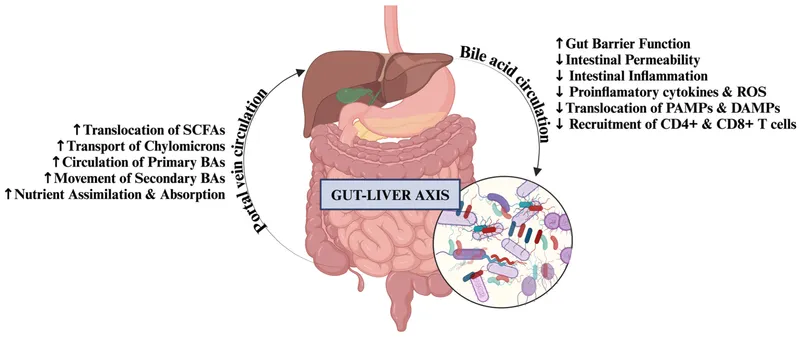
Emerging research reveals gut-liver axis as critical pathway in disease progression. Microbiome alterations, intestinal permeability, and bacterial translocation create inflammatory milieu that accelerates fibrosis and promotes complications.
Therapeutic targets include microbiome modulation with probiotics, intestinal barrier restoration with zinc supplementation, and anti-inflammatory strategies with pentoxifylline. Precision medicine approaches using genetic markers and metabolomics guide personalized treatment strategies.
Understanding these multi-system interactions transforms liver disease management from organ-specific treatment to comprehensive care addressing systemic manifestations and preventing complications through integrated therapeutic approaches.
🌐 Multi-System Integration: The Hepatic Network
🎯 Clinical Mastery Arsenal: Rapid Assessment Tools
📌 Remember: RAPID - Recognize patterns, Assess severity, Prioritize interventions, Identify complications, Determine disposition
- Essential Clinical Assessment Framework
- History: alcohol use, medication exposure, family history, travel
- Physical: jaundice, hepatomegaly, splenomegaly, ascites, encephalopathy
- Laboratory: ALT/AST pattern, bilirubin fractionation, synthetic function
- Acute presentation: ALT >500, rapid onset, exposure history
- Chronic presentation: spider angiomata, palmar erythema, gynecomastia
- Decompensation: ascites, bleeding, confusion
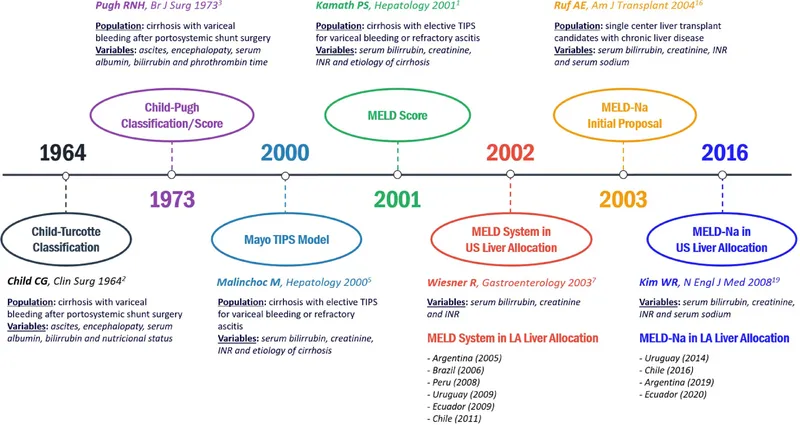
⭐ Clinical Pearl: MELD-Na score provides superior prognostic accuracy compared to traditional MELD - incorporates serum sodium to account for dilutional hyponatremia in advanced disease
Rapid Diagnostic Algorithms enable systematic evaluation of liver disease presentations. Pattern recognition guides targeted testing and immediate management decisions.
| Clinical Scenario | Key Features | Immediate Actions | Diagnostic Priority |
|---|---|---|---|
| Acute Hepatitis | ALT >1000, rapid onset | Acetaminophen level, viral serologies | Toxin exposure |
| Acute Liver Failure | INR >1.5 + encephalopathy | ICU admission, transplant evaluation | Etiology identification |
| Variceal Bleeding | Hematemesis + cirrhosis | IV octreotide, urgent endoscopy | Hemodynamic stability |
| Spontaneous Peritonitis | Ascites + fever | Diagnostic paracentesis, empiric antibiotics | Infection confirmation |
| Hepatic Encephalopathy | Confusion + liver disease | Lactulose, precipitant search | Grade assessment |
High-Yield Clinical Pearls for immediate application:
- Laboratory Interpretation Mastery
- ALT/AST >1000: acute hepatitis (viral, toxic, ischemic)
- ALP >3x normal: cholestatic pattern (biliary obstruction)
- INR >1.5: synthetic dysfunction (acute liver failure risk)
- Albumin <3.0: chronic liver disease (portal hypertension risk)
- Bilirubin >3.0: significant hepatic dysfunction (jaundice threshold)
⭐ Clinical Pearl: TIPS procedure reduces variceal rebleeding by 70% but increases hepatic encephalopathy risk by 30% - careful patient selection based on MELD score and encephalopathy history optimizes outcomes
Emergency Management Protocols ensure optimal outcomes in acute presentations:
- Acute Liver Failure Protocol
- Immediate: ICU admission, neurologic monitoring, coagulopathy correction
- Within 6 hours: transplant center contact, etiology workup, prognostic scoring
- Ongoing: cerebral edema monitoring, renal support, infection surveillance
This clinical mastery arsenal transforms complex liver disease into manageable clinical scenarios where systematic assessment and evidence-based protocols ensure optimal patient outcomes and clinical excellence.
🎯 Clinical Mastery Arsenal: Rapid Assessment Tools
Practice Questions: Liver disease
Test your understanding with these related questions
A 60-year-old rock musician presents to the office because he has been feeling increasingly tired for the past 6 months. He has a history of intravenous drug use and alcohol abuse. He states that he feels quite tired, but he otherwise has no complaints. Physical examination is noncontributory. His laboratory values are normal other than moderately elevated liver enzymes. Which of the following additional tests should you order first?













