Hematology (anemias, clotting disorders)

On this page
🩸 The Hematologic Command Center: Mastering Blood's Complex Orchestra
Blood is your body's most accessible organ, yet its disorders reveal themselves through subtle patterns that separate novice from expert clinicians. You'll master the systematic approach to anemias and clotting disorders by building from cellular physiology through coagulation cascades to diagnostic reasoning that transforms confusing lab panels into clear clinical pictures. We'll equip you with pattern recognition skills to rapidly differentiate microcytic from macrocytic anemias, distinguish bleeding from clotting disorders, and deploy evidence-based treatments confidently. This isn't memorization-it's learning to think like a hematologist while managing the blood diseases you'll encounter daily in any practice setting.
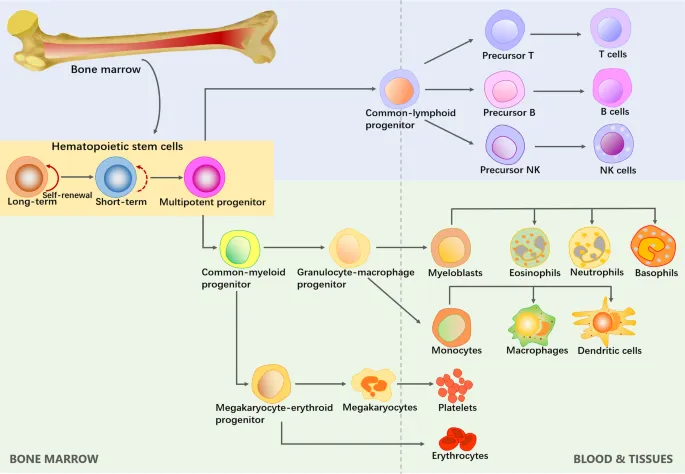
The Cellular Foundation: Blood's Essential Components
The hematologic system operates through four primary cellular divisions, each with distinct functions and clinical significance:
-
Erythrocytes (Red Blood Cells)
- Normal count: 4.5-5.5 million/μL (men), 4.0-5.0 million/μL (women)
- Lifespan: 120 days with continuous turnover
- Primary function: Oxygen transport via 280 million hemoglobin molecules per cell
- Hemoglobin concentration: 14-18 g/dL (men), 12-16 g/dL (women)
- Oxygen saturation: 95-100% under normal conditions
- Hematocrit: 42-52% (men), 37-47% (women)
-
Leukocytes (White Blood Cells)
- Total count: 4,500-11,000/μL with specific subset distributions
- Neutrophils: 50-70% (2,500-7,000/μL) - first responders to bacterial infections
- Lymphocytes: 20-40% (1,500-4,000/μL) - adaptive immunity orchestrators
- T-cells: 60-80% of lymphocytes
- B-cells: 10-20% of lymphocytes
- NK cells: 5-15% of lymphocytes
📌 Remember: HEMP for hematopoietic growth factors - Hematopoietin (EPO), Erythropoietin, M-CSF (macrophage), Platelets (thrombopoietin). EPO increases 100-1000x during severe anemia, M-CSF rises 10-50x during infections, and thrombopoietin maintains platelet counts at 150,000-450,000/μL.
- Thrombocytes (Platelets)
- Normal count: 150,000-450,000/μL with 7-10 day lifespan
- Primary hemostasis: Initial plug formation within 1-3 minutes
- Secondary hemostasis: Coagulation cascade activation in 3-8 minutes
- Bleeding time: 2-7 minutes (normal platelet function)
- Clot retraction: 50-100% within 2 hours
| Cell Type | Normal Count | Lifespan | Primary Function | Clinical Threshold |
|---|---|---|---|---|
| RBC | 4.0-5.5 million/μL | 120 days | Oxygen transport | <10 g/dL (severe anemia) |
| Neutrophils | 2,500-7,000/μL | 6-8 hours | Bacterial defense | <500/μL (neutropenia) |
| Platelets | 150,000-450,000/μL | 7-10 days | Hemostasis | <50,000/μL (bleeding risk) |
| Lymphocytes | 1,500-4,000/μL | Days-years | Adaptive immunity | <1,000/μL (immunodeficiency) |
| Monocytes | 200-800/μL | 1-3 days | Tissue macrophages | >1,000/μL (inflammation) |
Hemoglobin Architecture: The Oxygen Transport Marvel
Hemoglobin represents one of medicine's most elegant molecular machines, with four globin chains (2α, 2β) each containing heme groups with central iron atoms. This quaternary structure enables cooperative oxygen binding with sigmoidal dissociation curves.
📌 Remember: SHIFT for oxygen-hemoglobin curve factors - Sickness (acidosis shifts right), Heat (hyperthermia shifts right), Increased 2,3-DPG (shifts right), Fever (shifts right), Temperature (hypothermia shifts left). Right shifts decrease oxygen affinity by 20-30%, enhancing tissue delivery during metabolic stress.
Normal hemoglobin variants demonstrate specific clinical patterns:
- HbA (α2β2): 95-98% of adult hemoglobin
- HbA2 (α2δ2): 2-3% - elevated in β-thalassemia trait (>3.5%)
- HbF (α2γ2): <1% in adults - protective in sickle cell disease when >20%
💡 Master This: Understanding hemoglobin's cooperative binding explains why oxygen saturation drops precipitously below 90% (P50 = 27 mmHg), creating the clinical principle that saturation <90% represents tissue hypoxia regardless of other vital signs.
The bone marrow produces 200 billion red blood cells daily, requiring 20-25 mg iron, 400 μg folate, and 2-3 μg vitamin B12. This massive production explains why nutritional deficiencies rapidly manifest as hematologic abnormalities, with iron deficiency developing within 2-4 weeks and B12 deficiency emerging over 2-4 years due to hepatic stores.
Connect these foundational blood components through the coagulation cascade to understand how cellular elements integrate with plasma proteins for comprehensive hemostatic control.
🩸 The Hematologic Command Center: Mastering Blood's Complex Orchestra
⚙️ The Hemostatic Engine: Coagulation's Molecular Machinery

Primary Hemostasis: The Platelet Response Network
Vascular injury triggers immediate platelet activation through three sequential steps: adhesion, activation, and aggregation. This process begins within seconds and completes initial hemostatic plug formation in 1-3 minutes.
Platelet adhesion requires von Willebrand factor (vWF) bridging between subendothelial collagen and platelet GPIb receptors. Under high shear conditions (>1,500 s⁻¹), this interaction becomes essential, explaining why vWF deficiency causes bleeding primarily in high-flow vessels (GI tract, nose).
📌 Remember: PLATELET activation pathway - Phospholipase A2, Lipooxygenase, Arachidonic acid, Thromboxane A2, Endoperoxides, Leukotriene synthesis, Eicosanoid production, TXA2 receptor binding. Aspirin irreversibly acetylates Ser530 on COX-1, blocking TXA2 synthesis for the platelet's 7-10 day lifespan.
| Platelet Function Test | Normal Range | Clinical Significance | Abnormal Threshold |
|---|---|---|---|
| Bleeding Time | 2-7 minutes | Primary hemostasis | >10 minutes |
| Platelet Aggregometry | >60% with ADP | Platelet function | <50% aggregation |
| PFA-100 Closure Time | 85-165 seconds | High shear function | >300 seconds |
| Thromboelastography | R: 5-10 min | Clot formation | R >15 minutes |
| vWF Activity | 50-200% | vWF function | <30% (bleeding risk) |
⭐ Clinical Pearl: Platelet dysfunction causes mucocutaneous bleeding (petechiae, epistaxis, menorrhagia) while coagulation factor defects produce deep tissue bleeding (hemarthroses, muscle hematomas). This distinction guides initial diagnostic workup with 85% accuracy.
Secondary Hemostasis: The Coagulation Cascade Symphony
The coagulation cascade amplifies initial platelet responses through enzymatic cascades generating thrombin and fibrin. Two pathways converge at Factor X activation: the tissue factor pathway (extrinsic) and contact activation pathway (intrinsic).
-
Extrinsic Pathway (Tissue Factor)
- Initiated by tissue factor (TF) exposure during vascular injury
- TF-VIIa complex activates Factor X within seconds
- Primary pathway for physiologic hemostasis
- Measured by Prothrombin Time (PT): 11-13 seconds
-
Intrinsic Pathway (Contact Activation)
- Triggered by Factor XII contact with negatively charged surfaces
- Involves Factors XII, XI, IX, VIII in sequential activation
- Amplifies coagulation but not essential for hemostasis
- Measured by Partial Thromboplastin Time (PTT): 25-35 seconds
📌 Remember: 1972 for intrinsic pathway factors - 12, 9 (IX), 7 (VII - actually extrinsic), 2 (II/prothrombin). Actually: 12, 11, 9, 8 activate sequentially. Factor VIII deficiency (Hemophilia A) affects 1:5,000 males, while Factor IX deficiency (Hemophilia B) affects 1:25,000 males.
💡 Master This: Thrombin generation follows explosive kinetics - initial picomolar concentrations amplify to nanomolar levels within minutes through positive feedback loops. Each Factor Xa molecule generates 1,000+ thrombin molecules, explaining why small factor deficiencies (<5% activity) cause severe bleeding.
Anticoagulant Control Systems: The Hemostatic Brakes
Natural anticoagulants prevent excessive clot formation through three major systems: antithrombin, protein C/S pathway, and tissue factor pathway inhibitor (TFPI). These systems maintain hemostatic balance and localize clot formation to injury sites.

-
Antithrombin System
- Inhibits thrombin, Factor Xa, IXa through serpin mechanism
- Heparin accelerates antithrombin activity 1,000-fold
- Deficiency (<50% activity) increases VTE risk 10-20x
-
Protein C Pathway
- Activated Protein C (APC) cleaves Factors Va, VIIIa
- Requires Protein S cofactor and thrombomodulin activation
- Factor V Leiden mutation creates APC resistance in 5% Caucasians
⭐ Clinical Pearl: Warfarin-induced skin necrosis occurs in Protein C deficiency because Protein C (half-life 6 hours) depletes faster than Factors II, IX, X (half-lives 24-72 hours), creating transient hypercoagulable state during warfarin initiation.
Understanding these hemostatic mechanisms reveals how inherited thrombophilias and acquired coagulopathies disrupt normal balance, leading to either bleeding disorders or thrombotic complications that define much of clinical hematology.
⚙️ The Hemostatic Engine: Coagulation's Molecular Machinery
🔧 The Diagnostic Arsenal: Pattern Recognition in Hematologic Disease
Anemia Classification: The Morphologic Approach
Anemia diagnosis begins with Mean Corpuscular Volume (MCV) classification, creating three primary categories with distinct differential diagnoses and specific MCV thresholds:
- Microcytic Anemia (MCV <80 fL)
- Iron deficiency: Most common cause (50% of anemias globally)
- Ferritin <15 ng/mL (women), <30 ng/mL (men)
- Transferrin saturation <16%
- RDW >15% (early finding)
- Thalassemia trait: MCV <70 fL with normal/elevated RBC count
- HbA2 >3.5% in β-thalassemia trait
- Mentzer index <13 (MCV/RBC count)
- Anemia of chronic disease: 25% of hospitalized patients
- Ferritin >100 ng/mL despite iron deficiency
- Hepcidin elevation blocks iron absorption
- Iron deficiency: Most common cause (50% of anemias globally)
📌 Remember: TAILS for microcytic anemia - Thalassemia, Anemia of chronic disease, Iron deficiency, Lead poisoning, Sideroblastic anemia. Iron deficiency shows low ferritin + high TIBC, thalassemia shows normal ferritin + normal TIBC, and ACD shows high ferritin + low TIBC.
| Anemia Type | MCV Range | Key Laboratory Findings | Diagnostic Threshold |
|---|---|---|---|
| Iron Deficiency | 60-80 fL | Ferritin <15 ng/mL, TIBC >450 μg/dL | Transferrin sat <16% |
| Thalassemia Trait | 55-75 fL | HbA2 >3.5%, normal ferritin | Mentzer index <13 |
| B12 Deficiency | 100-130 fL | B12 <200 pg/mL, MMA >0.4 μmol/L | Homocysteine >15 μmol/L |
| Folate Deficiency | 100-130 fL | Folate <2 ng/mL, normal MMA | Homocysteine >15 μmol/L |
| Hemolytic Anemia | 80-110 fL | LDH >600 U/L, haptoglobin <25 mg/dL | Reticulocytes >3% |
Bleeding Disorder Evaluation: Hemostatic Pattern Recognition
Bleeding disorders present with characteristic patterns based on underlying defects: platelet disorders cause mucocutaneous bleeding, while coagulation defects produce deep tissue hemorrhage. Initial screening uses PT/PTT to localize pathway abnormalities.
-
Platelet-Type Bleeding Pattern
- Petechiae, purpura on dependent areas
- Mucosal bleeding: epistaxis, menorrhagia, GI bleeding
- Immediate post-surgical bleeding
- Normal PT/PTT with prolonged bleeding time
-
Coagulation-Type Bleeding Pattern
- Deep tissue hematomas in muscles, joints
- Delayed post-surgical bleeding (hours to days)
- Hemarthroses (especially knees, elbows, ankles)
- Abnormal PT and/or PTT
📌 Remember: HEMOPHILIA bleeding sites - Hemarthroses, Ecchymoses (deep), Muscle hematomas, Operative bleeding (delayed), Peritoneal bleeding, Hematuria, Intracranial hemorrhage, Lifelong history, Inherited pattern, Abnormal PTT.
Thrombocytopenia Diagnostic Framework
Thrombocytopenia evaluation requires systematic approach distinguishing decreased production, increased destruction, and sequestration. Platelet count thresholds determine bleeding risk and management urgency.
-
Production Defects
- Bone marrow failure: aplastic anemia, malignancy
- Nutritional deficiencies: B12, folate
- Drug-induced: chemotherapy, alcohol
- Bone marrow biopsy shows decreased megakaryocytes
-
Destruction/Consumption
- Immune thrombocytopenic purpura (ITP): isolated thrombocytopenia
- Thrombotic thrombocytopenic purpura (TTP): pentad of findings
- Disseminated intravascular coagulation (DIC): consumption coagulopathy
- Bone marrow shows increased megakaryocytes
💡 Master This: TTP pentad occurs in <10% of cases, but thrombocytopenia + microangiopathic hemolytic anemia + neurologic symptoms mandate immediate plasmapheresis. ADAMTS13 activity <10% confirms diagnosis, but treatment cannot await results due to >90% mortality if untreated.
| Thrombocytopenia Cause | Platelet Count | Key Features | Diagnostic Test |
|---|---|---|---|
| ITP | 20,000-80,000/μL | Isolated, no splenomegaly | Clinical diagnosis |
| TTP | 10,000-40,000/μL | MAHA + neuro symptoms | ADAMTS13 <10% |
| DIC | 50,000-100,000/μL | ↑PT/PTT, ↑D-dimer | Fibrinogen <150 mg/dL |
| Hypersplenism | 50,000-120,000/μL | Splenomegaly, pancytopenia | Spleen size >12 cm |
| Drug-induced | Variable | Temporal relationship | Drug rechallenge |
🔧 The Diagnostic Arsenal: Pattern Recognition in Hematologic Disease
🔍 The Differential Matrix: Systematic Hematologic Discrimination
Microcytic Anemia Discrimination Matrix
Microcytic anemias share MCV <80 fL but demonstrate distinct laboratory patterns enabling definitive differentiation. Iron studies, hemoglobin electrophoresis, and family history provide discriminating data points.
| Parameter | Iron Deficiency | Thalassemia Trait | Anemia of Chronic Disease | Lead Poisoning | Sideroblastic |
|---|---|---|---|---|---|
| Ferritin | <15 ng/mL | 50-200 ng/mL | >100 ng/mL | Normal | >500 ng/mL |
| TIBC | >450 μg/dL | 250-400 μg/dL | <250 μg/dL | Normal | <250 μg/dL |
| Transferrin Sat | <16% | 20-30% | <16% | Normal | >50% |
| RBC Count | ↓↓ (3.0-4.0) | Normal/↑ (4.5-6.0) | ↓ (3.5-4.5) | ↓ (3.5-4.5) | ↓ (3.0-4.0) |
| HbA2 | Normal (2-3%) | ↑ (>3.5%) | Normal | Normal | Normal |
| Family History | Negative | Positive | Variable | Environmental | Variable |
| Response to Iron | Excellent | None | Poor | None | None |
The Shine-Lal index provides additional discrimination: (MCV² × MCH)/100. Values <1,530 suggest thalassemia trait, while >1,530 indicates iron deficiency with 85% accuracy.
⭐ Clinical Pearl: Thalassemia trait patients maintain normal ferritin and show target cells on blood smear, while iron deficiency demonstrates pencil cells and low ferritin. Inappropriate iron therapy in thalassemia trait can cause iron overload over years.
Macrocytic Anemia Differentiation Framework
Macrocytic anemias (MCV >100 fL) divide into megaloblastic and non-megaloblastic categories based on vitamin B12/folate status and bone marrow morphology. Methylmalonic acid (MMA) and homocysteine provide definitive biochemical discrimination.
-
B12 Deficiency Discrimination
- Serum B12 <200 pg/mL with MMA >0.4 μmol/L
- Neurologic symptoms: peripheral neuropathy, subacute combined degeneration
- Intrinsic factor antibodies in 50-70% of pernicious anemia
- Schilling test (historical): <10% excretion corrected by intrinsic factor
-
Folate Deficiency Discrimination
- Serum folate <2 ng/mL or RBC folate <140 ng/mL
- Normal MMA with elevated homocysteine >15 μmol/L
- No neurologic symptoms (distinguishes from B12 deficiency)
- Rapid response to folate supplementation (reticulocytosis in 3-5 days)
📌 Remember: FOLD for folate deficiency causes - Folic acid antagonists (methotrexate), Old age with poor nutrition, Liver disease/alcohol, Dialysis and increased demands (pregnancy, hemolysis). Folate stores last 2-4 months vs B12 stores lasting 2-4 years.
Hemolytic Anemia Classification System
Hemolytic anemias require systematic classification into intrinsic (hereditary) vs extrinsic (acquired) causes, using family history, age of onset, and specific laboratory markers for discrimination.
| Category | Specific Disorder | Key Laboratory Finding | Diagnostic Test | Prevalence |
|---|---|---|---|---|
| Membrane | Hereditary Spherocytosis | ↑MCHC >36 g/dL | Osmotic fragility | 1:2,000 |
| Enzyme | G6PD Deficiency | Bite cells, blister cells | G6PD assay | 1:10 (Mediterranean) |
| Hemoglobin | Sickle Cell Disease | Sickle cells, ↑HbS | Hb electrophoresis | 1:365 (African American) |
| Immune | Autoimmune HA | Spherocytes, ↑bilirubin | Direct Coombs | 1:80,000 |
| Microangiopathic | TTP/HUS | Schistocytes >1% | ADAMTS13 activity | 1:100,000 |
| Infection | Malaria | Parasites in RBCs | Blood smear | Endemic areas |
💡 Master This: Direct Coombs test detects antibodies bound to RBCs, while indirect Coombs detects free antibodies in serum. IgG-positive suggests warm autoimmune hemolytic anemia, while C3-positive indicates cold agglutinin disease or complement-mediated hemolysis.
Understanding these discrimination matrices transforms pattern recognition from memorization to systematic analysis, enabling confident differentiation between morphologically similar conditions that require distinct therapeutic approaches.
🔍 The Differential Matrix: Systematic Hematologic Discrimination
⚖️ The Therapeutic Command Center: Evidence-Based Hematologic Management
Iron Deficiency Management: Precision Repletion Strategies
Iron deficiency treatment requires systematic approach addressing underlying causes while optimizing iron absorption and monitoring response parameters. Route selection depends on severity, tolerance, and urgency of correction.
Oral Iron Optimization Protocol:
- Elemental iron dose: 100-200 mg daily (divided doses reduce GI side effects)
- Absorption enhancers: Vitamin C 500 mg increases absorption 3-4x
- Timing: 1 hour before meals or 2 hours after for maximum absorption
- Avoid: Calcium, tea, coffee within 2 hours (reduce absorption 50-90%)
📌 Remember: IRON absorption inhibitors - Infection/inflammation, Rice/grains (phytates), Other minerals (Ca, Zn), Normal stomach acid required. Proton pump inhibitors reduce iron absorption by 65% through decreased gastric acid production.
| Iron Preparation | Elemental Iron Content | Absorption Rate | Side Effect Profile | Cost Ratio |
|---|---|---|---|---|
| Ferrous Sulfate | 65 mg/325 mg tablet | 15-20% | High GI (30-40%) | 1x |
| Ferrous Gluconate | 36 mg/325 mg tablet | 12-15% | Moderate GI (20-30%) | 2x |
| Ferrous Fumarate | 106 mg/325 mg tablet | 18-25% | High GI (35-45%) | 1.5x |
| Polysaccharide Iron | 150 mg/capsule | 10-15% | Low GI (10-15%) | 4x |
| IV Iron Sucrose | 100-200 mg/dose | 95-100% | Minimal GI (<5%) | 20x |
IV Iron Indications (Evidence-Based):
- Oral iron intolerance (GI side effects >grade 2)
- Malabsorption syndromes (celiac, IBD, gastric bypass)
- Chronic kidney disease with eGFR <30 mL/min
- Heart failure with LVEF <40% (improves symptoms regardless of anemia)
- Urgent correction needed (surgery within 4-6 weeks)
⭐ Clinical Pearl: Iron sucrose 200 mg IV weekly for 5 doses (total 1,000 mg) provides equivalent iron repletion to 6 months oral therapy with >90% tolerance vs 60% oral tolerance. Ferritin increases 100-200 ng/mL per 200 mg IV iron dose.
Anticoagulation Precision: Risk-Benefit Optimization
Anticoagulation management requires continuous risk-benefit assessment using validated scoring systems and laboratory monitoring to optimize thrombosis prevention while minimizing bleeding complications.
Atrial Fibrillation Anticoagulation Decision Framework:
- CHA2DS2-VASc Score Calculation
- Congestive heart failure (1 point)
- Hypertension (1 point)
- Age ≥75 years (2 points)
- Diabetes (1 point)
- Stroke/TIA history (2 points)
- Vascular disease (1 point)
- Age 65-74 years (1 point)
- Sex category (female) (1 point)
📌 Remember: CHADS scoring for stroke risk - CHF (2.5x risk), Hypertension (1.8x risk), Age >75 (2.5x risk), Diabetes (1.7x risk), Stroke history (2.5x risk). Score ≥2 mandates anticoagulation unless high bleeding risk.
| CHA2DS2-VASc Score | Annual Stroke Risk | Anticoagulation Recommendation | Evidence Level |
|---|---|---|---|
| 0 (men) | 0.2% | No anticoagulation | Class I |
| 1 (women) | 0.3% | No anticoagulation | Class I |
| 1 (men) | 0.9% | Consider anticoagulation | Class IIa |
| 2 (women) | 1.3% | Anticoagulation recommended | Class I |
| ≥2 | 2.2-15.2% | Anticoagulation recommended | Class I |
- DOAC preferred: CrCl >30 mL/min, no mechanical valves, patient preference
- Warfarin required: Mechanical heart valves, severe mitral stenosis, CrCl <15 mL/min
- DOAC dosing: Apixaban 5 mg BID (reduce to 2.5 mg BID if ≥2 criteria: age ≥80, weight ≤60 kg, creatinine ≥1.5 mg/dL)
💡 Master This: HAS-BLED score predicts major bleeding risk: Hypertension, Abnormal liver/kidney function, Stroke history, Bleeding history, Labile INRs, Elderly (>65), Drugs/alcohol. Score ≥3 indicates high bleeding risk (>3% annually) requiring enhanced monitoring.
Thrombocytopenia Management Algorithms
Thrombocytopenia treatment depends on underlying mechanism, bleeding severity, and platelet count thresholds. ITP management exemplifies evidence-based escalation from observation to immunosuppression.
ITP Treatment Escalation Protocol:
- Platelet count >30,000/μL: Observation with activity restrictions
- Platelet count 10,000-30,000/μL: Corticosteroids (prednisone 1 mg/kg daily)
- Platelet count <10,000/μL: High-dose steroids + IVIG 1 g/kg × 2 days
- Refractory disease: Splenectomy or thrombopoietin receptor agonists
⭐ Clinical Pearl: Platelet transfusion thresholds vary by clinical scenario: <10,000/μL for spontaneous bleeding risk, <50,000/μL for major surgery, <100,000/μL for neurosurgery/ophthalmologic surgery. Each platelet unit increases count by 5,000-10,000/μL in average adult.
Understanding these therapeutic frameworks enables systematic management of complex hematologic conditions while optimizing outcomes through evidence-based decision making and appropriate monitoring strategies.
⚖️ The Therapeutic Command Center: Evidence-Based Hematologic Management
🔗 The Integration Matrix: Multi-System Hematologic Orchestration
Cardio-Hematologic Integration: The Circulation-Blood Interface
Cardiovascular and hematologic systems demonstrate intimate physiologic coupling, where cardiac output, vascular integrity, and blood rheology create interdependent relationships affecting oxygen delivery and hemostatic balance.
Anemia-Cardiac Compensation Mechanisms:
- Hemoglobin <10 g/dL triggers cardiac output increase of 20-30%
- Stroke volume increases through enhanced venous return and reduced afterload
- Heart rate increases 10-15 bpm per 1 g/dL hemoglobin decrease
- Coronary blood flow increases 50-100% to maintain myocardial oxygen delivery
📌 Remember: CARDIAC compensation in anemia - Cardiac output ↑, Afterload ↓ (viscosity), Rate ↑, Diastolic filling ↑, Inotropic state ↑, Arterial vasodilation, Coronary flow ↑. Severe anemia (Hb <7 g/dL) can precipitate high-output heart failure in patients with underlying cardiac disease.
Thrombosis-Cardiovascular Risk Stratification:
- Atrial fibrillation increases stroke risk 5-fold (2-7% annually)
- Mechanical heart valves require INR 2.5-3.5 (higher intensity than tissue valves)
- Acute coronary syndromes mandate dual antiplatelet therapy for 12 months
- Peripheral arterial disease increases cardiovascular mortality 3-4x
| Cardiovascular Condition | Thrombotic Risk | Anticoagulation Target | Bleeding Risk Modifier |
|---|---|---|---|
| Atrial Fibrillation | 2-7% annually | INR 2.0-3.0 | Age >75 (+2 points) |
| Mechanical Valve | 4-8% annually | INR 2.5-3.5 | Valve position (mitral > aortic) |
| Acute MI | 2-4% in 30 days | Dual antiplatelet | GI bleeding history |
| Heart Failure | 1-3% annually | Consider if EF <35% | Renal dysfunction |
| Cardiomyopathy | Variable | Case-dependent | Liver disease |
Nephro-Hematologic Convergence: Kidney-Blood Interactions
Renal disease profoundly affects hematologic parameters through erythropoietin deficiency, uremic bleeding, mineral metabolism disorders, and drug clearance alterations requiring specialized management approaches.
Chronic Kidney Disease Anemia Management:
- EPO deficiency begins when eGFR <30 mL/min (Stage 4 CKD)
- Target hemoglobin: 10-11 g/dL (avoid >11.5 g/dL due to cardiovascular risk)
- Iron requirements: Transferrin saturation >20%, ferritin >100 ng/mL
- ESA dosing: Epoetin alfa 50-100 units/kg 3x weekly initially
📌 Remember: KIDNEY effects on hematology - Kidney EPO production ↓, Iron absorption ↓, Drug clearance ↓, Nutritional deficiencies, Electrolyte imbalances, Yuremic bleeding. Uremic bleeding occurs when BUN >60 mg/dL through platelet dysfunction and vWF abnormalities.
Anticoagulation in Renal Disease:
- CrCl >50 mL/min: Standard DOAC dosing
- CrCl 30-50 mL/min: Reduced DOAC doses (apixaban 2.5 mg BID)
- CrCl 15-30 mL/min: Warfarin preferred (INR monitoring)
- CrCl <15 mL/min: Warfarin only (DOACs contraindicated)
| eGFR Range | Anemia Prevalence | EPO Deficiency | Iron Management | ESA Response |
|---|---|---|---|---|
| >60 | 5-10% | Rare | Standard oral | Normal |
| 45-60 | 10-20% | Mild | Enhanced absorption | Slightly ↓ |
| 30-45 | 20-40% | Moderate | IV preferred | Reduced |
| 15-30 | 40-70% | Severe | IV required | Poor |
| <15 | 70-90% | Profound | IV + frequent monitoring | Very poor |
Hepato-Hematologic Synthesis: Liver-Blood Production Networks
Hepatic disease creates complex hematologic abnormalities through synthetic dysfunction, portal hypertension, hypersplenism, and altered drug metabolism requiring integrated management strategies.
Liver Disease Coagulation Abnormalities:
- Factor synthesis defects: II, V, VII, IX, X (vitamin K-dependent factors)
- Protein C/S deficiency: Paradoxical thrombosis risk despite prolonged PT/PTT
- Fibrinogen abnormalities: Dysfibrinogenemia with functional defects
- Platelet abnormalities: Hypersplenism + decreased thrombopoietin
Portal Hypertension Hematologic Effects:
- Splenomegaly causes pancytopenia through sequestration
- Platelet count typically 50,000-100,000/μL (rarely <30,000/μL)
- Leukopenia with WBC 2,000-4,000/μL
- Anemia from GI bleeding + hypersplenism + nutritional deficiencies
⭐ Clinical Pearl: INR elevation in liver disease doesn't predict bleeding risk due to balanced deficiency of procoagulant and anticoagulant factors. Thromboelastography provides better assessment of hemostatic function than standard coagulation tests.
Understanding these multi-system interactions enables comprehensive hematologic care that addresses underlying pathophysiology while optimizing therapeutic interventions across multiple organ systems simultaneously.
🔗 The Integration Matrix: Multi-System Hematologic Orchestration
🎯 The Clinical Mastery Arsenal: Rapid-Fire Hematologic Excellence
Essential Hematologic Thresholds: The Numbers That Matter
Critical Laboratory Values for Immediate Action:
-
Hemoglobin Crisis Points
- <7 g/dL: Transfusion threshold for most patients
- <8 g/dL: Transfusion threshold for cardiac disease
- <10 g/dL: Symptomatic anemia requiring investigation
- >18 g/dL: Polycythemia requiring phlebotomy consideration
-
Platelet Emergency Thresholds
- <10,000/μL: Spontaneous bleeding risk - immediate intervention
- <50,000/μL: Major surgery contraindication
- <100,000/μL: Neurosurgery/eye surgery contraindication
- >1,000,000/μL: Thrombotic risk from platelet dysfunction
📌 Remember: BLEEDING platelet thresholds - Brain surgery <100K, Liver biopsy <60K, Endoscopy <50K, Emergency surgery <50K, Dental extraction <30K, IM injections <20K, No procedures <10K, GI bleeding risk <5K.
| Clinical Scenario | Hemoglobin Threshold | Platelet Threshold | INR Threshold | Action Required |
|---|---|---|---|---|
| Emergency Surgery | <8 g/dL | <50,000/μL | >1.5 | Immediate correction |
| Neurosurgery | <10 g/dL | <100,000/μL | >1.3 | Pre-op optimization |
| Cardiac Surgery | <8 g/dL | <100,000/μL | >1.4 | Specialized protocols |
| GI Bleeding | <7 g/dL | <50,000/μL | >2.0 | Urgent reversal |
| ICU Patient | <7 g/dL | <20,000/μL | >2.5 | Critical intervention |
The "See This, Think That" Clinical Correlations:
- Schistocytes + Thrombocytopenia → Think TTP/HUS → Order ADAMTS13
- Spherocytes + Jaundice → Think Hereditary Spherocytosis → Osmotic fragility
- Target Cells + Microcytosis → Think Thalassemia → Hemoglobin electrophoresis
- Bite Cells + Hemolysis → Think G6PD Deficiency → G6PD enzyme assay
- Rouleaux + Back Pain → Think Multiple Myeloma → SPEP/UPEP
📌 Remember: SCHISTOCYTES differential - Stomach cancer (microangiopathic), Cancer (any), HUS/TTP, Infection (malaria), Severe hypertension, Thrombotic disorders, Other MAHA causes, Cardiac (mechanical valves), Yakult (just kidding - Young age suggests HUS), Transfusion reactions, Eclampsia, Sepsis with DIC.
Emergency Hematologic Protocols
Life-Threatening Scenarios Requiring Immediate Action:
-
TTP/HUS Management
- Plasmapheresis within 4-6 hours of diagnosis
- Daily plasma exchange until platelet count >150,000/μL × 2 days
- Avoid platelet transfusions (may worsen thrombosis)
- Mortality >90% if untreated, <10% with prompt treatment
-
DIC Management Protocol
- Treat underlying cause (sepsis, malignancy, trauma)
- Platelet transfusion if <50,000/μL and bleeding
- FFP if INR >1.5 and bleeding
- Cryoprecipitate if fibrinogen <100 mg/dL
⭐ Clinical Pearl: Massive transfusion protocol (>10 units RBC in 24 hours) requires 1:1:1 ratio of RBC:FFP:platelets to prevent dilutional coagulopathy. Tranexamic acid 1 g IV within 3 hours of trauma reduces mortality by 1.5%.
Quick Reference Formulas
Essential Calculations for Clinical Practice:
- Reticulocyte Index = (Reticulocyte % × Patient Hct) ÷ (45 × Maturation Factor)
- Corrected WBC = (WBC × 100) ÷ (100 + NRBC count)
- Platelet Increment = (Post-transfusion - Pre-transfusion) × BSA ÷ Platelets transfused
- Iron Deficit = Weight (kg) × (15 - Patient Hb) × 2.4 + 500 mg
💡 Master This: Rule of 3s in hematology - Hematocrit ≈ 3 × Hemoglobin, RBC count ≈ Hemoglobin ÷ 3, Each unit RBC raises Hb by 1 g/dL and Hct by 3%. Each platelet unit raises count by 5,000-10,000/μL.
| Emergency Situation | First-Line Treatment | Dosing | Expected Response | Monitoring |
|---|---|---|---|---|
| Severe ITP | IVIG + Steroids | 1 g/kg × 2d + Pred 1 mg/kg | Plt >50K in 48-72h | Daily CBC |
| TTP | Plasmapheresis | 1-1.5 plasma volumes daily | Plt >150K × 2d | Daily CBC, LDH |
| DIC | Treat cause + Support | FFP/Plt/Cryo PRN | Stabilize coags | q6h labs |
| Massive Bleeding | MTP activation | 1:1:1 ratio products | Hemostasis | Real-time labs |
| Sickle Crisis | Hydration + Analgesia | 1.5× maintenance + opioids | Pain control | Pain scores |
🎯 The Clinical Mastery Arsenal: Rapid-Fire Hematologic Excellence
Practice Questions: Hematology (anemias, clotting disorders)
Test your understanding with these related questions
A 25-year-old woman is being evaluated due to complaint of fatigue and voiding pink urine. The laboratory results are as follows: Hb 6.7 Red blood cell count 3.0 x 1012/L Leukocyte count 5,000/mm3 Platelets 170 x 109/L Reticulocyte count 6% Hematocrit 32% The physician thinks that the patient is suffering from an acquired mutation in hematopoietic stem cells, which is confirmed by flow cytometry analysis that revealed these cells are CD 55 and CD 59 negative. However, the physician is interested in knowing the corrected reticulocyte count before starting the patient on eculizumab. What value does the physician find after calculating the corrected reticulocyte count?













