Thalassemias US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Thalassemias. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Thalassemias US Medical PG Question 1: A 3-year-old boy is brought to the physician because of a 1-week history of yellowish discoloration of his eyes and skin. He has had generalized fatigue and mild shortness of breath for the past month. Three weeks ago, he was treated for a urinary tract infection with antibiotics. His father underwent a splenectomy during childhood. Examination shows pale conjunctivae and jaundice. The abdomen is soft and nontender; there is nontender splenomegaly. Laboratory studies show:
Hemoglobin 9.1 g/dL
Mean corpuscular volume 89 μm3
Mean corpuscular hemoglobin 32 pg/cell
Mean corpuscular hemoglobin concentration 37.8% Hb/cell
Leukocyte count 7800/mm3
Platelet count 245,000/mm3
Red cell distribution width 22.8% (N=13%–15%)
Serum
Bilirubin
Total 13.8 mg/dL
Direct 1.9 mg/dL
Lactate dehydrogenase 450 U/L
Which of the following is the most likely pathophysiology of these findings?
- A. Deficient glucose-6 phosphate dehydrogenase
- B. Decreased synthesis of alpha chains of hemoglobin
- C. Increased hemoglobin S
- D. Decreased spectrin in the RBC membrane (Correct Answer)
- E. Deficiency of pyruvate kinase
Thalassemias Explanation: ***Decreased spectrin in the RBC membrane***
- This presentation is highly suggestive of **hereditary spherocytosis**, characterized by **defects in red blood cell membrane proteins** like **spectrin**, ankyrin, or band 3 protein.
- The patient's features—**jaundice**, **anemia**, **splenomegaly**, elevated **indirect bilirubin**, increased **LDH**, high **MCHC**, and a family history of splenectomy (often a treatment for hereditary spherocytosis)—point to this diagnosis.
*Deficient glucose-6 phosphate dehydrogenase*
- **G6PD deficiency** typically presents with **hemolytic anemia** following exposure to **oxidative stressors** (e.g., certain drugs, fava beans, infections).
- While an infection might trigger hemolysis, the chronic nature of the symptoms, **splenomegaly**, and high MCHC are less typical for G6PD deficiency.
*Decreased synthesis of alpha chains of hemoglobin*
- This describes **alpha thalassemia**, which would present with **microcytic hypochromic anemia** (low MCV, low MCH) rather than the normal MCV and high MCHC seen here.
- While chronic hemolysis can occur in severe forms, the specific lab values do not align with thalassemia.
*Increased hemoglobin S*
- **Sickle cell anemia** involves **hemoglobin S**, leading to sickling of red blood cells under hypoxic conditions.
- Patients typically experience **vaso-occlusive crises** and different RBC morphology (e.g., sickle cells, target cells) and usually a normocytic or macrocytic anemia, which is not fully consistent with the high MCHC seen here in isolation.
*Deficiency of pyruvate kinase*
- **Pyruvate kinase deficiency** causes **hemolytic anemia** due to impaired glycolysis, leading to insufficient ATP production in RBCs.
- While it presents with chronic hemolysis, **splenomegaly**, and jaundice, it typically does not cause the characteristic **high MCHC** seen in hereditary spherocytosis, nor does it typically present with a positive family history of splenectomy in father.
Thalassemias US Medical PG Question 2: A 44-year-old male immigrant presents to his primary care physician for a new patient visit. The patient reports chronic fatigue but states that he otherwise feels well. His past medical history is not known, and he is not currently taking any medications. The patient admits to drinking 7 alcoholic beverages per day and smoking 1 pack of cigarettes per day. His temperature is 99.4°F (37.4°C), blood pressure is 157/98 mmHg, pulse is 99/min, respirations are 18/min, and oxygen saturation is 100% on room air. Physical exam demonstrates mild pallor but is otherwise not remarkable. Laboratory studies are ordered as seen below.
Hemoglobin: 9 g/dL
Hematocrit: 33%
Leukocyte count: 6,500/mm^3 with normal differential
Platelet count: 190,000/mm^3
Mean corpuscular volume (MCV): 60 femtoliters
Free iron: 272 mcg/dL
Total iron binding capacity (TIBC): 175 mcg/dL
Ferritin: 526 ng/mL
Reticulocyte count: 2.8%
Which of the following is the most likely diagnosis?
- A. Folate deficiency
- B. Beta-thalassemia (Correct Answer)
- C. Iron deficiency
- D. B12 deficiency
- E. Hemolytic anemia
Thalassemias Explanation: ***Beta-thalassemia***
- The patient presents with **microcytic anemia** (MCV 60 fL) and **elevated ferritin**, **high free iron**, and **low TIBC**, which are characteristic of thalassemia due to ineffective erythropoiesis and iron overload.
- A **reticulocyte count of 2.8%** (elevated for the degree of anemia) indicates the bone marrow is attempting to compensate, consistent with a hemolytic process like thalassemia.
*Folate deficiency*
- Folate deficiency typically causes **macrocytic anemia** (elevated MCV), which is not seen here; the patient has microcytic anemia.
- Alcohol abuse can cause folate deficiency, but the lab values for iron studies and MCV are inconsistent with this diagnosis.
*Iron deficiency*
- Iron deficiency anemia would present with **low ferritin**, **low free iron**, and **high TIBC**, which are opposite to the patient's lab results.
- Although the patient has microcytic anemia, the iron study profile rules out iron deficiency.
*B12 deficiency*
- Vitamin B12 deficiency also causes **macrocytic anemia** (elevated MCV), often with neurological symptoms, neither of which are observed in this patient.
- The patient's microcytic anemia and iron study results contradict a diagnosis of B12 deficiency.
*Hemolytic anemia*
- While beta-thalassemia is a form of hemolytic anemia, the term "hemolytic anemia" alone is too broad and does not specify the underlying cause, especially with the provided iron studies and MCV.
- Other common causes of hemolytic anemia, like autoimmune hemolytic anemia or G6PD deficiency, would require different diagnostic presentations or specific tests not consistent with the given lab values.
Thalassemias US Medical PG Question 3: A 13-month-old girl is brought to the pediatric clinic by her mother due to progressive abdominal distension, poor feeding, and failure to thrive. The perinatal history was uneventful. The family emigrated from Sudan 8 years ago. The vital signs include: temperature 36.8°C (98.2°F), blood pressure 100/55 mm Hg, and pulse 99/min. The physical examination shows conjunctival pallor, hepatosplenomegaly, and parietal and frontal bossing of the skull. The laboratory test results are as follows:
Hemoglobin 8.7 g/dL
Mean corpuscular volume 62 μm3
Red cell distribution width 12.2% (normal value is 11.5–14.5%)
Reticulocyte count 2.1 %
Leucocyte count 10,200/mm3
Platelet count 392,000/mm3
The peripheral blood smear shows microcytic red cells, target cells, and many nucleated red cells. Which of the following is the most likely diagnosis?
- A. Sickle cell disease
- B. Beta-thalassemia major (Correct Answer)
- C. Congenital dyserythropoietic anaemia
- D. Alpha-thalassemia major
- E. Glucose-6-phosphate dehydrogenase deficiency
Thalassemias Explanation: ***Beta-thalassemia major***
- The presentation of severe **microcytic anemia**, **hepatosplenomegaly**, **bone deformities** (**frontal and parietal bossing**), and **failure to thrive** in an infant from an endemic area (Sudan) is classic for **beta-thalassemia major** (Cooley's anemia).
- The reticulocyte count of 2.1% is **inadequate for the degree of anemia**, reflecting **ineffective erythropoiesis**, a hallmark of thalassemia, while the peripheral smear findings of **target cells** and **nucleated red cells** are also characteristic.
*Sickle cell disease*
- While sickle cell disease is also common in individuals of African descent, it typically presents with **vaso-occlusive crises**, **dactylitis**, or **splenic sequestration crises**, rather than the prominent bone deformities and extreme microcytic anemia seen here.
- The peripheral smear would show **sickle cells**, which are not mentioned.
*Congenital dyserythropoietic anaemia*
- This is a rare group of genetic disorders characterized by **ineffective erythropoiesis** and **morphological abnormalities** of erythroblasts in the bone marrow.
- Although it can cause anemia and hepatosplenomegaly, the distinct skeletal changes like frontal bossing are less typical, and the peripheral smear findings usually include **anisopoikilocytosis** rather than prominent target cells and nucleated red cells in the absence of severe marrow stress.
*Alpha-thalassemia major*
- This condition, also known as **hydrops fetalis**, is usually **lethal in utero** or shortly after birth due to severe anemia and massive edema.
- It is incompatible with a 13-month-old surviving infant.
*Glucose-6-phosphate dehydrogenase deficiency*
- This condition causes **hemolytic anemia** typically triggered by **oxidative stress** (e.g., certain foods like fava beans, medications, or infections).
- It presents with **acute hemolytic episodes** and generally leads to **normocytic or macrocytic anemia** with Heinz bodies, not the chronic microcytic anemia with skeletal deformities seen here.
Thalassemias US Medical PG Question 4: A 4-year-old girl is brought to the doctor by her mother with the complaint of hearing loss, which her mother noticed a few days ago when the girl stopped responding to her name. The mother is anxious and says, “I want my child to get better even if it requires admission to the hospital.” Her family moved to a 70-year-old family home in Flint, Michigan, in 2012. The girl has a known history of beta-thalassemia trait. She has never been treated for hookworm, as her mother states that they maintain “good hygiene standards” at home. On examination, the girl currently uses only 2-syllable words. She is in the 70th percentile for height and 50th for weight. A Rinne test reveals that the girl’s air conduction is greater than her bone conduction in both ears. She does not respond when the doctor calls her name, except when he is within her line of sight. Her lab parameters are:
Hemoglobin 9.9 gm%
Mean corpuscular volume 80 fl
Red blood cell distribution width (RDW) 15.9%
Serum ferritin 150 ng/ml
Total iron binding capacity 320 µg/dL
A peripheral smear shows a microcytic hypochromic anemia with basophilic stippling and a few target cells. Which of the following is the next best step in the management of this patient?
- A. Multivitamins with iron supplementation
- B. Chelation therapy if the blood lead level is more than 25 µg/dL
- C. Blood transfusion
- D. Treatment for hookworm
- E. Remove and prevent the child from exposure to the source of lead (Correct Answer)
Thalassemias Explanation: ***Remove and prevent the child from exposure to the source of lead***
- The patient's presentation with **hearing loss**, developmental delay (only using two-syllable words), and anemia with **basophilic stippling** and **microcytic hypochromic anemia** strongly suggests **lead poisoning**. The history of living in an old home in Flint, Michigan further supports this, as older homes and the Flint water crisis are known sources of lead exposure.
- The most crucial step in managing lead poisoning is to **eliminate the source of lead exposure** to prevent further accumulation and toxicity.
*Multivitamins with iron supplementation*
- While the patient has **anemia**, the peripheral smear findings of **basophilic stippling** and family history of residence in Flint, Michigan point towards lead intoxication as the underlying cause, not simple iron deficiency.
- Iron supplementation alone would not address the **neurotoxic effects** of lead or the root cause of the anemia.
*Chelation therapy if the blood lead level is more than 25 µg/dL*
- **Chelation therapy** is indicated for significantly elevated blood lead levels, typically above 45 µg/dL, and sometimes considered between 25-44 µg/dL depending on symptoms and clinical context.
- However, the **most important initial step** is still to remove the source of lead exposure, as chelation without source removal is less effective and may lead to re-exposure and continued toxicity.
*Blood transfusion*
- The patient has a **hemoglobin of 9.9 gm%**, which, while anemic, is not critically low enough to warrant immediate blood transfusion unless there are signs of severe symptomatic anemia or hemodynamic instability.
- Addressing the **underlying cause (lead poisoning)** and providing supportive care is prioritized over transfusion for this level of anemia.
*Treatment for hookworm*
- Although hookworm can cause **iron deficiency anemia**, the patient's normal **serum ferritin (150 ng/ml)** and absence of other signs like eosinophilia make hookworm an unlikely cause of her anemia.
- The presence of **basophilic stippling** and the history of potential lead exposure strongly point away from hookworm as the primary diagnosis.
Thalassemias US Medical PG Question 5: A 3080-g (6-lb 13-oz) male newborn is delivered at term to a 27-year-old woman, gravida 2, para 1. Pregnancy was uncomplicated. He appears pale. His temperature is 36.8°C (98.2°F), pulse is 167/min, and respirations are 56/min. Examination shows jaundice of the skin and conjunctivae. The liver is palpated 2–3 cm below the right costal margin, and the spleen is palpated 1–2 cm below the left costal margin. The lungs are clear to auscultation. No murmurs are heard. His hemoglobin concentration is 10.6 g/dL and mean corpuscular volume is 73 μm3. Hemoglobin DNA testing shows 3 missing alleles. Which of the following laboratory findings is most likely present in this patient?
- A. Increased hemoglobin Barts concentration (Correct Answer)
- B. Elevated HbA2
- C. Low reticulocyte count
- D. Low serum ferritin
- E. Elevated HbF
Thalassemias Explanation: **Increased hemoglobin Barts concentration**
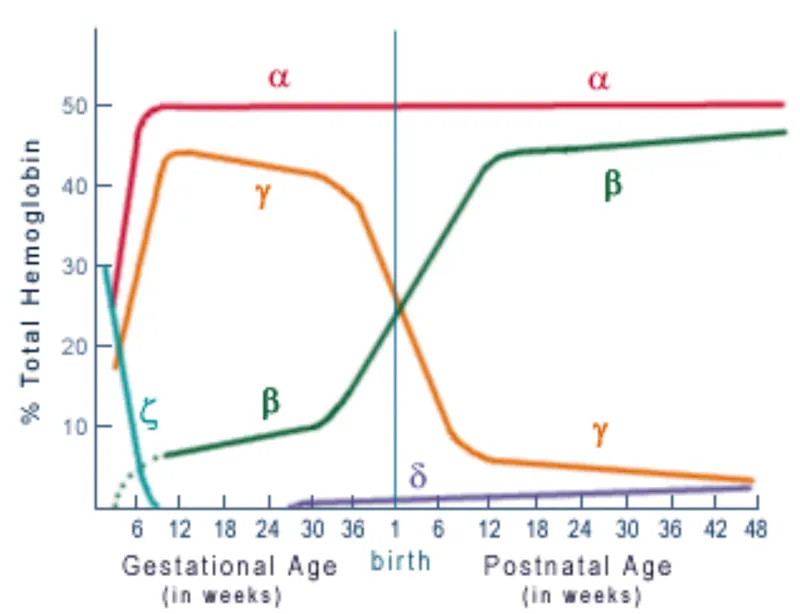
- The clinical presentation with **pallor**, **jaundice**, hepatosplenomegaly, and **microcytic anemia** (Hb 10.6 g/dL, MCV 73 fL) in a newborn, along with **3 missing alpha globin alleles** (Genomic DNA testing shows 3 missing alleles for the alpha-globin gene indicating alpha-thalassemia intermedia), is highly suggestive of **HbH disease**, a form of alpha-thalassemia.
- **Hemoglobin Barts** (γ4) is typically seen in fetuses and newborns with severe alpha-thalassemia, particularly **hydrops fetalis (4-gene deletion)**, but can also be present in HbH disease (3-gene deletion) due to the presence of excess gamma chains that cannot form HbF and instead form gamma tetramers (Hb Barts).
*Elevated HbA2*
- **Elevated HbA2** is characteristic of **beta-thalassemia minor**, not alpha-thalassemia, as increased quantities of delta chains (δ) compensate for reduced beta globin production.
- In alpha-thalassemia, the primary issue is reduced alpha globin chains, and HbA2 levels are typically normal or even low.
*Low reticulocyte count*
- A low reticulocyte count would indicate **hypoproliferative anemia**, where the bone marrow is not producing enough red blood cells.
- In hemolytic conditions like **alpha-thalassemia**, the bone marrow attempts to compensate for red blood cell destruction, leading to an **elevated reticulocyte count** due to increased erythropoiesis.
*Low serum ferritin*
- Low serum ferritin is a hallmark of **iron deficiency anemia**.
- While alpha-thalassemia is a microcytic anemia, the underlying pathology is a **globin chain imbalance** rather than iron deficiency; iron stores are typically normal or even elevated due to ineffective erythropoiesis and repeated transfusions.
*Elevated HbF*
- **Elevated HbF** (fetal hemoglobin, α2γ2) can be seen in some conditions like **beta-thalassemia** (as a compensatory mechanism) or hereditary persistence of fetal hemoglobin.
- In alpha-thalassemia, the problem is with alpha-globin chain synthesis, which is required for both HbA and HbF; thus, HbF levels are usually not significantly elevated and may even be lower than expected relative to healthy newborns if the alpha chain deficiency is severe.
Thalassemias US Medical PG Question 6: A 57-year-old woman comes to the physician because of a 3-month history of easy fatigability and dyspnea on exertion. Menopause occurred 5 years ago. Her pulse is 105/min and blood pressure is 100/70 mm Hg. Physical examination shows pallor of the nail beds and conjunctivae. A peripheral blood smear shows small, pale red blood cells. Further evaluation is most likely to show which of the following findings?
- A. Increased concentration of HbA2
- B. Decreased serum haptoglobin concentration
- C. Positive stool guaiac test (Correct Answer)
- D. Dry bone marrow tap
- E. Increased serum methylmalonic acid concentration
Thalassemias Explanation: ***Positive stool guaiac test***
* The patient's symptoms of **fatigability**, **dyspnea on exertion**, and signs like **pallor**, along with a peripheral blood smear showing **small, pale red blood cells** (**microcytic hypochromic anemia**), are highly indicative of **iron deficiency anemia**.
* In a 57-year-old postmenopausal woman, the most common cause of **iron deficiency anemia** is **chronic blood loss from the gastrointestinal (GI) tract**, which would be detected by a **positive stool guaiac test**.
*Increased concentration of HbA2*
* An increased concentration of **HbA2** is characteristic of **beta-thalassemia minor**, a genetic disorder, which presents as microcytic anemia, but the clinical context and age make iron deficiency due to blood loss more likely in this patient.
* While both can cause microcytic anemia, the patient's acute presentation of symptoms and postmenopausal status strongly point to an acquired cause like chronic blood loss rather than a lifelong genetic condition.
*Decreased serum haptoglobin concentration*
* **Decreased serum haptoglobin concentration** is a marker of **hemolytic anemia**, where red blood cells are prematurely destroyed, leading to the release of free hemoglobin that binds to haptoglobin.
* The patient's peripheral smear finding of **small, pale red blood cells** (microcytic hypochromic) is inconsistent with hemolysis as the primary cause; hemolytic anemias often present with normocytic or macrocytic red blood cells.
*Dry bone marrow tap*
* A **dry bone marrow tap** is typically associated with **myelofibrosis** or sometimes **hairy cell leukemia** or severe aplastic anemia, where the bone marrow is fibrotic or hypocellular and cannot be aspirated.
* Iron deficiency anemia, while causing anemia, does not typically lead to a dry bone marrow tap; the marrow would usually be hypercellular with erythroid hyperplasia, reflecting the body's attempt to compensate for the anemia.
*Increased serum methylmalonic acid concentration*
* An **increased serum methylmalonic acid concentration** is a specific marker for **vitamin B12 deficiency**, which causes **megaloblastic (macrocytic) anemia**.
* The patient's peripheral blood smear findings of **small, pale red blood cells** (microcytic hypochromic) are inconsistent with **vitamin B12 deficiency**, which leads to **large red blood cells**.
Thalassemias US Medical PG Question 7: A 21-year-old woman comes to the physician for an annual health maintenance examination. She has no particular health concerns. Laboratory studies show:
Hemoglobin 11.2 g/dL
Mean corpuscular volume 74 μm3
Mean corpuscular hemoglobin concentration 30% Hb/cell
Red cell distribution width 14% (N=13–15)
Genetic analysis shows a point mutation in intron 1 of a gene on the short arm of chromosome 11. A process involving which of the following components is most likely affected in this patient?
- A. TATA-rich nucleotide sequence
- B. Transfer RNA
- C. Heat shock protein 60
- D. Small nuclear ribonucleoprotein (Correct Answer)
- E. MicroRNA
Thalassemias Explanation: ***Small nuclear ribonucleoprotein***
- The patient's lab results (low 11.2 g/dL **hemoglobin**, low 74 µm3 **MCV**, and low 30% **MCHC**) indicate **microcytic, hypochromic anemia**, consistent with **thalassemia**.
- A point mutation in **intron 1** of a gene suggests a problem with **RNA splicing**, which is mediated by **small nuclear ribonucleoproteins (snRNPs)** as part of the spliceosome.
*TATA-rich nucleotide sequence*
- The **TATA box** is located in the **promoter region** of genes and is involved in the initiation of **transcription**, not splicing.
- A mutation in the TATA box would affect the **rate of transcription** or gene expression, not the processing of mRNA after transcription.
*Transfer RNA*
- **tRNA** molecules are essential for **protein translation** by carrying specific amino acids to the ribosome.
- A problem with tRNA would affect the **synthesis of proteins**, not the processing of pre-mRNA.
*Heat shock protein 60*
- **Heat shock proteins** are molecular **chaperones** involved in the proper **folding of proteins** and preventing protein aggregation.
- A defect in HSP60 would lead to misfolded proteins, not impaired mRNA splicing.
*MicroRNA*
- **MicroRNAs (miRNAs)** are small non-coding RNA molecules that regulate gene expression by **silencing mRNA** or inhibiting **translation**.
- While miRNAs regulate gene expression, they are not directly involved in the **splicing of introns** from pre-mRNA.
Thalassemias US Medical PG Question 8: A 4-year-old boy is brought to the physician for the evaluation of fatigue since he returned from visiting family in South Africa one week ago. The day after he returned, he had fever, chills, and diffuse joint pain for 3 days. His symptoms improved with acetaminophen. He was born at term and has been healthy. His immunizations are up-to-date. His temperature is 37.6°C (99.68°F), pulse is 100/min, and blood pressure is 100/60 mm Hg. Examination shows conjunctival pallor. The remainder of the examination shows no abnormalities. Laboratory studies show:
Hemoglobin 10.8 g/dL
Mean corpuscular volume 68 μm3
Red cell distribution width 14% (N = 13%–15%)
Hemoglobin A2 6% (N < 3.5%)
A peripheral smear shows microcytic, hypochromic erythrocytes, some of which have a darkly stained center and peripheral rim, separated by a pale ring. Which of the following is the most appropriate next step in the management of this patient?
- A. Oral pyridoxine
- B. Iron supplementation
- C. Reassurance (Correct Answer)
- D. Folic acid therapy
- E. Oral succimer
Thalassemias Explanation: ***Reassurance***
- The patient's presentation with **microcytic anemia**, elevated **Hemoglobin A2 (6%)**, and **target cells** on peripheral smear is highly suggestive of **beta-thalassemia trait** (minor). This genetic condition is more common in individuals with Mediterranean, African, Middle Eastern, or South Asian ancestry.
- Beta-thalassemia trait is a **benign condition** that does not typically require specific medical intervention. The mild anemia does not usually cause significant symptoms or complications, and patients can live normal lives without treatment.
- The elevated HbA2 is the key diagnostic finding that distinguishes thalassemia trait from iron deficiency anemia.
*Oral pyridoxine*
- **Pyridoxine (Vitamin B6)** supplementation is indicated for **sideroblastic anemia**, which can also cause microcytic anemia.
- However, sideroblastic anemia typically presents with **ring sideroblasts** in the bone marrow and does not have the characteristic elevated HbA2 seen in beta-thalassemia trait.
*Iron supplementation*
- **Iron deficiency anemia** is a common cause of microcytic hypochromic anemia, but it would present with **low ferritin** and **low or normal HbA2** (not elevated).
- In this case, iron supplementation would not be appropriate and could potentially be harmful due to the risk of **iron overload** in thalassemia syndromes, even in the trait form.
- The elevated HbA2 and normal RDW help distinguish thalassemia trait from iron deficiency.
*Folic acid therapy*
- **Folic acid** is primarily used in the management of **macrocytic anemias** or in conditions with high red blood cell turnover, such as **hemolytic anemias** or major thalassemia syndromes requiring chronic transfusions.
- It is not indicated for beta-thalassemia trait, which is a microcytic anemia with normal red blood cell turnover and no significant hemolysis.
*Oral succimer*
- **Succimer** is a chelating agent used to treat **lead poisoning**, which can cause microcytic anemia with basophilic stippling.
- There are no clinical or laboratory findings in this patient (e.g., **basophilic stippling**, developmental delays, neurological symptoms, abdominal pain) to suggest lead poisoning.
Thalassemias US Medical PG Question 9: A 23-year-old Sicilian male presents to his primary care physician complaining of lethargy, joint pain, and urinary frequency. Vitals signs include T 98.7 F, HR 96 bpm, BP 135/71 mm/Hg, RR 18 breaths/minute, O2 99%. Laboratory findings include: random glucose 326 mg/dL, Hemoglobin 7.1, and elevated reticulocyte count and transferrin saturation. The patient is not surprised that his "blood level is low" and suggests that he might need another transfusion. An echocardiogram demonstrates restrictive cardiomyopathy. The disorder with which this patient presents can be characterized by which of the following?
- A. Mutations resulting in copper accumulation
- B. Presence of the fetal hemoglobin
- C. Absence of the hemoglobin alpha-chain
- D. Mutation resulting in increased iron absorption
- E. Absence of the hemoglobin beta-chain (Correct Answer)
Thalassemias Explanation: ***Absence of the hemoglobin beta-chain***
- The patient's symptoms (lethargy, joint pain, elevated glucose, restrictive cardiomyopathy, high transferrin saturation, and need for transfusions) in a **Sicilian male** are highly suggestive of **beta-thalassemia major**, which involves a reduced or absent production of hemoglobin beta-chains.
- This leads to ineffective erythropoiesis, **chronic anemia**, and subsequent **iron overload** due to frequent transfusions and increased intestinal iron absorption.
*Mutation resulting in increased iron absorption*
- This describes **hereditary hemochromatosis**, which can present with increased iron absorption, joint pain, and diabetes, but typically does not involve the severe, transfusion-dependent anemia and high reticulocyte count seen in this patient.
- While iron overload is present in this patient, it's primarily secondary to the underlying anemia and transfusions, not a primary mutation in iron absorption regulation.
*Absence of the hemoglobin alpha-chain*
- This describes **alpha-thalassemia**. The most severe form, **hydrops fetalis**, is incompatible with life, and milder forms present differently, often without the severe, transfusion-dependent anemia and systemic iron overload from transfusions seen here.
- Beta-thalassemia is more common in Mediterranean populations and aligns better with the clinical picture of profound anemia, extramedullary hematopoiesis, and resultant iron overload.
*Presence of the fetal hemoglobin*
- While patients with thalassemia may have **increased fetal hemoglobin (HbF)** as a compensatory mechanism, its *presence* is not the primary characteristic of the disease itself, but rather a response to the deficient or absent adult hemoglobin chain production.
- The fundamental genetic defect lies in the reduced or absent synthesis of adult hemoglobin chains (alpha or beta).
*Mutations resulting in copper accumulation*
- This describes **Wilson's disease**, which involves copper accumulation and can affect the liver, brain, and other organs, but does not present with severe anemia requiring transfusions or the specific type of iron overload-related cardiomyopathy and diabetes seen here.
- The laboratory findings of high reticulocytes, low hemoglobin, and high transferrin saturation specifically point to a primary hematological disorder with secondary iron loading rather than a copper metabolism disorder.
Thalassemias US Medical PG Question 10: A 24-year-old Asian woman comes to the office complaining of fatigue. She states that for weeks she has noticed a decrease in her energy. She is a spin instructor, and she has been unable to teach. She said that when she was bringing groceries up the stairs yesterday she experienced some breathlessness and had to rest after ascending 1 flight. She denies chest pain, palpitations, or dyspnea at rest. She has occasional constipation. She recently became vegan 3 months ago following a yoga retreat abroad. The patient has no significant medical history and takes no medications. She was adopted, and her family history is non-contributory. She has never been pregnant. Her last menstrual period was 3 days ago, and her periods are regular. She is sexually active with her boyfriend of 2 years and uses condoms consistently. She drinks a glass of red wine each evening with dinner. She denies tobacco use or other recreational drug use. Her temperature is 99°F (37.2°C), blood pressure is 104/74 mmHg and pulse is 95/min. Oxygen saturation is 98% while breathing ambient air. On physical examination, bilateral conjunctiva are pale. Her capillary refill is 3 seconds. A complete blood count is drawn, as shown below:
Hemoglobin: 10 g/dL
Hematocrit: 32%
Leukocyte count: 10,000/mm^3 with normal differential
Platelet count: 200,000/mm^3
A peripheral smear shows hypochromic red blood cells and poikilocytosis. A hemoglobin electrophoresis reveals a minor reduction in hemoglobin A2. Which of the following is most likely to be seen on the patient’s iron studies?
- A. Decreased serum iron and increased TIBC (Correct Answer)
- B. Normal serum iron and normal TIBC
- C. Increased serum iron and decreased TIBC
- D. Increased serum ferritin and increased iron saturation
- E. Decreased serum iron and decreased TIBC
Thalassemias Explanation: ***Decreased serum iron and increased TIBC***
- The patient's symptoms (fatigue, breathlessness, pale conjunctiva, prolonged capillary refill) and lab results (hemoglobin 10 g/dL, hematocrit 32%, hypochromic red blood cells, poikilocytosis) are highly indicative of **iron deficiency anemia (IDA)**.
- The **decreased hemoglobin A2** on electrophoresis further supports IDA, as HbA2 levels are typically reduced in iron deficiency (whereas they are elevated in beta-thalassemia trait).
- In IDA, the body lacks iron, leading to **decreased serum iron** and a compensatory **increased total iron-binding capacity (TIBC)** as the body tries to maximize iron absorption and transport.
*Normal serum iron and normal TIBC*
- This profile is typically seen in individuals without iron metabolic disturbances, which contradicts the patient's clear signs and symptoms of **anemia** and likely iron deficiency.
- Normal iron studies would not explain the **hypochromic microcytic red blood cells** found on the peripheral smear.
*Increased serum iron and decreased TIBC*
- This pattern is characteristic of **iron overload conditions** such as hemochromatosis or sideroblastic anemia, which are inconsistent with the patient's presentation of fatigue and anemia.
- Decreased TIBC indicates the body has sufficient or excess iron and does not need to increase iron binding protein production.
*Decreased serum iron and decreased TIBC*
- This finding is most commonly associated with **anemia of chronic disease (ACD)**, where inflammatory mediators lead to iron sequestration, resulting in both low serum iron and reduced TIBC.
- While the patient has some signs of anemia, her recent switch to a **vegan diet** and the absence of a chronic inflammatory condition make IDA more likely than ACD.
*Increased serum ferritin and increased iron saturation*
- **Increased serum ferritin** would indicate iron overload or inflammation, while **increased iron saturation** suggests there is plenty of iron available for binding.
- These findings are contrary to the classic picture of **iron deficiency anemia**, where ferritin (the storage form of iron) would be low, and iron saturation would be reduced due to insufficient iron.
More Thalassemias US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.