Hemolytic anemias US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Hemolytic anemias. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Hemolytic anemias US Medical PG Question 1: A 3-year-old boy is brought to the physician because of a 1-week history of yellowish discoloration of his eyes and skin. He has had generalized fatigue and mild shortness of breath for the past month. Three weeks ago, he was treated for a urinary tract infection with antibiotics. His father underwent a splenectomy during childhood. Examination shows pale conjunctivae and jaundice. The abdomen is soft and nontender; there is nontender splenomegaly. Laboratory studies show:
Hemoglobin 9.1 g/dL
Mean corpuscular volume 89 μm3
Mean corpuscular hemoglobin 32 pg/cell
Mean corpuscular hemoglobin concentration 37.8% Hb/cell
Leukocyte count 7800/mm3
Platelet count 245,000/mm3
Red cell distribution width 22.8% (N=13%–15%)
Serum
Bilirubin
Total 13.8 mg/dL
Direct 1.9 mg/dL
Lactate dehydrogenase 450 U/L
Which of the following is the most likely pathophysiology of these findings?
- A. Deficient glucose-6 phosphate dehydrogenase
- B. Decreased synthesis of alpha chains of hemoglobin
- C. Increased hemoglobin S
- D. Decreased spectrin in the RBC membrane (Correct Answer)
- E. Deficiency of pyruvate kinase
Hemolytic anemias Explanation: ***Decreased spectrin in the RBC membrane***
- This presentation is highly suggestive of **hereditary spherocytosis**, characterized by **defects in red blood cell membrane proteins** like **spectrin**, ankyrin, or band 3 protein.
- The patient's features—**jaundice**, **anemia**, **splenomegaly**, elevated **indirect bilirubin**, increased **LDH**, high **MCHC**, and a family history of splenectomy (often a treatment for hereditary spherocytosis)—point to this diagnosis.
*Deficient glucose-6 phosphate dehydrogenase*
- **G6PD deficiency** typically presents with **hemolytic anemia** following exposure to **oxidative stressors** (e.g., certain drugs, fava beans, infections).
- While an infection might trigger hemolysis, the chronic nature of the symptoms, **splenomegaly**, and high MCHC are less typical for G6PD deficiency.
*Decreased synthesis of alpha chains of hemoglobin*
- This describes **alpha thalassemia**, which would present with **microcytic hypochromic anemia** (low MCV, low MCH) rather than the normal MCV and high MCHC seen here.
- While chronic hemolysis can occur in severe forms, the specific lab values do not align with thalassemia.
*Increased hemoglobin S*
- **Sickle cell anemia** involves **hemoglobin S**, leading to sickling of red blood cells under hypoxic conditions.
- Patients typically experience **vaso-occlusive crises** and different RBC morphology (e.g., sickle cells, target cells) and usually a normocytic or macrocytic anemia, which is not fully consistent with the high MCHC seen here in isolation.
*Deficiency of pyruvate kinase*
- **Pyruvate kinase deficiency** causes **hemolytic anemia** due to impaired glycolysis, leading to insufficient ATP production in RBCs.
- While it presents with chronic hemolysis, **splenomegaly**, and jaundice, it typically does not cause the characteristic **high MCHC** seen in hereditary spherocytosis, nor does it typically present with a positive family history of splenectomy in father.
Hemolytic anemias US Medical PG Question 2: A 2-year-old girl with a history of SS-hemoglobin is brought to her pediatrician by her mother, who noted an abdominal mass. On exam, the girl's spleen is palpably enlarged, and her palms and conjunctiva are noted to be extremely pale. Serum haptoglobin levels are decreased. Which of the following is the most likely cause of this patient's symptoms?
- A. Intravascular hemolysis
- B. Extravascular hemolysis (Correct Answer)
- C. Hemolytic uremic syndrome
- D. Complement-mediated hemolysis
- E. Decreased red blood cell production
Hemolytic anemias Explanation: ***Extravascular hemolysis***
- This patient presents with **anemia** (pale conjunctiva and palms) and an **enlarged spleen**, which are classic signs of **extravascular hemolysis**. **Sickle cell disease** is a condition known to cause chronic hemolysis through this mechanism, where damaged red blood cells are removed by the reticuloendothelial system, primarily in the spleen.
- In **extravascular hemolysis**, red blood cells are destroyed outside of the bloodstream by macrophages in the spleen, liver, and bone marrow. While this process releases less **free hemoglobin** into circulation than intravascular hemolysis, **chronic hemolysis in sickle cell disease still leads to decreased haptoglobin levels** due to ongoing red cell destruction and some degree of intravascular hemolysis.
- The **splenomegaly** in this young patient is characteristic, as the spleen becomes engorged with sequestered and destroyed sickled red blood cells before it undergoes autoinfarction in later childhood.
*Intravascular hemolysis*
- **Intravascular hemolysis** involves the destruction of red blood cells within the circulation, leading to the release of **free hemoglobin**.
- While sickle cell disease does have some intravascular component, the **primary mechanism is extravascular**, and the presence of **significant splenomegaly** with palpable abdominal mass points to splenic sequestration and extravascular destruction as the dominant process.
*Hemolytic uremic syndrome*
- **Hemolytic uremic syndrome (HUS)** is characterized by a triad of **microangiopathic hemolytic anemia**, **thrombocytopenia**, and **acute kidney injury**.
- While it involves hemolysis, the patient's presentation lacks **thrombocytopenia** and evidence of **renal failure**, making HUS less likely. Additionally, HUS is not typically associated with **sickle cell disease**.
*Complement-mediated hemolysis*
- **Complement-mediated hemolysis** is seen in conditions like **paroxysmal nocturnal hemoglobinuria (PNH)** or some autoimmune hemolytic anemias.
- This typically results in **intravascular hemolysis** due to complement activation on red cell surfaces. The patient's known diagnosis of **sickle cell disease** makes this mechanism less likely as the primary cause.
*Decreased red blood cell production*
- Conditions with **decreased red blood cell production**, such as **aplastic anemia** or **pure red cell aplasia**, would present with anemia but not typically with an **enlarged spleen** or evidence of significant red blood cell destruction.
- The patient's history of **SS-hemoglobin (sickle cell disease)** and **decreased haptoglobin** point towards a hemolytic process rather than bone marrow suppression as the primary cause of anemia.
Hemolytic anemias US Medical PG Question 3: A 27-year-old African American man presents to a primary care physician for a routine checkup as a new patient. The patient states that he has been doing well lately and recently was promoted at his job. He states that 2 weeks ago he went to the ED for severe pain and was treated with morphine and oral fluids and discharged home that night. This had happened once before and he was treated similarly. The patient states that he drinks 7 to 8 alcoholic beverages per night and smokes 1 pack of cigarettes per day. The patient states that he has been gaining weight recently due to a diet consisting mostly of fast food. Basic labs are ordered as seen below.
Hemoglobin: 8 g/dL
Hematocrit: 28%
Mean corpuscular volume: 72 um^3
Leukocyte count: 6,500/mm^3 with normal differential
Platelet count: 157,000/mm^3
Serum:
Na+: 139 mEq/L
Cl-: 100 mEq/L
K+: 4.3 mEq/L
HCO3-: 25 mEq/L
BUN: 20 mg/dL
Glucose: 99 mg/dL
Creatinine: 1.1 mg/dL
LDH: 540 U/L
Ca2+: 10.2 mg/dL
AST: 12 U/L
ALT: 10 U/L
Which of the following is the best explanation of this patient's laboratory abnormalities?
- A. Folate deficiency
- B. Extravascular hemolysis (Correct Answer)
- C. Chronic inflammation
- D. Ineffective erythropoiesis
- E. Vitamin B12 deficiency
Hemolytic anemias Explanation: ***Extravascular hemolysis***
- The patient's presentation with **recurrent severe pain episodes** requiring ED visits, low hemoglobin (8 g/dL), elevated LDH (540 U/L), and normal leukocyte and platelet counts in an African American man strongly suggests **sickle cell disease**.
- In sickle cell disease, abnormal hemoglobin leads to fragile red blood cells that are prematurely destroyed in the **spleen and liver** (extravascular hemolysis), resulting in **anemia** and **elevated LDH**.
- The **microcytic anemia** (MCV 72 um³) is atypical for uncomplicated sickle cell disease and likely represents **concurrent iron deficiency** (from chronic hemoglobinuria and urinary iron loss) or **coexistent thalassemia trait**, both common in patients with chronic hemolytic anemias.
*Folate deficiency*
- Folate deficiency typically causes a **macrocytic anemia** (elevated MCV > 100 um³), which contradicts the patient's **microcytic anemia** (MCV 72 um³).
- While folate requirements can increase in chronic hemolytic states, the primary laboratory abnormality here points away from macrocytic anemia.
*Chronic inflammation*
- Anemia of chronic inflammation usually presents with **normocytic or mildly microcytic anemia** and low serum iron, but it typically does not cause the markedly elevated **LDH** (540 U/L) seen in this patient.
- The recurrent severe pain crises requiring ED visits and morphine are characteristic of **vaso-occlusive crises** in sickle cell disease, not simple anemia of chronic inflammation.
*Ineffective erythropoiesis*
- **Ineffective erythropoiesis** means that red blood cells are produced but are defective and destroyed in the bone marrow before release, often seen in conditions like **myelodysplastic syndromes** or **thalassemia major**.
- While it can cause anemia and elevated LDH, the **episodic severe pain crises** requiring ED visits and morphine are pathognomonic for **sickle cell vaso-occlusive crises**, not ineffective erythropoiesis.
- The clinical presentation strongly points to peripheral hemolysis rather than primarily ineffective production.
*Vitamin B12 deficiency*
- Like folate deficiency, vitamin B12 deficiency results in **macrocytic anemia** (elevated MCV > 100 um³), not the **microcytic anemia** (MCV 72 um³) observed in this patient.
- It's also associated with neurological symptoms (paresthesias, ataxia, dementia), which are not mentioned here.
Hemolytic anemias US Medical PG Question 4: A 21-year-old college student is admitted to the emergency department with complaints of pharyngitis, headache, and a persistent, non-productive, dry, hacking cough. The patient complains of feeling tired and fatigued and denies fever/chills. On physical examination, her mucosa is pale. A complete blood count is remarkable for decreased hemoglobin. The physician suspects viral pneumonia, but the sputum culture tests come back with the following description: ‘fried-egg shaped colonies on sterol-containing media, and mulberry-shaped colonies on media containing sterols’. A direct Coombs test comes back positive. Which of the following statements is true regarding the complications associated with Mycoplasma pneumoniae?
- A. It is similarly associated with systemic lupus erythematosus
- B. The underlying mechanism is complement-independent.
- C. Red blood cells bind to IgG in warm temperatures > 37°C (98.6°F)
- D. Red blood cells bind to IgM in cold temperatures < 37°C (98.6°F) (Correct Answer)
- E. It primarily causes intravascular hemolysis rather than extravascular hemolysis
Hemolytic anemias Explanation: ***Red blood cells bind to IgM in cold temperatures < 37°C (98.6°F)***
- This patient's presentation, including **non-productive cough**, **fatigue**, and **positive Coombs test**, along with the characteristic **"fried-egg"** and **"mulberry" colonies** (consistent with *Mycoplasma pneumoniae*), indicates a secondary **cold agglutinin hemolytic anemia**.
- In response to *Mycoplasma pneumoniae* infection, the body produces **IgM antibodies** that react with **I antigen** on red blood cells, leading to agglutination and hemolysis preferentially at temperatures below **37°C (98.6°F)** in cooler areas of the body.
*It is similarly associated with systemic lupus erythematosus*
- **Systemic lupus erythematosus** (SLE) is an autoimmune disease primarily associated with **warm autoimmune hemolytic anemia (AIHA)**, where IgG antibodies are active at body temperature.
- While both can cause anemia, the immunological mechanism and primary antibodies involved are distinct.
*The underlying mechanism is complement-independent.*
- **Cold agglutinin disease** is a **complement-dependent** process; IgM antibodies cause agglutination, which then activates the classical complement pathway leading to C3b deposition on red blood cells.
- The opsonized red blood cells are subsequently cleared by macrophages in the liver and spleen (extravascular hemolysis) or lysed intravascularly by the full complement cascade.
*Red blood cells bind to IgG in warm temperatures > 37°C (98.6°F)*
- This describes **warm autoimmune hemolytic anemia (AIHA)**, where **IgG antibodies** bind to red blood cells optimally at **body temperature (37°C)**.
- The patient's condition, with a positive direct Coombs test but features suggestive of *Mycoplasma pneumoniae* and positive complement, is indicative of **cold agglutinin disease**, which is mediated by IgM.
*It primarily causes intravascular hemolysis rather than extravascular hemolysis*
- Although some **intravascular hemolysis** can occur, **cold agglutinin disease** caused by *Mycoplasma pneumoniae* primarily results in **extravascular hemolysis**, particularly in the liver and spleen.
- Macrophages in these organs recognize and clear red blood cells opsonized with C3b, which is deposited after IgM binds and activates complement, leading to red blood cell destruction outside the bloodstream.
Hemolytic anemias US Medical PG Question 5: A 25-year-old man of Mediterranean descent makes an appointment with his physician because his skin and sclera have become yellow. He complains of fatigue and fever that started at the same time icterus appeared. On examination, he is tachycardic and tachypneic. The oxygen (O2) saturation is < 90%. He has increased unconjugated bilirubin, hemoglobinemia, and an increased number of reticulocytes in the peripheral blood. What is the most likely diagnosis?
- A. Microcytic anemia caused by iron deficiency
- B. Aplastic anemia
- C. Autoimmune hemolytic anemia (AIHA)
- D. Anemia caused by renal failure
- E. Hemolytic anemia caused by glucose-6-phosphate dehydrogenase deficiency (G6PD deficiency) (Correct Answer)
Hemolytic anemias Explanation: ***Hemolytic anemia caused by glucose-6-phosphate dehydrogenase deficiency (G6PD deficiency)***
- The patient's presentation with **jaundice**, **fatigue**, fever, **tachycardia**, **tachypnea**, and **low oxygen saturation** points to an acute hemolytic crisis.
- The laboratory findings of **increased unconjugated bilirubin**, **hemoglobinemia** (evidence of red blood cell destruction), and **increased reticulocytes** (bone marrow's attempt to compensate for red blood cell loss) are classic for hemolytic anemia. A young man of **Mediterranean descent** makes G6PD deficiency a strong possibility, as it is common in this population and can be triggered by various factors leading to oxidative stress.
*Microcytic anemia caused by iron deficiency*
- **Iron deficiency anemia** typically presents with **microcytic hypochromic red blood cells**, and while it causes fatigue and pallor, it does not typically lead to acute jaundice and hemoglobinemia.
- Reticulocyte count is usually normal or only mildly elevated, not significantly increased as seen in rapid red blood cell destruction.
*Aplastic anemia*
- **Aplastic anemia** is characterized by **pancytopenia** (decreased red blood cells, white blood cells, and platelets) due to bone marrow failure.
- It does not present with signs of hemolytic crisis such as jaundice, hemoglobinemia, or increased reticulocytes.
*Autoimmune hemolytic anemia (AIHA)*
- While AIHA causes **hemolytic anemia** with similar lab findings (jaundice, increased unconjugated bilirubin, reticulocytosis), the context of a young man of **Mediterranean descent** makes G6PD deficiency a more likely primary consideration, especially without specific triggers for AIHA or a positive direct Coombs test result.
- AIHA involves autoantibodies against red blood cells.
*Anemia caused by renal failure*
- Anemia due to **renal failure** is primarily caused by decreased production of **erythropoietin** leading to **normocytic, normochromic anemia**.
- It does not involve acute hemolysis, jaundice, hemoglobinemia, or increased reticulocytes.
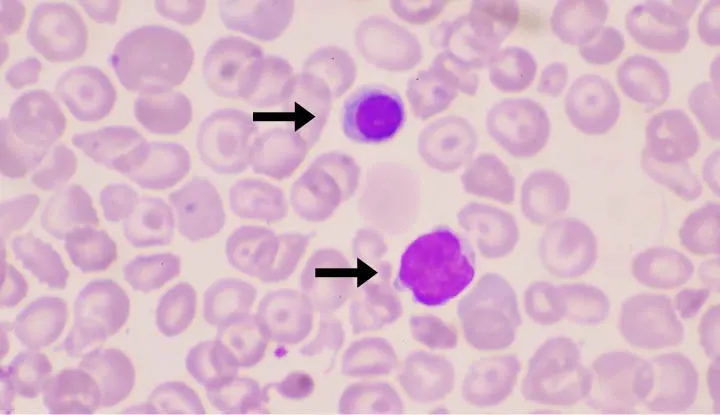
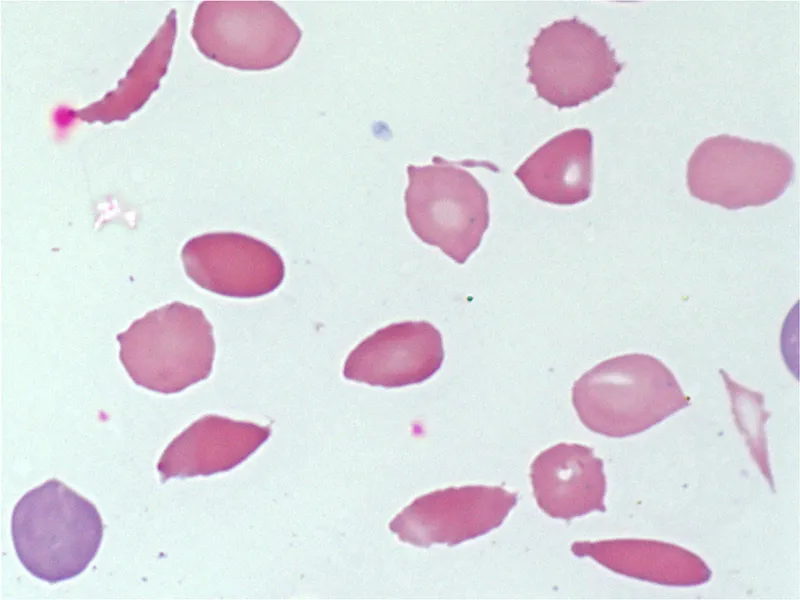
Hemolytic anemias US Medical PG Question 6: A 58-year old man comes to the emergency department because of progressively worsening shortness of breath and fatigue for 3 days. During the last month, he has also noticed dark colored urine. One month ago, he underwent mechanical aortic valve replacement for high-grade aortic stenosis. A photomicrograph of a peripheral blood smear from the patient is shown. Which of the following findings is most likely to be seen in this patient?
- A. Elevated lactate dehydrogenase (Correct Answer)
- B. Low ferritin
- C. Low platelets
- D. Elevated haptoglobin
- E. Low unconjugated bilirubin
Hemolytic anemias Explanation: ***Elevated lactate dehydrogenase***
- The patient has **mechanical hemolysis** from his prosthetic aortic valve, evidenced by schistocytes on blood smear and dark urine (hemoglobinuria).
- **LDH is markedly elevated** in intravascular hemolysis due to release from lysed red blood cells.
- LDH is the **most direct and sensitive marker** of ongoing hemolysis, making it the best answer.
*Low ferritin*
- While ferritin **can be low** in chronic mechanical hemolysis due to urinary iron loss (hemosiderinuria), this develops over months to years.
- This patient's valve was placed only 1 month ago, making significant iron depletion unlikely.
- More importantly, **LDH elevation is more specific** for acute hemolysis than ferritin changes.
*Low platelets*
- Thrombocytopenia is not a typical feature of **mechanical hemolysis** alone.
- Low platelets would suggest microangiopathic processes like **TTP or HUS**, which present differently.
- Platelet counts are typically normal in isolated mechanical hemolysis.
*Elevated haptoglobin*
- **Haptoglobin is consumed** (decreased, not elevated) in hemolysis as it binds free hemoglobin released into circulation.
- **Low or undetectable haptoglobin** is characteristic of intravascular hemolysis.
- Elevated haptoglobin would contradict the diagnosis of hemolytic anemia.
*Low unconjugated bilirubin*
- Unconjugated bilirubin is **elevated** (not low) in hemolysis due to breakdown of heme from lysed red blood cells.
- The dark urine suggests hemoglobinuria, and there would also be elevated indirect bilirubin.
- Low unconjugated bilirubin is inconsistent with hemolytic anemia.
Hemolytic anemias US Medical PG Question 7: A 4-year-old boy is brought to the physician because of yellowish discoloration of his eyes and skin for 5 days. He has had generalized fatigue and mild shortness of breath over the past 2 months. Two weeks ago, he was treated for a urinary tract infection with antibiotics. His father has a history of undergoing a splenectomy in his childhood. Examination shows pale conjunctivae and jaundice. The abdomen is soft and nontender; the spleen is palpated 4 to 5 cm below the left costal margin. Laboratory studies show:
Hemoglobin 9.9 g/dL
Mean corpuscular volume 88 μm3
Mean corpuscular hemoglobin 31.7 pg/cell
Mean corpuscular hemoglobin concentration 37.0% Hb/cell
Leukocyte count 6600/mm3
Platelet count 233,000/mm3
Red cell distribution width 24.3% (N = 13–15)
Serum
Bilirubin
Total 12.3 mg/dL
Direct 1.8 mg/dL
Lactate dehydrogenase 401 U/L
Which of the following is the most likely cause of these findings?
- A. Decreased synthesis of alpha chains of hemoglobin
- B. Thrombotic microangiopathy
- C. Decreased CD55 and CD59 in RBC
- D. Defective spectrin in the RBC membrane (Correct Answer)
- E. Deficient glucose-6 phosphate dehydrogenase
Hemolytic anemias Explanation: ***Defective spectrin in the RBC membrane***
- This presentation is highly suggestive of **hereditary spherocytosis**, an autosomal dominant disorder characterized by **defects in red blood cell membrane proteins** like spectrin, ankyrin, or band 3.
- The patient exhibits classic signs of **hemolytic anemia** (jaundice, fatigue, splenomegaly, elevated unconjugated bilirubin, high LDH, high RDW), a family history of splenectomy, and a high MCHC, all consistent with hereditary spherocytosis.
*Decreased synthesis of alpha chains of hemoglobin*
- This describes **alpha-thalassemia**, which is caused by reduced or absent alpha-globin chain production, leading to **microcytic, hypochromic anemia**.
- While it can cause hemolysis, the **normal MCV** (88 μm3) and **high MCHC** observed in this patient make alpha-thalassemia less likely.
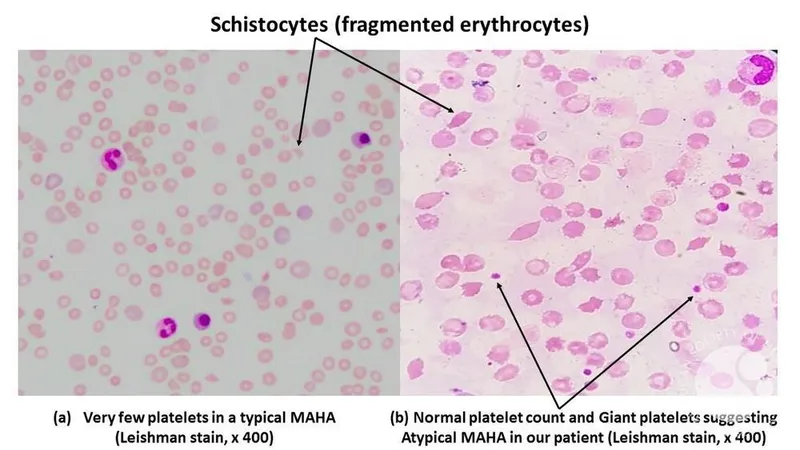
*Thrombotic microangiopathy*
- This is a group of disorders characterized by **microangiopathic hemolytic anemia**, **thrombocytopenia**, and **organ damage** due to microvascular thrombi.
- While it causes hemolytic anemia (high LDH, jaundice), the patient's **normal platelet count** and lack of severe multi-organ involvement make this diagnosis unlikely.
*Decreased CD55 and CD59 in RBC*
- This is the hallmark of **paroxysmal nocturnal hemoglobinuria (PNH)**, an acquired clonal stem cell disorder resulting in unregulated complement activation on red blood cells.
- PNH causes hemolytic anemia and often presents with **hemoglobinuria**, **thrombosis**, and bone marrow failure, which are not described in this case, and usually has a normal MCHC.
*Deficient glucose-6 phosphate dehydrogenase*
- **G6PD deficiency** is an X-linked disorder causing episodic hemolytic anemia, typically triggered by **oxidative stress** from certain foods (fava beans), drugs (sulfa drugs, antimalarials), or infections.
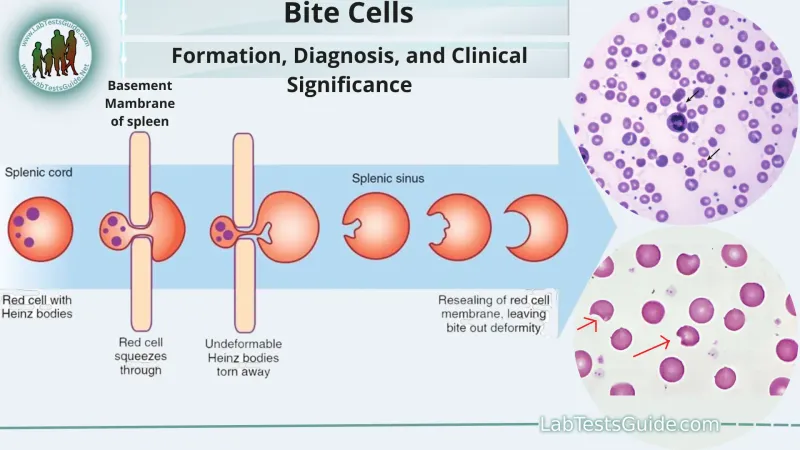
- While the patient was treated with antibiotics, his symptoms are prolonged, and the absence of specific triggers or evidence of **Heinz bodies** (oxidized hemoglobin seen in G6PD deficiency) makes this less likely.
Hemolytic anemias US Medical PG Question 8: A 55-year-old man comes to the physician because of progressive daytime sleepiness and exertional dyspnea for the past 6 months. Physical examination shows conjunctival pallor and several subcutaneous purple spots on his legs. His hemoglobin concentration is 8.5 g/dL, leukocyte count is 3,000/mm3, and platelet count is 16,000/mm3. Which of the following laboratory values is most likely to be increased in this patient?
- A. Reticulocyte count
- B. Lactate dehydrogenase concentration
- C. Transferrin concentration
- D. Erythropoietin concentration (Correct Answer)
- E. Haptoglobin concentration
Hemolytic anemias Explanation: ***Erythropoietin concentration***
- The patient presents with severe **anemia (Hb 8.5 g/dL)**, **leukopenia**, and **thrombocytopenia**, indicating **pancytopenia**. This severe decrease in red blood cells will trigger the kidneys to produce more **erythropoietin** in an attempt to stimulate red blood cell production in the bone marrow.
- Given the pancytopenia, the underlying pathology is likely bone marrow failure (e.g., aplastic anemia, myelodysplastic syndrome), which would explain why the bone marrow is not responding effectively to increased erythropoietin.
*Reticulocyte count*
- A low or inappropriately normal reticulocyte count in the presence of severe anemia would suggest **bone marrow suppression** or ineffective erythropoiesis, which is consistent with the pancytopenia observed.
- An **increased reticulocyte count** would typically be seen in hemolytic anemia or acute blood loss, where the bone marrow is hyperactive in response to RBC destruction or loss.
*Lactate dehydrogenase concentration*
- **Elevated LDH** is a marker of **cellular damage** or high cell turnover, commonly seen in hemolytic anemia or certain hematologic malignancies.
- While it could be elevated in some conditions causing pancytopenia, it's not the most direct or specific compensatory response to chronic anemia as erythropoietin.
*Transferrin concentration*
- **Transferrin** levels usually **increase** in **iron deficiency anemia** as the body attempts to maximize iron absorption and transport.
- While anemia is present, the broad pancytopenia suggests a bone marrow problem rather than a primary iron deficiency, and increased transferrin isn't a direct compensatory mechanism for severe pancytopenia.
*Haptoglobin concentration*
- **Haptoglobin levels decrease** in conditions involving **intravascular hemolysis**, as haptoglobin binds to free hemoglobin and is then cleared from circulation.
- There is no evidence in the clinical picture to suggest hemolysis as the primary cause of anemia; the pancytopenia points to bone marrow pathology.
Hemolytic anemias US Medical PG Question 9: A 24-year-old woman, otherwise healthy, presents with a non-productive cough, sore throat, and myalgia. The patient reports that her symptoms started gradually 2 weeks ago and have not improved. She has no significant past medical history and no current medications. She is a college student and denies any recent overseas travel. The patient received the flu vaccine this year, and her 2-part PPD required for school was negative. She does not smoke, drink, or use recreational drugs. The patient denies being sexually active. The vital signs include: temperature 37.0°C (98.6°F), blood pressure 110/75 mm Hg, pulse 98/min, respirations 20/min, and oxygen saturation 99% on room air. On physical exam, the patient is alert and cooperative. The cardiac exam is normal. There are rales present bilaterally over both lung fields. The skin and conjunctiva are pale. The laboratory tests are pending. The chest X-ray is shown in the image. Which of the following laboratory findings would also commonly be found in this patient?
- A. Elevated cold agglutinin titers (Correct Answer)
- B. Heinz bodies on peripheral smear
- C. Low serum levels of complement
- D. Low serum ferritin and serum iron
- E. Schistocytes on peripheral smear
Hemolytic anemias Explanation: ***Elevated cold agglutinin titers***
- The clinical presentation (non-productive cough, sore throat, myalgia, bilateral rales, pallor) in a young, otherwise healthy patient, along with the chest X-ray showing bilateral patchy infiltrates, is consistent with **atypical pneumonia**, most likely caused by ***Mycoplasma pneumoniae***.
- ***Mycoplasma pneumoniae*** infection can induce **autoimmune hemolytic anemia** through the production of **cold agglutinins** (IgM antibodies that agglutinate RBCs at cold temperatures). This occurs in approximately 50% of Mycoplasma infections, though clinically significant hemolysis is less common.
- Laboratory findings include **elevated cold agglutinin titers** (typically >1:64), **positive direct Coombs test** (IgM and complement), **spherocytes** on peripheral smear (from extravascular hemolysis), elevated indirect bilirubin, and decreased haptoglobin.
- The pallor noted on exam reflects the hemolytic anemia caused by these cold-reactive autoantibodies.
*Low serum ferritin and serum iron*
- **Iron deficiency anemia** presents with pallor and fatigue, but results from chronic blood loss, inadequate dietary intake, or malabsorption, not from acute atypical pneumonia.
- While anemia is present in this case, it's **hemolytic anemia** (from cold agglutinins), not iron deficiency. Iron studies would typically be normal or show elevated ferritin (acute phase reactant).
*Heinz bodies on peripheral smear*
- **Heinz bodies** are inclusions of denatured hemoglobin seen in **G6PD deficiency** after oxidative stress or with unstable hemoglobin variants.
- While G6PD deficiency causes hemolytic anemia, it's not associated with *Mycoplasma pneumoniae* infection and doesn't explain the respiratory symptoms or chest X-ray findings.
*Schistocytes on peripheral smear*
- **Schistocytes** are fragmented RBCs indicating **microangiopathic hemolytic anemia** (TTP, HUS, DIC) where RBCs are mechanically sheared in damaged microvasculature.
- *Mycoplasma pneumoniae* causes **immune-mediated extravascular hemolysis** via cold agglutinins, not microangiopathic intravascular hemolysis. Spherocytes, not schistocytes, would be seen.
*Low serum levels of complement*
- Low complement levels indicate complement consumption in **immune complex diseases** (SLE, post-infectious glomerulonephritis) or alternative pathway activation (C3 glomerulonephritis).
- While cold agglutinins can activate complement (causing hemolysis), this is a **local effect on RBC surfaces**, not systemic complement depletion. Serum complement levels typically remain normal in cold agglutinin disease.
Hemolytic anemias US Medical PG Question 10: A 68-year-old woman comes to the physician because of increasing heartburn for the last few months. During this period, she has taken ranitidine several times a day without relief and has lost 10 kg (22 lbs). She has retrosternal pressure and burning with every meal. She has had heartburn for several years and took ranitidine as needed. She has hypertension. She has smoked one pack of cigarettes daily for the last 40 years and drinks one glass of wine occasionally. Other current medications include amlodipine and hydrochlorothiazide. She appears pale. Her height is 163 cm (5 ft 4 in), her weight is 75 kg (165 lbs), BMI is 27.5 kg/m2. Her temperature is 37.2°C (98.96°F), pulse is 78/min, and blood pressure is 135/80 mm Hg. Cardiovascular examination shows no abnormalities. Abdominal examination shows mild tenderness to palpation in the epigastric region. Bowel sounds are normal. The remainder of the examination shows no abnormalities. Laboratory studies show:
Hemoglobin 10.2 g/dL
Mean corpuscular volume 78 μm
Mean corpuscular hemoglobin 23 pg/cell
Leukocyte count 9,500/mm3
Platelet count 330,000/mm3
Serum
Na+ 137 mEq/L
K+ 3.8 mEq/L
Cl- 100 mEq/L
HCO3- 25 mEq/L
Creatinine 1.2 mg/dL
Lactate dehydrogenase 260 U/L
Alanine aminotransferase 18 U/L
Aspartate aminotransferase 15 U/L
Lipase (N < 280 U/L) 40 U/L
Troponin I (N < 0.1 ng/mL) 0.029 ng/mL
An ECG shows normal sinus rhythm without ST-T changes. Which of the following is the most appropriate next step in the management of this patient?
- A. 24-hour esophageal pH monitoring
- B. Esophagogastroduodenoscopy (Correct Answer)
- C. Barium swallow
- D. Trial of proton-pump inhibitor
- E. Esophageal manometry
Hemolytic anemias Explanation: ***Esophagogastroduodenoscopy***
- This patient presents with **alarm symptoms** (weight loss, iron deficiency anemia, persistent heartburn unresponsive to ranitidine) that warrant an immediate investigation for underlying malignancy or severe mucosal damage.
- **EGD directly visualizes the esophagus, stomach, and duodenum**, allowing for biopsies of suspicious lesions, which is crucial given her risk factors (smoking, chronic GERD, age).
*24-hour esophageal pH monitoring*
- This test is primarily used to **diagnose GERD** in patients with typical symptoms but normal endoscopy, or to guide treatment for refractory GERD.
- It is not the appropriate first step here because the patient has alarm symptoms, which necessitate direct visualization and biopsy to rule out serious pathology.
*Barium swallow*
- A barium swallow can identify **structural abnormalities** such as strictures, diverticula, or large masses but has limited utility for detecting subtle mucosal changes or early malignancy.
- It does not allow for **biopsy**, which is essential for definitive diagnosis in a patient with alarm symptoms.
*Trial of proton-pump inhibitor*
- A trial of PPIs is appropriate for patients with **typical GERD symptoms** without alarm features, as a diagnostic and therapeutic intervention.
- However, this patient has already tried ranitidine (an H2 blocker) without relief and exhibits multiple **alarm symptoms**, making empirical treatment insufficient and potentially dangerous by delaying diagnosis.
*Esophageal manometry*
- Esophageal manometry assesses **esophageal motility** and sphincter function, useful for diagnosing motility disorders like achalasia or diffuse esophageal spasm.
- It is indicated if a motility disorder is suspected, usually *after* ruling out structural causes with EGD, and does not address the immediate concern of underlying malignancy or severe damage raised by the patient's alarm symptoms.
More Hemolytic anemias US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.