Heart failure

On this page
💓 The Failing Heart Engine: Understanding Cardiac Decompensation
Heart failure isn't simply a weak pump-it's a complex syndrome where the heart triggers cascading compensatory mechanisms that ultimately worsen the very problem they're trying to solve. You'll learn how reduced cardiac output activates neurohormonal systems, why patients develop distinct clinical phenotypes based on ejection fraction, and how modern therapies interrupt these pathological cycles to reduce mortality. By mastering the physiology behind decompensation and the evidence-based treatment hierarchy, you'll confidently recognize, classify, and manage one of medicine's most prevalent and deadly conditions.
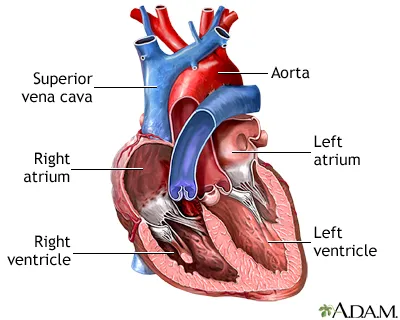
The heart's pumping mechanism depends on three fundamental components working in perfect synchrony:
-
Preload - venous return determining ventricular filling
- Normal central venous pressure: 2-8 mmHg
- Optimal left ventricular end-diastolic pressure: 8-12 mmHg
- Frank-Starling mechanism optimizes contractility based on stretch
-
Afterload - arterial resistance against ventricular ejection
- Normal systemic vascular resistance: 800-1200 dynes·sec/cm⁵
- Aortic systolic pressure typically 100-140 mmHg
- Increased afterload reduces stroke volume exponentially
-
Contractility - intrinsic myocardial force generation
- Normal ejection fraction: 55-70%
- Stroke volume: 60-80 mL per beat
- Cardiac index: 2.5-4.0 L/min/m²
📌 Remember: PUMP - Preload, Unloading (afterload), Myocardial contractility, Perfusion pressure. These four factors determine cardiac output through the equation: CO = HR × SV, where stroke volume depends on preload, afterload, and contractility.
| Parameter | Normal Range | Mild HF | Moderate HF | Severe HF | Clinical Significance |
|---|---|---|---|---|---|
| Ejection Fraction | 55-70% | 45-54% | 30-44% | <30% | Primary classification metric |
| BNP (pg/mL) | <100 | 100-400 | 400-1000 | >1000 | Diagnostic and prognostic |
| Cardiac Index | 2.5-4.0 | 2.0-2.4 | 1.5-1.9 | <1.5 | Hemodynamic assessment |
| NYHA Class | - | I-II | II-III | III-IV | Functional capacity |
| 1-year Mortality | <1% | 5-10% | 15-25% | 30-50% | Risk stratification |
- Sympathetic activation - increases heart rate by 20-40% and contractility
- Renin-angiotensin-aldosterone system - retains sodium and water
- Ventricular remodeling - chamber dilation and wall thickening
⭐ Clinical Pearl: BNP levels correlate directly with ventricular wall stress. Values >400 pg/mL indicate significant heart failure with 95% sensitivity, while levels >1000 pg/mL predict 30-day readmission risk of 25%.
💡 Master This: Heart failure is not a single disease but a syndrome resulting from any structural or functional cardiac abnormality that impairs ventricular filling or ejection. The key is recognizing that symptoms develop when compensatory mechanisms become maladaptive, typically when ejection fraction drops below 40% or filling pressures exceed 18 mmHg.
Understanding these foundational concepts provides the framework for recognizing the two major heart failure phenotypes and their distinct pathophysiological mechanisms.
💓 The Failing Heart Engine: Understanding Cardiac Decompensation
🔄 Compensatory Mechanisms: The Heart's Emergency Response
Immediate Compensatory Responses (0-24 hours):
-
Sympathetic nervous system activation
- Norepinephrine levels increase 3-5 fold within hours
- Heart rate increases by 15-30 beats per minute
- Contractility enhanced through β1-adrenergic stimulation
- Peripheral vasoconstriction maintains blood pressure
-
Frank-Starling mechanism
- Increased venous return stretches myocardial fibers
- Enhanced contractility up to optimal fiber length
- Stroke volume maintained despite reduced ejection fraction
- Effective until filling pressures exceed 20 mmHg
Intermediate Responses (24 hours - weeks):
-
Renin-angiotensin-aldosterone system (RAAS)
- Renin release increases 2-3 fold within 24 hours
- Angiotensin II levels rise 4-6 fold
- Aldosterone secretion increases 3-5 fold
- Sodium retention of 2-4 mEq/day above normal
-
Antidiuretic hormone (ADH) release
- Plasma ADH levels increase 2-4 fold
- Free water retention despite hyponatremia
- Contributes to 5-15% of total volume expansion
📌 Remember: RAAS HARM - Renin release, Angiotensin conversion, Aldosterone secretion, Sodium retention leads to Hypertrophy, Arrhythmias, Remodeling, Mortality. This system initially helps but ultimately accelerates heart failure progression.
| Compensatory Mechanism | Timeline | Initial Benefit | Long-term Consequence | Therapeutic Target |
|---|---|---|---|---|
| Sympathetic Activation | Minutes | ↑ CO by 20-30% | Arrhythmias, ischemia | β-blockers |
| Frank-Starling | Minutes | ↑ SV by 15-25% | Pulmonary congestion | Diuretics |
| RAAS Activation | Hours | Maintain BP | Remodeling, fibrosis | ACE-I/ARB |
| Ventricular Remodeling | Weeks | ↑ Wall stress tolerance | Progressive dysfunction | Aldosterone antagonists |
| Myocyte Hypertrophy | Months | ↑ Contractile mass | Diastolic dysfunction | Exercise training |
-
Ventricular remodeling
- Chamber dilation increases wall stress by 50-100%
- Wall thickness increases by 20-40% (concentric hypertrophy)
- Spherical shape change reduces ejection efficiency
- Mitral regurgitation develops in 60-80% of patients
-
Myocardial fibrosis
- Collagen deposition increases 3-5 fold
- Myocyte apoptosis rate increases 10-20 fold
- Capillary density decreases by 20-30%
- Conduction abnormalities develop in 40-60%
⭐ Clinical Pearl: Plasma norepinephrine levels >900 pg/mL (normal: 200-400 pg/mL) predict 2-year mortality of 50% in heart failure patients. This biomarker reflects the degree of sympathetic activation and correlates with disease severity better than ejection fraction alone.
The transition from compensated to decompensated heart failure occurs when these mechanisms can no longer maintain adequate cardiac output. Key thresholds include:
- Ejection fraction decline below 35% despite maximal compensation
- Filling pressures consistently above 18 mmHg
- Cardiac index falling below 2.0 L/min/m²
- Exercise capacity limited to <4 METs (metabolic equivalents)
💡 Master This: The compensatory mechanisms in heart failure follow the principle of "too much of a good thing." Initially life-saving responses become the primary drivers of disease progression. Successful heart failure therapy targets these maladaptive pathways: β-blockers counter sympathetic excess, ACE inhibitors block RAAS activation, and diuretics reverse volume overload.
These compensatory mechanisms set the stage for understanding the distinct pathophysiological patterns that define heart failure with reduced versus preserved ejection fraction.
🔄 Compensatory Mechanisms: The Heart's Emergency Response
🎯 Clinical Recognition: Decoding the Heart Failure Syndrome
Primary Symptom Patterns:
-
Exertional dyspnea (present in 95% of patients)
- NYHA Class II: symptoms with moderate exertion (climbing 2 flights)
- NYHA Class III: symptoms with minimal exertion (walking 1 block)
- NYHA Class IV: symptoms at rest or with any activity
- Correlates with peak VO₂ consumption: Class I >20 mL/kg/min, Class IV <10 mL/kg/min
-
Orthopnea and paroxysmal nocturnal dyspnea
- Orthopnea sensitivity 89%, specificity 74% for heart failure
- PND occurs when supine position increases venous return by 500-800 mL
- Symptoms develop when pulmonary capillary wedge pressure exceeds 18 mmHg
-
Fatigue and exercise intolerance
- Reduced cardiac reserve limits peak cardiac output to <8 L/min
- Exercise capacity measured in METs: normal >10, severe HF <4
- Correlates with skeletal muscle perfusion and oxygen extraction
📌 Remember: FACES - Fatigue, Ankle swelling, Cough (especially nocturnal), Exertional dyspnea, Shortness of breath when lying flat. These five symptoms capture 85% of heart failure presentations when systematically assessed.
Physical Examination Findings:
-
Elevated jugular venous pressure
- JVP >8 cm H₂O has 81% sensitivity for elevated filling pressures
- Hepatojugular reflux increases JVP by >3 cm in 75% of HF patients
- Correlates with right atrial pressure and predicts hospitalization risk
-
Pulmonary rales/crackles
- Present in 60-70% of acute heart failure cases
- Bilateral basilar crackles suggest pulmonary capillary pressure >25 mmHg
- May be absent in chronic HF due to lymphatic compensation
-
S3 gallop rhythm
- Sensitivity 61%, specificity 90% for systolic dysfunction
- Indicates elevated filling pressures and poor compliance
- Associated with 2-fold increased risk of cardiovascular events
-
Peripheral edema
- Requires 5-10 pounds of fluid retention before becoming apparent
- Bilateral, pitting, starting in dependent areas
- Correlates with total body sodium excess of 300-500 mEq
| Clinical Finding | Sensitivity | Specificity | Positive LR | Negative LR | Clinical Utility |
|---|---|---|---|---|---|
| Exertional dyspnea | 95% | 35% | 1.5 | 0.1 | High sensitivity, poor specificity |
| Orthopnea | 89% | 74% | 3.4 | 0.15 | Good discriminator |
| JVP >8 cm | 81% | 80% | 4.1 | 0.24 | Excellent when present |
| S3 gallop | 61% | 90% | 6.1 | 0.43 | Highly specific |
| Bilateral rales | 70% | 78% | 3.2 | 0.38 | Moderate utility |
- Young patient + acute dyspnea + clear lungs → Think HFpEF or flash pulmonary edema
- Elderly + gradual onset + ankle swelling → Think chronic systolic HF
- Rapid weight gain (>3 lbs in 2 days) → Think volume overload requiring diuresis
- Dyspnea + chest pain + elevated troponin → Think acute coronary syndrome with HF
- Bilateral edema + JVP elevation + hepatomegaly → Think right heart failure
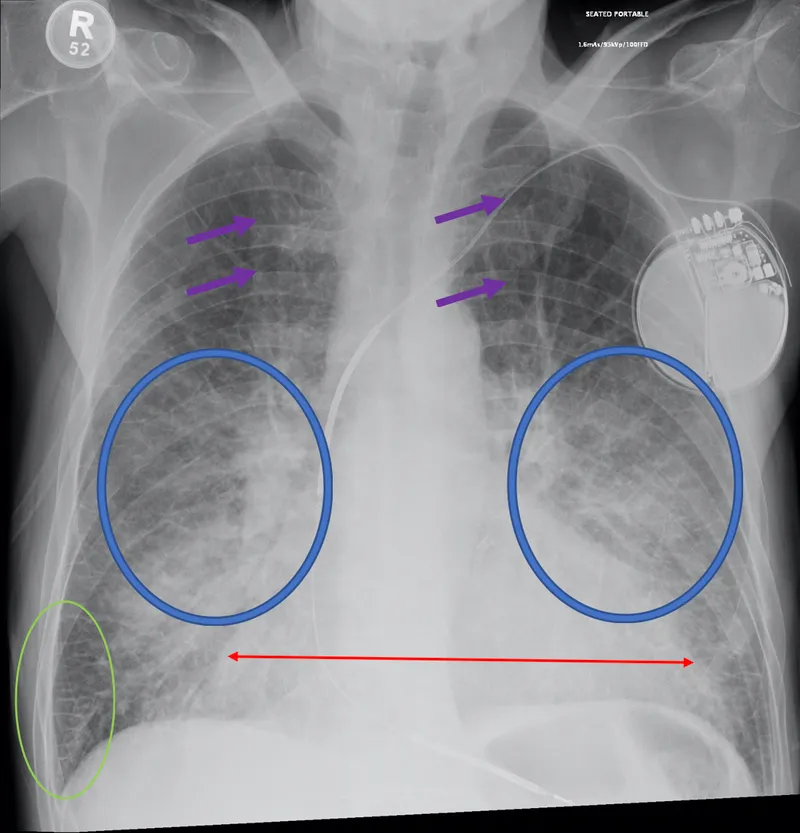
Red Flag Presentations Requiring Immediate Evaluation:
- Acute onset dyspnea with oxygen saturation <90%
- Chest pain with heart failure symptoms (rule out ACS)
- Syncope or presyncope (evaluate for arrhythmias)
- Rapid weight gain >5 pounds in 1 week
- Altered mental status (consider cardiogenic shock)
⭐ Clinical Pearl: The combination of BNP >400 pg/mL plus two clinical criteria (dyspnea, edema, elevated JVP) has 96% sensitivity and 84% specificity for heart failure diagnosis. This approach reduces diagnostic uncertainty and guides appropriate therapy initiation.
Differential Diagnosis Considerations:
- Pulmonary causes: COPD, pneumonia, pulmonary embolism
- Renal causes: Chronic kidney disease, nephrotic syndrome
- Hepatic causes: Cirrhosis with ascites
- Venous causes: Chronic venous insufficiency
- Medication-related: NSAIDs, calcium channel blockers, thiazolidinediones
💡 Master This: Heart failure diagnosis requires integration of clinical presentation, physical examination, and objective testing. No single finding confirms or excludes the diagnosis. The key is recognizing symptom patterns that suggest elevated filling pressures or reduced cardiac output, then confirming with echocardiography and natriuretic peptides.
This clinical recognition framework provides the foundation for understanding how heart failure manifests differently based on underlying pathophysiology and ejection fraction status.
🎯 Clinical Recognition: Decoding the Heart Failure Syndrome
⚖️ HFrEF vs HFpEF: The Tale of Two Failures
Heart Failure with Reduced Ejection Fraction (HFrEF):
Pathophysiology:
- Primary defect: Impaired systolic contraction
- Ejection fraction: <40% (normal 55-70%)
- Stroke volume: Reduced to 40-60 mL (normal 70-80 mL)
- Compensatory mechanism: Chamber dilation to maintain stroke volume
- Wall stress: Markedly elevated due to increased radius
Underlying Etiologies:
-
Ischemic cardiomyopathy (60-70% of cases)
- Prior myocardial infarction with scar formation
- Chronic coronary artery disease with hibernating myocardium
- Acute coronary syndromes with large territory involvement
-
Non-ischemic dilated cardiomyopathy (30-40% of cases)
- Idiopathic dilated cardiomyopathy
- Viral myocarditis (especially Coxsackievirus, adenovirus)
- Toxic cardiomyopathy (alcohol, chemotherapy, cocaine)
- Genetic mutations (titin, lamin A/C, dystrophin)
📌 Remember: DILATED - Drug toxicity, Ischemia, Lamin mutations, Alcohol, Titin defects, Enteroviruses, Dystrophin abnormalities. These seven categories account for 85% of HFrEF cases and guide specific therapeutic interventions.
| Characteristic | HFrEF | HFpEF | Clinical Significance |
|---|---|---|---|
| Ejection Fraction | <40% | ≥50% | Primary classification criterion |
| Age (mean) | 65 years | 75 years | HFpEF more common in elderly |
| Gender | 70% male | 60% female | Different risk factor profiles |
| Comorbidities | CAD (70%) | HTN (90%), DM (50%) | Guides targeted therapy |
| 5-year Mortality | 50-60% | 35-45% | HFrEF has worse prognosis |
| Hospitalization Rate | 35%/year | 30%/year | Similar healthcare burden |
| Response to ACE-I | ↓ mortality 20% | No proven benefit | Evidence-based therapy differs |
Pathophysiology:
- Primary defect: Impaired diastolic relaxation and filling
- Ejection fraction: ≥50% with normal systolic function
- Left ventricular compliance: Reduced by 40-60%
- Filling pressures: Elevated (>15 mmHg at rest, >25 mmHg with exercise)
- Chamber geometry: Concentric remodeling with thick walls
Underlying Mechanisms:
-
Diastolic dysfunction
- Impaired active relaxation (energy-dependent process)
- Increased passive stiffness from fibrosis
- Elevated filling pressures despite normal volumes
-
Systemic inflammation
- Elevated CRP, IL-6, TNF-α levels
- Endothelial dysfunction with reduced NO bioavailability
- Oxidative stress promoting myocardial fibrosis
-
Comorbidity-driven pathophysiology
- Hypertension in 90% of patients
- Diabetes mellitus in 50% of patients
- Obesity (BMI >30) in 60% of patients
- Chronic kidney disease in 40% of patients
Key Diagnostic Differences:
-
Echocardiographic patterns
- HFrEF: Dilated chambers, reduced wall motion, low EF
- HFpEF: Normal chamber size, preserved wall motion, elevated E/e' ratio >15
-
Biomarker profiles
- HFrEF: BNP typically >1000 pg/mL, troponin often elevated
- HFpEF: BNP 100-500 pg/mL, normal troponin unless acute
-
Exercise hemodynamics
- HFrEF: Reduced cardiac output response, chronotropic incompetence
- HFpEF: Exaggerated blood pressure response, rapid E/e' increase
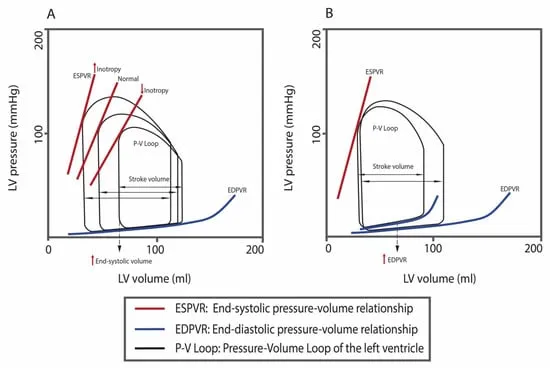
⭐ Clinical Pearl: H2FPEF score predicts HFpEF probability: Heavy (BMI >30, 2 points), Hypertensive (2 points), Fib (atrial fibrillation, 3 points), Pulmonary hypertension (1 point), Elderly (>60 years, 1 point), Filling pressures (E/e' >9, 1 point). Score ≥6 indicates 90% probability of HFpEF.
Therapeutic Implications:
-
HFrEF evidence-based therapy
- ACE inhibitors reduce mortality by 20-25%
- β-blockers reduce mortality by 30-35%
- Aldosterone antagonists reduce mortality by 15-20%
- SGLT2 inhibitors reduce hospitalizations by 25-30%
-
HFpEF management challenges
- No proven mortality benefit from ACE inhibitors or ARBs
- Limited evidence for β-blockers except rate control
- Focus on comorbidity management and symptom control
- SGLT2 inhibitors show promise for hospitalization reduction
💡 Master This: HFrEF and HFpEF represent fundamentally different disease processes requiring distinct therapeutic approaches. HFrEF responds to neurohormonal blockade that improves survival, while HFpEF management focuses on comorbidity optimization and symptom relief. The key is accurate phenotyping through comprehensive echocardiographic assessment and clinical context.
Understanding these phenotypic differences guides the selection of appropriate evidence-based therapies and sets realistic treatment expectations for each patient population.
⚖️ HFrEF vs HFpEF: The Tale of Two Failures
🎯 Evidence-Based Treatment Arsenal: The Therapeutic Hierarchy
The Four Pillars of HFrEF Therapy:
Pillar 1: ACE Inhibitors/ARBs/ARNI
-
Mechanism: Block renin-angiotensin system, reduce afterload and preload
-
Mortality benefit: 20-25% reduction in cardiovascular death
-
Target doses:
- Lisinopril 20-40 mg daily
- Enalapril 10-20 mg twice daily
- Losartan 50-150 mg daily
- Sacubitril/valsartan 97/103 mg twice daily
-
Monitoring requirements:
- Serum creatinine increase <30% acceptable
- Potassium levels <5.5 mEq/L
- Blood pressure >90 mmHg systolic
📌 Remember: ACE-ARNI - Angiotensin blockade, Creatinine monitoring, Electrolyte surveillance, Afterload reduction, Remodeling prevention, Natriuretic enhancement, Improved survival. These mechanisms explain the 25% mortality reduction seen with optimal RAAS blockade.
| Medication Class | Mortality Reduction | Hospitalization Reduction | Key Trials | Target Dose Achievement |
|---|---|---|---|---|
| ACE Inhibitors | 20-25% | 15-20% | SOLVD, CONSENSUS | 60-70% of patients |
| ARBs | 15-20% | 10-15% | CHARM, Val-HeFT | 70-80% of patients |
| ARNI | 16% vs ACE-I | 21% vs ACE-I | PARADIGM-HF | 50-60% of patients |
| β-blockers | 30-35% | 25-30% | MERIT-HF, CIBIS | 80-90% of patients |
| Aldosterone Antagonists | 15-20% | 10-15% | RALES, EMPHASIS | 70-80% of patients |
-
Evidence-based agents: Metoprolol succinate, carvedilol, bisoprolol
-
Mortality benefit: 30-35% reduction in sudden cardiac death
-
Mechanism: Counteract sympathetic excess, improve diastolic filling
-
Target doses:
- Metoprolol succinate 200 mg daily
- Carvedilol 25 mg twice daily (50 mg if >85 kg)
- Bisoprolol 10 mg daily
-
Initiation strategy: Start low, go slow
- Begin at 25% of target dose
- Double dose every 2-4 weeks as tolerated
- Monitor heart rate >50 bpm, blood pressure >90 mmHg
Pillar 3: Aldosterone Receptor Antagonists
-
Agents: Spironolactone, eplerenone
-
Mortality benefit: 15-20% reduction in cardiovascular death
-
Mechanism: Block aldosterone-mediated fibrosis and potassium wasting
-
Dosing:
- Spironolactone 25-50 mg daily
- Eplerenone 25-50 mg daily
-
Monitoring: Potassium and creatinine at 1 week, 1 month, then quarterly
-
Contraindications: Creatinine >2.5 mg/dL, potassium >5.0 mEq/L
Pillar 4: SGLT2 Inhibitors
- Agents: Dapagliflozin, empagliflozin
- Evidence: 25% reduction in cardiovascular death and heart failure hospitalization
- Mechanism: Diuretic effect, metabolic benefits, direct cardiac protection
- Dosing: Dapagliflozin 10 mg daily, empagliflozin 10 mg daily
- Benefits: Independent of diabetes status, rapid onset within 30 days
Additional Evidence-Based Therapies:
-
Ivabradine (if heart rate >70 bpm on maximum β-blocker)
- 18% reduction in heart failure hospitalizations
- Dose: 5-7.5 mg twice daily
- Monitor for bradycardia and visual disturbances
-
Hydralazine/Isosorbide dinitrate (African American patients)
- 43% mortality reduction in African Americans
- Combination therapy: Hydralazine 75 mg + ISDN 40 mg three times daily
-
Vericiguat (recent hospitalization or elevated natriuretic peptides)
- 10% reduction in cardiovascular death and heart failure hospitalization
- Dose: 10 mg daily
⭐ Clinical Pearl: Quadruple therapy (ACE-I/ARB + β-blocker + aldosterone antagonist + SGLT2 inhibitor) reduces 5-year mortality from 50% to 25% when implemented at target doses. The challenge is systematic implementation and dose optimization over 3-6 months.
HFpEF Management Approach:
- Symptom management: Diuretics for volume overload
- Comorbidity optimization:
- Blood pressure control <130/80 mmHg
- Diabetes management (HbA1c <7%)
- Weight management (target BMI <30)
- SGLT2 inhibitors: 26% reduction in heart failure hospitalizations
- Exercise training: Improves quality of life and functional capacity
💡 Master This: Heart failure therapy success depends on systematic implementation of evidence-based medications at optimal doses, not just prescription initiation. The goal is achieving target doses of all four pillars within 3-6 months, with careful monitoring for side effects and dose adjustments based on clinical response.
This therapeutic foundation enables advanced interventions including device therapy and mechanical circulatory support for patients with persistent symptoms despite optimal medical management.
🎯 Evidence-Based Treatment Arsenal: The Therapeutic Hierarchy
🔧 Advanced Interventions: Beyond Pills and Lifestyle
Device Therapy Landscape:
Implantable Cardioverter Defibrillators (ICDs):
-
Primary prevention indications:
- Ejection fraction ≤35% despite 3 months optimal medical therapy
- Life expectancy >1 year with reasonable functional status
- NYHA Class II-III symptoms on optimal therapy
-
Mortality benefit: 23% reduction in sudden cardiac death
-
Patient selection criteria:
- Ischemic cardiomyopathy: 40 days post-MI minimum
- Non-ischemic cardiomyopathy: 3 months optimal medical therapy
- Exclude patients with <1 year life expectancy
-
Complications: Device infection (1-2%), inappropriate shocks (10-15%), lead complications (5-10%)
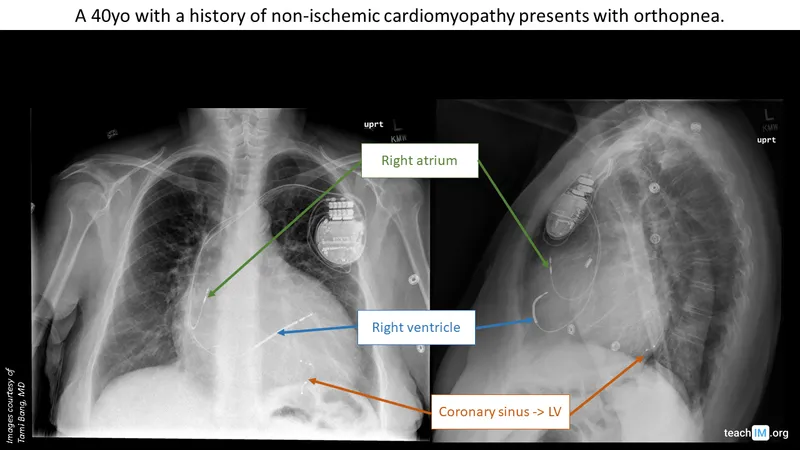
Cardiac Resynchronization Therapy (CRT):
-
Indications:
- Ejection fraction ≤35%
- QRS duration ≥150 ms (Class I) or 120-149 ms (Class IIa)
- Left bundle branch block morphology preferred
- NYHA Class II-IV symptoms despite optimal medical therapy
-
Clinical benefits:
- 25-30% reduction in heart failure hospitalizations
- 15-20% mortality reduction
- 5-10% absolute improvement in ejection fraction
- Improved quality of life scores by 15-20 points
-
Response predictors:
- LBBB morphology: 70-80% response rate
- QRS ≥150 ms: 65-75% response rate
- Female gender: 80-85% response rate
- Non-ischemic etiology: 75-80% response rate
📌 Remember: CRT-LBBB - Cardiac resynchronization for Reduced ejection fraction, Timing delays, Left bundle branch block, Broad QRS >150 ms, Better outcomes in females. These criteria identify patients with 70-80% likelihood of clinical improvement.
| Device Type | Indication | Mortality Benefit | Hospitalization Reduction | Cost-Effectiveness |
|---|---|---|---|---|
| ICD | EF ≤35%, life expectancy >1 year | 23% | Minimal | $50,000/QALY |
| CRT-P | EF ≤35%, QRS ≥150 ms, NYHA III-IV | 15-20% | 25-30% | $43,000/QALY |
| CRT-D | Combined ICD + CRT indications | 25-30% | 30-35% | $48,000/QALY |
| LVAD | Advanced HF, transplant candidate | 50% at 2 years | 80-90% | $200,000/QALY |
-
Bridge to transplant indications:
- Advanced heart failure despite optimal therapy
- Cardiac index <2.0 L/min/m²
- Pulmonary capillary wedge pressure >20 mmHg
- Transplant candidate with prolonged wait time
-
Destination therapy criteria:
- Advanced heart failure, not transplant candidate
- Age typically >65 years or significant comorbidities
- Functional status allowing rehabilitation
-
Outcomes with modern devices:
- 2-year survival: 70-80% (vs 25% with medical therapy)
- Quality of life: Significant improvement in 85% of patients
- Functional capacity: NYHA Class improvement by 2-3 classes
LVAD Complications and Management:
-
Bleeding (20-30% of patients annually)
- GI bleeding most common due to acquired von Willebrand syndrome
- Requires anticoagulation balance with warfarin (INR 2.0-3.0)
-
Thrombosis (5-10% annually)
- Pump thrombosis requires urgent evaluation
- Prevention through optimal anticoagulation and pump speed
-
Infection (15-25% annually)
- Driveline infections most common
- Requires aggressive antibiotic therapy, rarely device removal
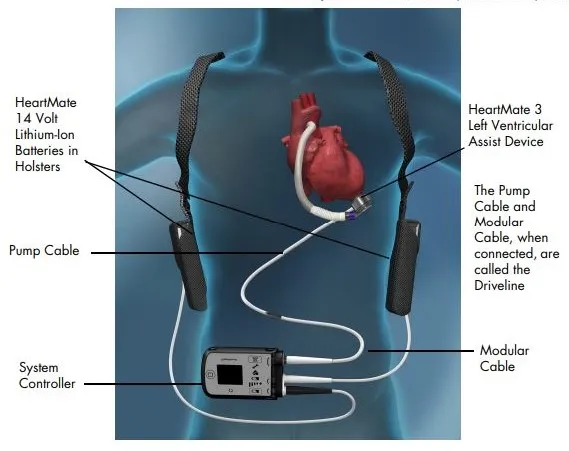
Heart Transplantation:
-
Candidate criteria:
- Advanced heart failure despite optimal therapy
- Age typically <65 years (varies by center)
- Absence of irreversible pulmonary hypertension
- Psychosocial stability and compliance
-
Outcomes:
- 1-year survival: 90-95%
- 10-year survival: 55-60%
- Median survival: 12-15 years
-
Limitations:
- Donor shortage: 3,000 transplants annually vs 50,000 potential candidates
- Average wait time: 6-12 months for Status 1A patients
⭐ Clinical Pearl: INTERMACS profiles stratify advanced heart failure severity: Profile 1 (cardiogenic shock) has 50% 1-year mortality without intervention, while Profile 4-7 patients have 80-90% 1-year survival with optimal medical therapy. This classification guides timing of advanced interventions.
Emerging Therapies:
- Cardiac contractility modulation: 25% improvement in peak VO₂
- Baroreflex activation therapy: 30% reduction in heart failure hospitalizations
- Transcatheter mitral valve repair: 47% reduction in heart failure hospitalizations
💡 Master This: Advanced heart failure interventions require careful patient selection based on functional status, comorbidities, and life expectancy. The goal is matching the right intervention to the right patient at the right time, with device therapy offering significant survival and quality of life benefits when appropriately applied.
These advanced interventions represent the culmination of comprehensive heart failure management, providing hope and improved outcomes for patients with end-stage disease.
🔧 Advanced Interventions: Beyond Pills and Lifestyle
🎯 Clinical Mastery Framework: Your Heart Failure Command Center
Essential Clinical Arsenal:
📌 Remember: OPTIMIZE-HF - Objective assessment (echo, BNP), Pharmacotherapy at target doses, Team-based care, Implantable devices when indicated, Monitoring and adjustment, Inpatient optimization, Zero tolerance for clinical inertia, Education and self-care, Hospitalization prevention, Follow-up within 7 days. This framework reduces 30-day readmissions by 25%.
| Clinical Scenario | Immediate Action | Key Threshold | Next Step | Monitoring Parameter |
|---|---|---|---|---|
| New HF diagnosis | Echo + BNP + CXR | EF <40% | Start ACE-I + β-blocker | Weekly until stable |
| Acute decompensation | IV diuretics | Weight loss 2-3 kg | Transition to PO | Daily weights |
| Medication optimization | Titrate to target | HR >50, SBP >90 | Add next pillar | Monthly labs |
| Device consideration | EF ≤35% × 3 months | QRS ≥150 ms | CRT evaluation | 6-month follow-up |
| Advanced HF | LVAD/transplant eval | INTERMACS 1-3 | Urgent referral | Weekly assessment |
-
History essentials (2 minutes):
- Symptom onset and progression
- Medication adherence and doses
- Recent weight changes (>3 lbs in 2 days)
- Exercise tolerance in METs
-
Physical examination priorities (3 minutes):
- Vital signs including orthostatics
- JVP assessment and hepatojugular reflux
- Cardiac auscultation for S3, murmurs
- Pulmonary examination for rales
- Extremity examination for edema
-
Diagnostic integration (5 minutes):
- BNP interpretation in clinical context
- Echocardiogram review for EF and function
- Chest X-ray for congestion and cardiomegaly
- Laboratory assessment for renal function
Medication Optimization Checklist:
⭐ Clinical Pearl: Target dose achievement of all four pillars (ACE-I/ARB, β-blocker, aldosterone antagonist, SGLT2 inhibitor) reduces 5-year mortality from 50% to 25%. The key is systematic titration over 3-6 months with careful monitoring for side effects and clinical response.
-
ACE-I/ARB titration:
- Start: 25% of target dose
- Increase: Every 2 weeks if tolerated
- Monitor: Creatinine <30% increase, K+ <5.5 mEq/L
- Target: Maximum tolerated dose
-
β-blocker optimization:
- Start: 25% of target dose
- Increase: Every 2-4 weeks
- Monitor: HR >50 bpm, SBP >90 mmHg
- Target: Evidence-based maximum doses
Prognostic Assessment Tools:
- Seattle Heart Failure Model: Predicts 1-3 year survival with 70% accuracy
- MAGGIC Risk Score: Estimates 3-year mortality using 13 variables
- INTERMACS Profiles: Classify advanced heart failure severity for device timing
💡 Master This: Heart failure management success depends on systematic implementation of evidence-based care through structured protocols, regular monitoring, and aggressive optimization of medical therapy. The goal is preventing hospitalizations while improving functional capacity and survival through comprehensive, team-based care.
🎯 Clinical Mastery Framework: Your Heart Failure Command Center
Practice Questions: Heart failure
Test your understanding with these related questions
An 18-year-old man presents to a rural emergency department after being stabbed multiple times. The patient's past medical history is notable for obesity, diabetes, chronic upper respiratory infections, a 10 pack-year smoking history, and heart failure. He is protecting his airway and he is oxygenating and ventilating well. His temperature is 97.6°F (36.4°C), blood pressure is 74/34 mmHg, pulse is 180/min, respirations are 24/min, and oxygen saturation is 98% on room air. The patient is started on whole blood and the surgeon on call is contacted to take the patient to the operating room. During the secondary survey, the patient complains of shortness of breath. His blood pressure is 54/14 mmHg, pulse is 200/min, respirations are 24/min, and oxygen saturation is 90% on room air. Physical exam is notable for bilateral wheezing on lung exam. The patient goes into cardiac arrest and after 30 minutes, attempts at resuscitation are terminated. Which of the following is associated with this patient's decompensation during resuscitation?












