IBD

On this page
🔥 The Inflammatory Bowel Disease Battlefield: Understanding the Gut's Civil War
Inflammatory bowel disease transforms the gut into a battlefield where the immune system attacks its own tissue, creating a chronic inflammatory cascade that extends far beyond the intestines. You'll master the molecular machinery driving Crohn's disease and ulcerative colitis, sharpen your diagnostic pattern recognition to distinguish IBD from clever mimics, and command the therapeutic algorithms that can induce remission and prevent devastating complications. This lesson builds your clinical judgment from pathophysiology through systemic manifestations, equipping you to recognize subtle presentations and deploy targeted treatments confidently.
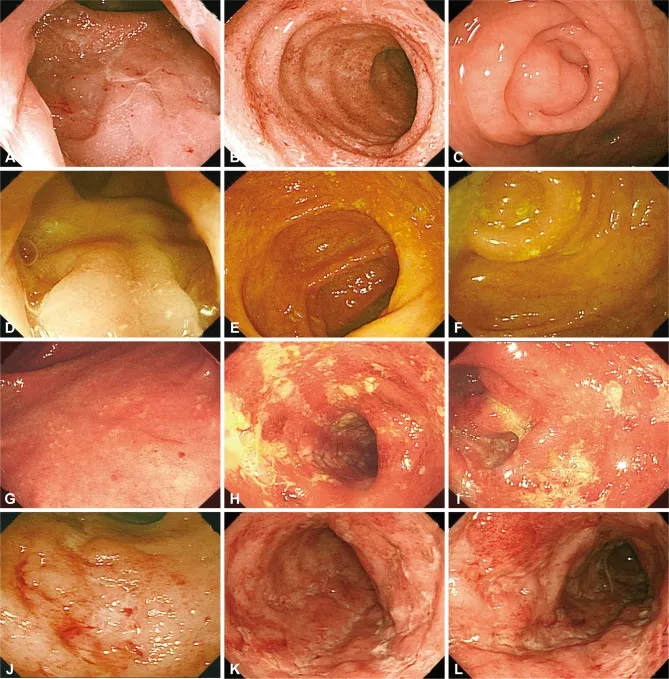
The IBD Spectrum: Two Distinct Warriors
IBD encompasses two primary entities with fundamentally different patterns of intestinal assault:
- Ulcerative Colitis (UC)
- Continuous mucosal inflammation starting from rectum
- Limited to colon and rectum (100% rectal involvement)
- Superficial mucosal and submucosal involvement only
- Crypt abscesses and epithelial damage predominate
- Goblet cell depletion creates characteristic appearance
- Pseudopolyps form from regenerating mucosa
- Crohn's Disease (CD)
- Discontinuous, transmural inflammation (skip lesions)
- Can affect any part of GI tract (mouth to anus)
- Full-thickness bowel wall involvement
- Granulomatous inflammation in 60-70% of cases
- Cobblestone appearance from deep ulcerations
- Strictures and fistulas from transmural damage
📌 Remember: CLOSEUP for UC features - Continuous, Limited to colon, Only mucosa/submucosa, Superficial ulcers, Epithelial damage, Uniform inflammation, Pseudopolyps
| Feature | Ulcerative Colitis | Crohn's Disease | Clinical Significance |
|---|---|---|---|
| Distribution | Continuous from rectum | Skip lesions, any GI location | UC: predictable surgical planning |
| Depth | Mucosa/submucosa only | Transmural involvement | CD: higher complication risk |
| Rectal Involvement | 100% of cases | 50% of cases | UC: always accessible by sigmoidoscopy |
| Granulomas | Rare (<5%) | Present in 60-70% | CD: pathognomonic when present |
| Smoking Effect | Protective (50% risk reduction) | Harmful (2x increased risk) | Opposite effects guide counseling |
Epidemiological Landscape: The Global IBD Surge
IBD demonstrates striking epidemiological patterns that reveal important pathogenic clues:
- Geographic Distribution
- Highest incidence in Northern Europe and North America
- 20-fold variation in incidence between countries
- Urban > rural populations (2-3x higher incidence)
- Demographic Patterns
- Bimodal age distribution: peaks at 20-30 and 60-70 years
- Slight female predominance in CD (1.3:1 ratio)
- Equal gender distribution in UC
- 10-15% family history in affected patients
💡 Master This: The "hygiene hypothesis" explains IBD's geographic pattern - reduced early-life microbial exposure in developed countries leads to altered immune system development and increased IBD susceptibility.
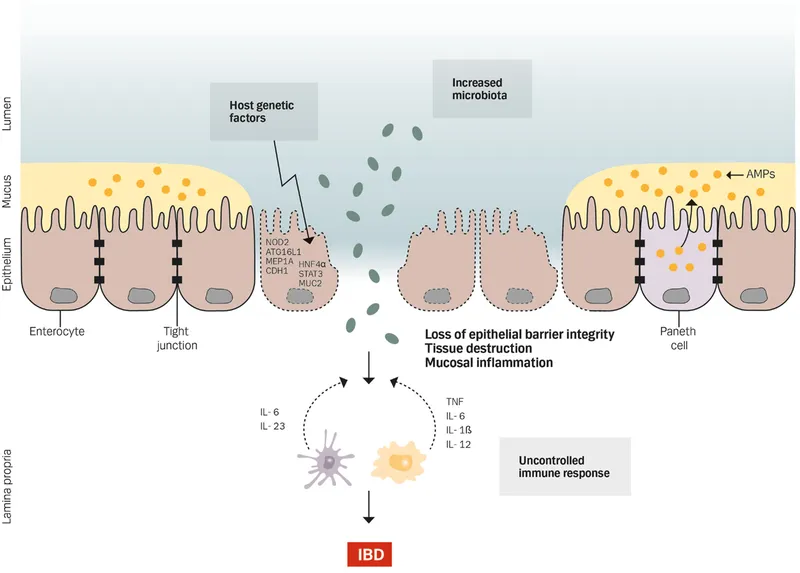
Genetic Architecture: The Susceptibility Blueprint
IBD genetics reveal the complex interplay between host susceptibility and environmental triggers:
- Genetic Risk Factors
- >200 IBD susceptibility loci identified through GWAS
- NOD2/CARD15 mutations in 15-20% of CD patients
- HLA-DRB1*0103 association with severe UC
- Twin concordance: 50% for CD, 15% for UC
- Functional Pathways
- Autophagy defects (ATG16L1, IRGM genes)
- Barrier function impairment (FUT2, CDH1)
- Immune regulation dysfunction (IL23R, JAK2)
⭐ Clinical Pearl: NOD2 mutations increase CD risk 3-fold in heterozygotes and 20-40 fold in homozygotes, with particular association with ileal disease and stricturing behavior.
The foundation of IBD pathogenesis rests on this genetic susceptibility framework, setting the stage for understanding how environmental triggers activate the inflammatory cascade that defines these chronic conditions.
🔥 The Inflammatory Bowel Disease Battlefield: Understanding the Gut's Civil War
⚙️ The Inflammatory Engine: Decoding IBD's Molecular Machinery
The Immune Dysregulation Cascade
IBD pathogenesis centers on the loss of immune tolerance to commensal bacteria, creating a state of chronic inflammation:
- T-Helper Cell Polarization
- Crohn's Disease: Th1/Th17 predominance
- IFN-γ and IL-17 drive transmural inflammation
- TNF-α levels 10-20x higher than normal
- Macrophage activation creates granulomatous response
- Ulcerative Colitis: Th2/Th17 pattern
- IL-13 and IL-5 promote mucosal inflammation
- IL-17 contributes to epithelial barrier disruption
- Neutrophil infiltration creates crypt abscesses
- Crohn's Disease: Th1/Th17 predominance
📌 Remember: CROHN'S cytokine profile - CD4+ Th1, Raised TNF-α, Overwhelming IFN-γ, High IL-17, NF-κB activation, Severe macrophage response
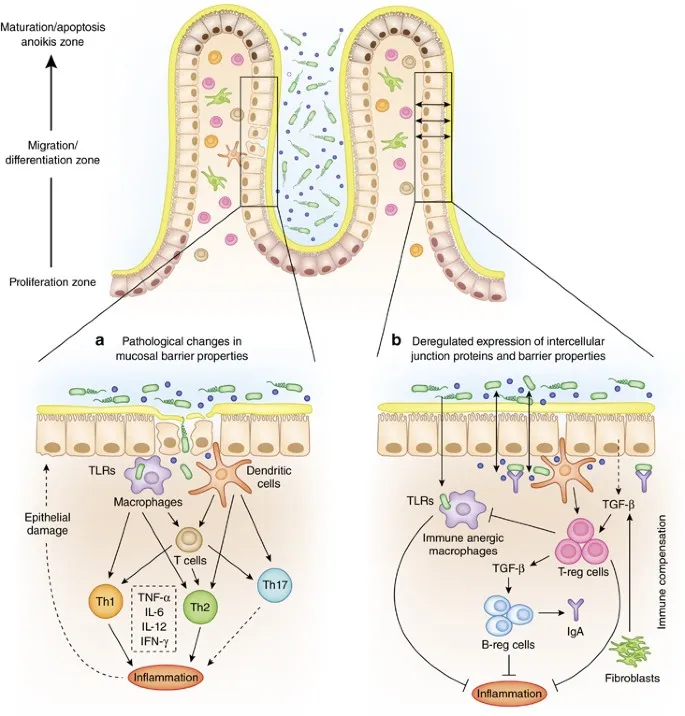
Barrier Function Breakdown: The Leaky Gut Phenomenon
Intestinal barrier dysfunction represents both cause and consequence of IBD inflammation:
| Barrier Component | Normal Function | IBD Dysfunction | Clinical Impact |
|---|---|---|---|
| Tight Junctions | Selective permeability | 50-70% reduction in claudin-1 | Bacterial translocation |
| Mucus Layer | Bacterial exclusion | 80% reduction in MUC2 | Direct epithelial contact |
| Antimicrobial Peptides | Microbial control | 60% reduction in defensins | Dysbiosis development |
| IgA Secretion | Immune exclusion | 40% reduction in secretory IgA | Loss of tolerance |
| Epithelial Turnover | Rapid repair (3-5 days) | Delayed by 2-3x | Persistent ulceration |
Microbiome Dysbiosis: The Microbial Imbalance
The gut microbiome undergoes dramatic shifts in IBD, contributing to disease perpetuation:
- Microbial Diversity Changes
- 50-75% reduction in overall bacterial diversity
- Firmicutes phylum decreased by 60-80%
- Proteobacteria increased by 300-500%
- Loss of beneficial Faecalibacterium prausnitzii (90% reduction)
- Functional Consequences
- Short-chain fatty acid production decreased by 70%
- Butyrate levels reduced to <25% of normal
- Increased sulfate-reducing bacteria (5-10x normal)
- Enhanced adherent-invasive E. coli in CD (40% of patients)
💡 Master This: Butyrate deficiency in IBD creates a vicious cycle - reduced SCFA production impairs colonocyte energy metabolism, weakening barrier function and perpetuating inflammation.
Autophagy Dysfunction: Cellular Housekeeping Gone Wrong
Defective autophagy represents a key mechanism in IBD pathogenesis, particularly in Crohn's disease:
- Autophagy Gene Variants
- ATG16L1 polymorphisms in 60% of CD patients
- IRGM variants increase CD risk 2-fold
- LRRK2 mutations affect 15% of CD cases
- Functional Consequences
- Impaired bacterial clearance from epithelial cells
- Defective antigen presentation by dendritic cells
- Accumulation of damaged organelles and protein aggregates
- Paneth cell dysfunction with reduced antimicrobial peptide secretion
⭐ Clinical Pearl: ATG16L1 variants specifically impair clearance of adherent-invasive E. coli, explaining the increased bacterial burden in CD patients with these mutations.
This molecular machinery creates a self-perpetuating cycle where genetic susceptibility, environmental triggers, and immune dysfunction converge to establish chronic intestinal inflammation. Understanding these mechanisms provides the foundation for targeted therapeutic approaches that can interrupt specific pathways in the inflammatory cascade.
⚙️ The Inflammatory Engine: Decoding IBD's Molecular Machinery
🎯 Pattern Recognition Mastery: The IBD Diagnostic Arsenal
The Clinical Presentation Matrix
IBD presents with overlapping but distinct symptom patterns that guide initial diagnostic suspicion:
- Ulcerative Colitis Presentation
- Bloody diarrhea in 95% of patients at diagnosis
- Tenesmus and urgency in 80-90% of cases
- Left lower quadrant pain predominates
- Fever in 40% during active disease
- Correlates with disease extent and severity
- >38.5°C suggests severe colitis requiring hospitalization
- Crohn's Disease Presentation
- Abdominal pain in 90% of patients (often right lower quadrant)
- Non-bloody diarrhea in 70% of cases
- Weight loss >10% in 60% at diagnosis
- Perianal disease in 30-40% of patients
- Fistulas, abscesses, skin tags
- May precede intestinal symptoms by months to years
📌 Remember: PAIN distinguishes presentations - Perianal disease (CD), Abdominal pain predominant (CD), Intestinal bleeding prominent (UC), Non-bloody diarrhea typical (CD)
Laboratory Pattern Recognition Framework
Laboratory findings provide crucial diagnostic clues and disease activity assessment:
| Laboratory Test | Ulcerative Colitis | Crohn's Disease | Diagnostic Significance |
|---|---|---|---|
| CRP Elevation | 50-60% of patients | 80-90% of patients | CD more inflammatory |
| ESR >30 mm/hr | 70% active disease | 85% active disease | Correlates with activity |
| Fecal Calprotectin | >250 μg/g in 90% | >250 μg/g in 95% | Excludes IBS effectively |
| Albumin <3.5 g/dL | 30% severe disease | 50% active disease | Malabsorption in CD |
| Iron Deficiency | 60-80% of patients | 40-60% of patients | Blood loss vs malabsorption |
Endoscopic Pattern Recognition: The Gold Standard
Endoscopic findings provide definitive diagnostic information and guide treatment decisions:
- Ulcerative Colitis Endoscopic Features
- Continuous inflammation starting from anal verge
- Loss of vascular pattern in 100% of active disease
- Granular, friable mucosa with contact bleeding
- Pseudopolyps in 20-30% of chronic cases
- Represent regenerating mucosa between ulcerated areas
- More common with longer disease duration
- Crohn's Disease Endoscopic Features
- Skip lesions with normal intervening mucosa
- Aphthous ulcers progressing to linear ulcerations
- Cobblestone appearance from deep ulcers and mucosal edema
- Strictures in 30-40% at diagnosis
💡 Master This: The "cobblestone" pattern in CD results from deep longitudinal ulcers intersecting with transverse ulcers, creating islands of edematous mucosa that resemble cobblestones.
Histological Discrimination Framework
Histological examination provides crucial diagnostic confirmation and subtype differentiation:
- Architectural Distortion Patterns
- Crypt branching and shortening in 80% of IBD cases
- Surface epithelial damage more prominent in UC
- Basal plasmacytosis (plasma cells in lower 1/3 of mucosa)
- Paneth cell metaplasia in left colon suggests IBD
- Inflammatory Cell Infiltration
- Chronic inflammation with lymphocytes and plasma cells
- Neutrophil infiltration creating crypt abscesses (UC > CD)
- Granulomas in 60-70% of CD cases
- Non-caseating epithelioid granulomas
- Must exclude infectious causes
⭐ Clinical Pearl: Granulomas in IBD are non-caseating and found in 60-70% of CD cases, but their absence doesn't exclude the diagnosis. They're most commonly found in rectal biopsies even when rectum appears endoscopically normal.
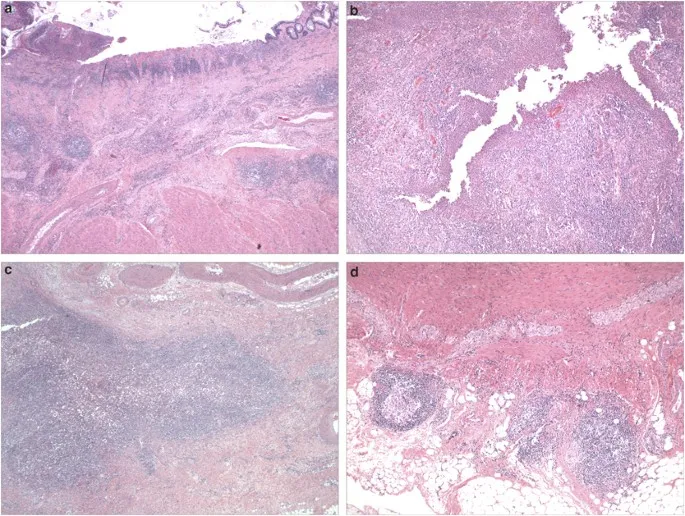
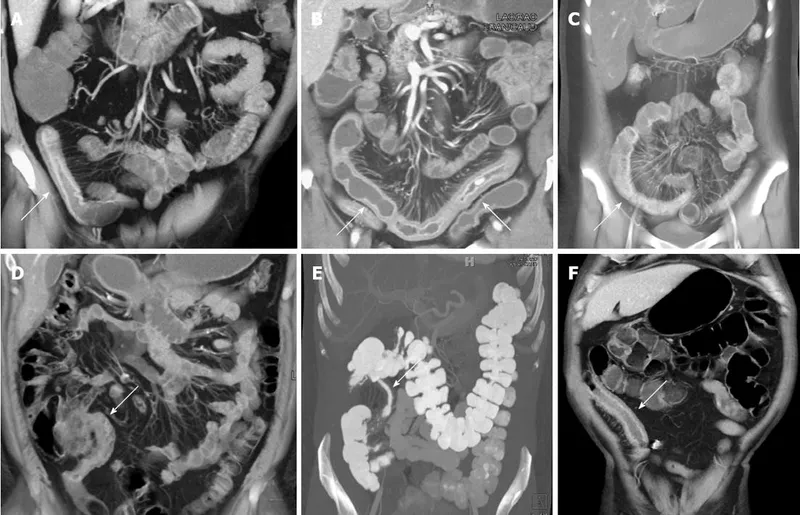
Imaging Pattern Recognition: Beyond the Mucosa
Cross-sectional imaging reveals transmural and extraintestinal disease features:
- CT Enterography Findings
- Bowel wall thickening >3mm suggests active inflammation
- Mural hyperenhancement indicates acute inflammation
- "Comb sign" (prominent mesenteric vessels) in 70% of active CD
- Strictures with prestenotic dilation
- MR Enterography Advantages
- Superior soft tissue contrast for fistula detection
- No radiation exposure for young patients
- Diffusion-weighted imaging detects active inflammation
- T2-weighted sequences identify fluid collections and abscesses
📌 Remember: COMB sign on CT - Comb-like mesenteric vessels, Obvious in active CD, Mesenteric fat stranding, Bowel wall thickening
This systematic approach to pattern recognition enables rapid, accurate IBD diagnosis while avoiding common diagnostic pitfalls. The integration of clinical, laboratory, endoscopic, and imaging patterns creates a comprehensive diagnostic framework that guides appropriate treatment initiation.
🎯 Pattern Recognition Mastery: The IBD Diagnostic Arsenal
🔬 Differential Diagnosis Mastery: Separating IBD from Imposters
Infectious Colitis: The Great Mimicker
Infectious causes represent the most important IBD mimics, requiring systematic exclusion before IBD diagnosis:
| Pathogen | Key Features | Distinguishing Characteristics | Timeline |
|---|---|---|---|
| C. difficile | Pseudomembranous colitis | Antibiotic exposure in 80% | 2-8 weeks post-antibiotics |
| Salmonella | Bloody diarrhea, fever | Food exposure history | 1-3 days incubation |
| Shigella | Dysentery syndrome | Person-to-person spread | 1-3 days incubation |
| Campylobacter | Bloody diarrhea | Poultry exposure common | 2-5 days incubation |
| E. coli O157:H7 | Hemorrhagic colitis | HUS risk in 10% children | 1-8 days incubation |
| Cytomegalovirus | Ulcerative colitis-like | Immunocompromised patients | Weeks to months |
Medication-Induced Colitis: The Iatrogenic Imposters
Drug-induced colitis can perfectly mimic IBD, requiring careful medication history review:
- NSAIDs-Induced Colitis
- Right-sided predominance in 60% of cases
- Diaphragm-like strictures pathognomonic
- Eosinophilia in 30% of patients
- Improvement within 4-8 weeks of discontinuation
- Checkpoint Inhibitor Colitis
- Lower GI toxicity in 10-15% of patients on anti-PD-1/PD-L1
- Onset typically 6-12 weeks after initiation
- Steroid-responsive in 80% of cases
- May require anti-TNF therapy for severe cases
- Mycophenolate Colitis
- Dose-dependent effect (>2g/day higher risk)
- Histological mimicry of IBD with chronic inflammation
- Reversible with dose reduction or discontinuation
💡 Master This: NSAID-induced colitis characteristically affects the cecum and ascending colon, opposite to UC's typical left-sided distribution, and creates diaphragm-like strictures not seen in IBD.
Ischemic Colitis: The Vascular Impostor
Ischemic colitis predominantly affects older patients but can occur in younger individuals with risk factors:
- Classic Presentation
- Sudden onset abdominal pain in >50 years old
- Left lower quadrant pain with bloody diarrhea
- Watershed areas most affected (splenic flexure, rectosigmoid)
- Thumbprinting on imaging from submucosal edema
- Risk Factors
- Age >65 years in 90% of cases
- Cardiovascular disease in 70% of patients
- Medications: oral contraceptives, vasoconstrictors
- Hypotension or dehydration
- Distinguishing Features
- Acute onset vs gradual IBD progression
- Segmental distribution following vascular territories
- Resolution in 80% within 2-4 weeks
- Lack of rectal involvement (unlike UC)
⭐ Clinical Pearl: Ischemic colitis spares the rectum in 95% of cases due to dual blood supply (superior and middle hemorrhoidal arteries), while UC involves the rectum in 100% of cases.
Microscopic Colitis: The Histological Deceiver
Microscopic colitis presents with chronic diarrhea but normal endoscopic appearance:
- Collagenous Colitis
- Subepithelial collagen band >10 μm thick
- Female predominance (9:1 ratio)
- Mean age 65 years at diagnosis
- Associated medications: NSAIDs, PPIs, SSRIs
- Lymphocytic Colitis
- >20 lymphocytes per 100 epithelial cells
- Equal gender distribution
- Younger age of onset (mean 45 years)
- Celiac disease association in 20% of cases
| Feature | Microscopic Colitis | IBD | Clinical Significance |
|---|---|---|---|
| Endoscopic Appearance | Normal mucosa | Visible inflammation | Key distinguishing feature |
| Diarrhea Character | Watery, >400mL/day | Often bloody in UC | Volume and consistency differ |
| Histology | Specific patterns | Chronic inflammation | Diagnostic gold standard |
| Treatment Response | Budesonide 90% response | Variable IBD therapies | Therapeutic trial diagnostic |
| Malignancy Risk | No increased risk | 2-3x increased risk | Long-term implications |
Neoplastic Conditions: The Malignant Masqueraders
Certain malignancies can present with IBD-like symptoms and endoscopic findings:
- Colorectal Adenocarcinoma
- Apple-core lesions on imaging
- Asymmetric wall thickening on CT
- Older age (mean 70 years) vs IBD (mean 30 years)
- Constitutional symptoms more prominent
- Lymphoma (Primary Intestinal)
- MALT lymphoma can mimic CD
- Mantle cell lymphoma causes multiple polyps
- B-symptoms in 40% of cases
- Monoclonal B-cell population on flow cytometry
⭐ Clinical Pearl: New-onset IBD-like symptoms in patients >60 years should prompt aggressive evaluation for malignancy, as primary IBD diagnosis is uncommon in this age group (<10% of new diagnoses).
This systematic approach to differential diagnosis ensures accurate IBD identification while avoiding misdiagnosis of treatable conditions that mimic inflammatory bowel disease. The key lies in maintaining high clinical suspicion and systematically excluding alternative diagnoses through appropriate testing.
🔬 Differential Diagnosis Mastery: Separating IBD from Imposters
⚕️ Treatment Strategy Command Center: Mastering IBD Therapeutic Algorithms
Treatment Goals: The Modern IBD Paradigm
Contemporary IBD management targets multiple endpoints beyond symptom relief:
- Clinical Remission Targets
- Symptom resolution: Normal bowel frequency, no bleeding
- Biomarker normalization: CRP <3 mg/L, fecal calprotectin <150 μg/g
- Quality of life restoration: IBDQ score >170
- Endoscopic Healing Goals
- Mayo endoscopic score ≤1 for UC
- SES-CD score reduction >50% for Crohn's disease
- Mucosal healing achieved in 40-60% with biologics
- Histological Remission
- Absence of neutrophils in epithelium and crypts
- Reduced chronic inflammation to near-normal levels
- Associated with sustained remission in 80% of patients
⭐ Clinical Pearl: Mucosal healing reduces hospitalization risk by 75% and surgery risk by 50% over 5 years, making it a crucial treatment target beyond symptom control.
Ulcerative Colitis Treatment Hierarchy
UC treatment follows a systematic approach based on disease extent and severity:
| Disease Severity | First-Line Therapy | Second-Line Options | Biologic Indications |
|---|---|---|---|
| Mild Proctitis | Mesalamine suppositories 1g daily | Topical steroids | Refractory to conventional |
| Mild Left-sided | Mesalamine 2.4-4.8g daily + topical | Oral steroids 40mg | 2+ steroid courses/year |
| Moderate Extensive | Mesalamine 4.8g + prednisolone 40mg | Immunomodulators | Steroid-dependent |
| Severe Colitis | IV steroids 1mg/kg (max 80mg) | Rescue therapy (infliximab/cyclosporine) | Day 3 if no improvement |
| Fulminant | IV steroids + rescue therapy | Emergency colectomy | Immediate consideration |
Crohn's Disease Treatment Algorithms
CD management requires consideration of disease location, behavior, and complications:
- Mild-Moderate Ileocolonic CD
- Budesonide 9mg daily for 8-12 weeks
- Mesalamine 4g daily (limited efficacy vs UC)
- Metronidazole 400mg TID for perianal disease
- Azathioprine 2-2.5mg/kg for maintenance
- Moderate-Severe CD
- Prednisolone 40-60mg daily for induction
- Early biologic therapy (anti-TNF, vedolizumab, ustekinumab)
- Combination therapy (biologic + immunomodulator)
- Therapeutic drug monitoring to optimize levels
- Complicated CD (Stricturing/Penetrating)
- Surgical evaluation for mechanical obstruction
- Anti-TNF therapy for inflammatory strictures
- Drainage of abscesses before biologic initiation
- Postoperative prophylaxis with biologics
💡 Master This: Combination therapy (anti-TNF + azathioprine) increases mucosal healing rates from 30% to 60% and reduces immunogenicity from 13% to 3%, but requires careful monitoring for lymphoma risk.
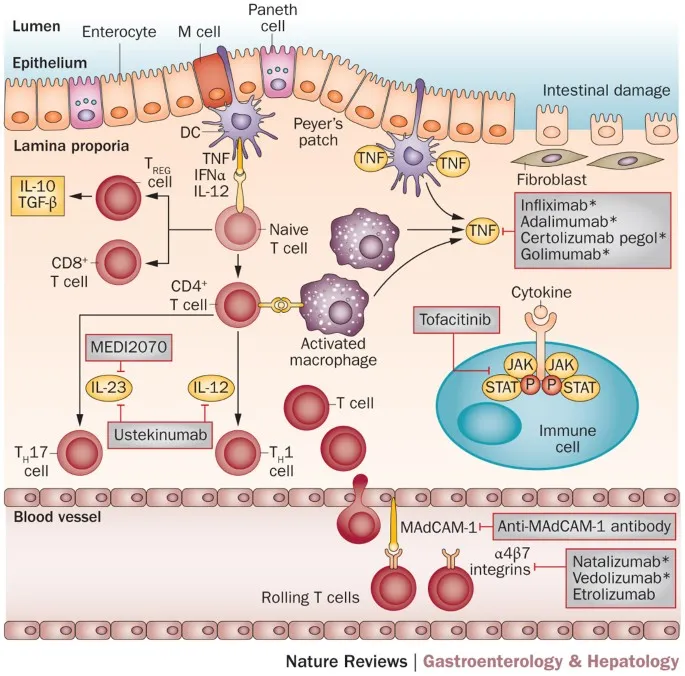
Biologic Therapy Selection Matrix
Choosing appropriate biologic therapy requires understanding mechanisms, efficacy, and safety profiles:
| Biologic Class | Mechanism | UC Efficacy | CD Efficacy | Key Advantages | Major Risks |
|---|---|---|---|---|---|
| Anti-TNF (Infliximab, Adalimumab) | TNF-α blockade | 60-70% response | 65-75% response | Rapid onset, fistula healing | Infection, lymphoma |
| Anti-Integrin (Vedolizumab) | Gut-selective adhesion | 50-60% response | 45-55% response | Gut-specific, low infection risk | Slower onset (12-16 weeks) |
| Anti-IL12/23 (Ustekinumab) | Cytokine blockade | 60% response | 55-65% response | Low immunogenicity | Limited long-term data |
| JAK Inhibitors (Tofacitinib) | Intracellular signaling | 65% response | Not approved | Oral administration | Thrombosis, infection |
| Anti-IL23 (Risankizumab) | IL-23p19 blockade | Under study | 70% response | High specificity | Limited experience |
Monitoring and Optimization Strategies
Successful IBD management requires systematic monitoring and treatment optimization:
- Therapeutic Drug Monitoring
- Anti-TNF trough levels: Infliximab >3-7 μg/mL, Adalimumab >5-8 μg/mL
- Anti-drug antibodies: Check if low levels with poor response
- Dose optimization: Increase dose or frequency based on levels
- Cost-effectiveness: Reduces treatment failures by 40%
- Disease Activity Monitoring
- Fecal calprotectin every 3-6 months
- CRP and CBC every 3 months on immunosuppression
- Endoscopy every 1-2 years for surveillance
- Cross-sectional imaging annually for CD complications
⭐ Clinical Pearl: Proactive therapeutic drug monitoring reduces treatment failure rates from 40% to 15% and decreases healthcare costs by $8,000-12,000 per patient annually through optimized dosing strategies.
This comprehensive treatment framework enables personalized IBD management that achieves deep remission while minimizing adverse effects. The key lies in early aggressive therapy, objective monitoring, and systematic treatment optimization based on measurable outcomes.
⚕️ Treatment Strategy Command Center: Mastering IBD Therapeutic Algorithms
🌐 IBD Complications Network: Navigating the Systemic Impact
Intestinal Complications: The Local Battlefield
IBD creates specific patterns of bowel complications that differ between UC and CD:
- Ulcerative Colitis Complications
- Toxic megacolon in 5-10% of severe attacks
- Colon diameter >6cm on plain radiograph
- Mortality 15-20% without prompt intervention
- Requires immediate surgical consultation
- Perforation risk 2-3% during severe flares
- Massive hemorrhage requiring transfusion in 5%
- Colorectal cancer risk 2-3x general population
- Toxic megacolon in 5-10% of severe attacks
- Crohn's Disease Complications
- Strictures in 50-70% over 20 years
- Inflammatory vs fibrotic (treatment differs)
- Balloon dilation effective for short strictures <4cm
- Fistulas in 30-50% of patients
- Perianal fistulas most common (90% of fistulizing disease)
- Enteroenteric and enterovesical fistulas
- Abscesses in 20-30% of patients
- Require drainage before biologic therapy initiation
- Strictures in 50-70% over 20 years
📌 Remember: COMPLICATIONS of CD - Cancer risk, Obstruction from strictures, Malnutrition, Perianal disease, Liver involvement, Infection/abscesses, Colorectal cancer, Arthritis, Thromboembolism, Iron deficiency, Osteoporosis, Nephrolithiasis, Skin manifestations
Extraintestinal Manifestations: The Systemic Network
EIMs occur in 25-40% of IBD patients and often parallel intestinal disease activity:
| System | Manifestation | IBD Association | Prevalence | Treatment Response |
|---|---|---|---|---|
| Musculoskeletal | Peripheral arthritis | Activity-related | 20-25% | Parallels gut inflammation |
| Musculoskeletal | Ankylosing spondylitis | Independent | 5-10% | Anti-TNF preferred |
| Dermatologic | Erythema nodosum | Activity-related | 10-15% | Improves with IBD treatment |
| Dermatologic | Pyoderma gangrenosum | Independent | 2-5% | Requires specific therapy |
| Ophthalmologic | Episcleritis | Activity-related | 5-10% | Topical steroids |
| Ophthalmologic | Uveitis | Independent | 2-5% | Urgent ophthalmology |
| Hepatobiliary | Primary sclerosing cholangitis | Independent | 5% (UC: 70%) | No proven therapy |
Musculoskeletal Manifestations: The Joint Connection
Arthritis represents the most common EIM, with distinct patterns requiring different management approaches:
- Type 1 Peripheral Arthritis
- Large joint oligoarthritis (<5 joints)
- Parallels intestinal disease activity
- Knees, ankles, wrists most commonly affected
- Self-limiting episodes lasting weeks to months
- Responds to IBD treatment
- Type 2 Peripheral Arthritis
- Small joint polyarthritis (≥5 joints)
- Independent of intestinal activity
- Metacarpophalangeal and PIP joints
- Chronic, persistent course
- May require specific arthritis therapy
- Axial Arthropathy
- Ankylosing spondylitis in 5-10% of IBD patients
- HLA-B27 positive in 70% of cases
- Independent of intestinal disease activity
- Anti-TNF therapy most effective treatment
💡 Master This: Peripheral arthritis Type 1 affects large joints, parallels gut inflammation, and improves with IBD treatment, while Type 2 affects small joints, runs independent course, and may require separate therapy.
Dermatologic Manifestations: Skin as Disease Mirror
Skin manifestations provide visible clues to IBD activity and complications:
- Erythema Nodosum
- Tender, raised nodules on anterior shins
- Parallels intestinal disease activity in 80%
- More common in CD (15%) than UC (10%)
- Resolves with IBD treatment
- Pyoderma Gangrenosum
- Painful ulcerative lesions with undermined borders
- Independent of intestinal activity
- Pathergy phenomenon (trauma triggers lesions)
- Requires aggressive immunosuppression
- Associated with UC in 70% of IBD cases
⭐ Clinical Pearl: Pyoderma gangrenosum shows pathergy - minor trauma triggers new lesions. Avoid surgical debridement as it worsens the condition. Treatment requires systemic immunosuppression with steroids or anti-TNF therapy.
Hepatobiliary Complications: The Liver-Gut Axis
Liver involvement in IBD ranges from mild enzyme elevation to life-threatening complications:
- Primary Sclerosing Cholangitis (PSC)
- Progressive bile duct fibrosis and stricturing
- "Beading" appearance on MRCP/ERCP
- Cholangiocarcinoma risk 10-15% lifetime
- Liver transplantation ultimate treatment
- IBD may worsen after liver transplant
- Drug-Induced Hepatotoxicity
- Azathioprine hepatotoxicity in 3-5% of patients
- Methotrexate fibrosis with long-term use
- Anti-TNF-induced autoimmune hepatitis (rare)
- Regular monitoring with LFTs every 3 months
Thromboembolic Complications: The Hypercoagulable State
IBD creates a 2-3 fold increased risk of venous thromboembolism through multiple mechanisms:
- Pathophysiology of Hypercoagulability
- Elevated factor VIII and fibrinogen during inflammation
- Decreased protein C and S levels
- Platelet activation and increased aggregation
- Immobilization during severe flares
- Clinical Manifestations
- Deep vein thrombosis in 6-8% of hospitalized patients
- Pulmonary embolism in 1-2% of severe cases
- Portal vein thrombosis in 1% of patients
- Cerebral venous thrombosis (rare but serious)
⭐ Clinical Pearl: Active IBD increases VTE risk 3-fold, and hospitalized IBD patients should receive prophylactic anticoagulation unless contraindicated by active bleeding.
This comprehensive understanding of IBD's systemic impact enables clinicians to anticipate, recognize, and manage the complex web of complications that extend far beyond the gastrointestinal tract, ultimately improving patient outcomes and quality of life.
🌐 IBD Complications Network: Navigating the Systemic Impact
🎯 IBD Mastery Arsenal: Your Clinical Command Center
Essential Diagnostic Thresholds: The Numbers That Matter
Critical values that guide IBD diagnosis and management decisions:
- Laboratory Cutoffs
- Fecal calprotectin >250 μg/g: 95% sensitivity for IBD vs IBS
- CRP >3 mg/L: Suggests active inflammation
- ESR >30 mm/hr: Correlates with disease activity
- Albumin <3.5 g/dL: Indicates severe disease/malnutrition
- Hemoglobin <10 g/dL: Significant anemia requiring investigation
- Endoscopic Scoring
- Mayo score ≤1: Endoscopic remission in UC
- SES-CD reduction >50%: Significant improvement in CD
- Rutgeerts score ≥i2: Postoperative CD recurrence risk
- Imaging Thresholds
- Bowel wall thickness >3mm: Suggests active inflammation
- Colon diameter >6cm: Toxic megacolon requiring surgery
📌 Remember: CRITICAL numbers - Calprotectin >250, Remission Mayo ≤1, Inflammation CRP >3, Toxic colon >6cm, Iron deficiency Hgb <10, CD activity SES-CD, Albumin severe <3.5, Lab monitoring q3 months
Rapid Treatment Decision Matrix
Immediate treatment selection based on disease severity and patient factors:
| Clinical Scenario | First-Line Treatment | Timeline | Monitoring |
|---|---|---|---|
| Mild UC proctitis | Mesalamine suppository 1g daily | 4-8 weeks | Symptoms, sigmoidoscopy |
| Moderate UC | Mesalamine 4.8g + prednisolone 40mg | 8-12 weeks | Weekly labs, taper steroids |
| Severe UC | IV methylprednisolone 1mg/kg | Day 3 assessment | Daily labs, rescue therapy |
| Mild-moderate CD | Budesonide 9mg daily | 8-12 weeks | Symptoms, CRP, calprotectin |
| Moderate-severe CD | Anti-TNF + azathioprine | 12-16 weeks | TDM, CBC, LFTs |
| Perianal CD | Anti-TNF + metronidazole | 16-24 weeks | MRI pelvis, examination |
Biologic Optimization Framework
Systematic approach to biologic therapy selection and optimization:
- Anti-TNF Selection Criteria
- First-line for extraintestinal manifestations
- Preferred for perianal Crohn's disease
- Avoid in patients with heart failure (NYHA III-IV)
- Screen for tuberculosis, hepatitis B/C
- Therapeutic Drug Monitoring Targets
- Infliximab trough: 3-7 μg/mL (higher for severe disease)
- Adalimumab trough: 5-8 μg/mL
- Anti-drug antibodies: Check if low levels + poor response
- Optimize dosing: Increase dose/frequency before switching
💡 Master This: Combination therapy (anti-TNF + immunomodulator) doubles mucosal healing rates and reduces immunogenicity by 75%, but requires careful lymphoma risk assessment in young males.
Emergency Recognition Patterns
Critical presentations requiring immediate intervention:
- Toxic Megacolon Signs
- Colon diameter >6cm on plain radiograph
- Systemic toxicity: fever, tachycardia, leukocytosis
- Abdominal distension with decreased bowel sounds
- Immediate surgical consultation required
- Severe Flare Criteria
- >6 bloody stools/day with systemic symptoms
- Pulse >90 bpm, temperature >37.8°C
- Hemoglobin <10.5 g/dL, ESR >30 mm/hr
- Hospitalization and IV steroids indicated
- Perforation Risk Factors
- Deep ulcerations on endoscopy
- Rapid symptom progression
- Immunosuppression masking symptoms
- High index of suspicion in severe disease
⚠️ Warning: Never perform colonoscopy in suspected toxic megacolon - risk of perforation >50%. Use flexible sigmoidoscopy or CT for assessment.
Monitoring Schedule Mastery
Systematic surveillance protocols for optimal IBD management:
- Active Disease Monitoring
- Symptoms: Weekly during flares
- Laboratory: CBC, CRP every 2-4 weeks
- Calprotectin: Every 4-8 weeks
- Endoscopy: 3-6 months after treatment change
- Stable Disease Surveillance
- Clinical assessment: Every 3-6 months
- Laboratory: CBC, CRP, LFTs every 3-6 months
- Calprotectin: Every 6-12 months
- Colonoscopy: Every 1-2 years for cancer surveillance
- Immunosuppression Monitoring
- Azathioprine: CBC, LFTs every 3 months
- Methotrexate: CBC, LFTs every 3 months + folate
- Anti-TNF: CBC every 3 months, TB screening annually
⭐ Clinical Pearl: Fecal calprotectin <150 μg/g predicts endoscopic remission with 85% accuracy, allowing non-invasive monitoring and reduced endoscopy frequency.
Surgical Timing Decision Points
Critical indicators for surgical consultation and intervention:
- Ulcerative Colitis Surgery Indications
- Medically refractory disease despite optimal therapy
- Toxic megacolon or perforation
- Massive hemorrhage requiring >6 units transfusion
- High-grade dysplasia or colorectal cancer
- Growth failure in pediatric patients
- Crohn's Disease Surgery Indications
- Mechanical obstruction from fibrotic strictures
- Abscesses not amenable to percutaneous drainage
- Complex perianal fistulas refractory to medical therapy
- Perforation or free air
- Failure to thrive despite optimal nutrition
This clinical arsenal provides the essential tools for mastering IBD diagnosis, treatment, and monitoring. Regular reference to these frameworks enables confident clinical decision-making and optimal patient outcomes in the complex landscape of inflammatory bowel disease management.
🎯 IBD Mastery Arsenal: Your Clinical Command Center
Practice Questions: IBD
Test your understanding with these related questions
A 33-year-old woman with Crohn’s disease colitis presents to her physician after 2 days of photophobia and blurred vision. She has had no similar episodes in the past. She has no abdominal pain or diarrhea and takes mesalazine, azathioprine, and prednisone as maintenance therapy. Her vital signs are within normal range. Examination of the eyes shows conjunctival injection. The physical examination is otherwise normal. Slit-lamp examination by an ophthalmologist shows evidence of inflammation in the anterior chamber. Which of the following is the most appropriate modification to this patient’s medication at this time?













