Gastroenterology

On this page
🔬 The Gastric Ecosystem: Helicobacter's Hidden Empire
You'll master the complete clinical approach to Helicobacter pylori, the resilient bacterium colonizing half the world's stomachs and driving peptic ulcers, gastritis, and gastric cancer. We'll trace how this pathogen survives stomach acid, triggers inflammation, and manifests in diverse symptoms, then build your diagnostic strategy from noninvasive tests to endoscopic confirmation. You'll learn evidence-based eradication regimens, navigate antibiotic resistance patterns, and integrate long-term monitoring to prevent complications and recurrence in your patients.
📌 Remember: SPIRAL - Spiral shape, Produces urease, Increases gastrin, Reduces somatostatin, Ammonia production, Lives in mucus layer. This organism's unique adaptations enable survival in the acidic gastric environment with pH 1.5-3.5.
The bacterium's survival strategy involves sophisticated acid-resistance mechanisms. H. pylori produces urease enzyme at concentrations 100-fold higher than other bacteria, converting urea to ammonia and creating alkaline microenvironments with pH 6.0-7.0 around bacterial colonies.
- Colonization Sites
- Gastric antrum: 85% of infections in duodenal ulcer patients
- Gastric corpus: 65% of infections in gastric ulcer patients
- Duodenal bulb: 40% of cases with gastric metaplasia
- Requires gastric-type epithelium for bacterial adherence
- Associated with 3-fold increased duodenal ulcer risk
⭐ Clinical Pearl: H. pylori infection increases gastric cancer risk by 2-6 fold, with intestinal-type adenocarcinoma showing strongest association. The bacterium is classified as a Class I carcinogen by WHO, responsible for 75% of gastric cancers worldwide.
| Virulence Factor | Prevalence | Clinical Association | Mechanism | Risk Increase |
|---|---|---|---|---|
| CagA protein | 60-70% | Gastric cancer | Cytotoxin injection | 2.8-fold |
| VacA toxin | 95% | Peptic ulcers | Vacuole formation | 1.9-fold |
| BabA adhesin | 70% | Severe gastritis | Epithelial binding | 2.1-fold |
| IceA1 allele | 45% | Duodenal ulcers | Inflammation | 1.6-fold |
| OipA protein | 80% | Corpus gastritis | Outer membrane | 1.4-fold |
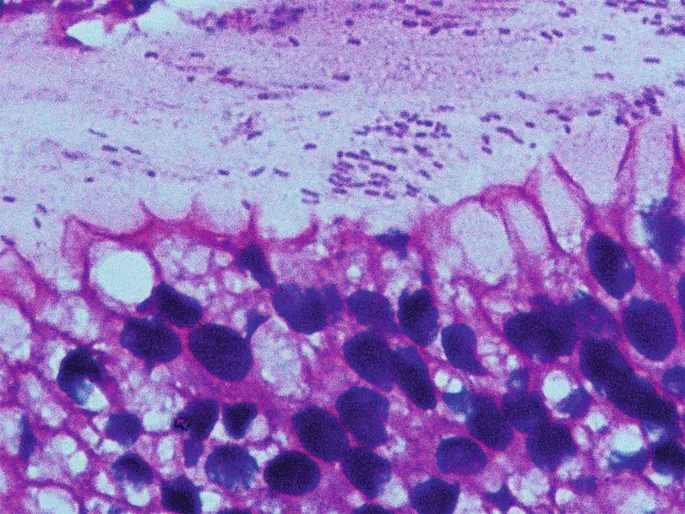
Connect this foundational understanding through bacterial pathogenesis mechanisms to understand how H. pylori transforms gastric physiology and triggers clinical manifestations.
🔬 The Gastric Ecosystem: Helicobacter's Hidden Empire
⚙️ The Inflammatory Cascade: Pathogenic Mechanisms Unleashed
📌 Remember: DAMAGE - Direct cytotoxicity, Ammonia production, Molecular mimicry, Autoimmune responses, Gastrin elevation, Epithelial barrier disruption. These mechanisms explain the 10-15 year progression from initial infection to peptic ulcer development.
The bacterium's Type IV secretion system injects CagA protein directly into gastric epithelial cells, where it undergoes tyrosine phosphorylation and disrupts cellular signaling pathways. CagA-positive strains increase peptic ulcer risk by 280% and gastric cancer risk by 290% compared to CagA-negative strains.
- Gastric Acid Regulation Disruption
- Antral-predominant gastritis: Increased gastrin secretion (3-5 fold elevation)
- Corpus-predominant gastritis: Decreased acid production (60-80% reduction)
- Pangastritis: Mixed pattern with moderate acid suppression
- Associated with highest gastric cancer risk (4-6 fold increase)
- Develops intestinal metaplasia in 40-60% of patients over 20 years
⭐ Clinical Pearl: Duodenal ulcer patients typically maintain normal or elevated gastric acid output despite H. pylori infection, while gastric ulcer patients show significant acid suppression (<10 mEq/hour vs normal 15-25 mEq/hour). This explains the inverse relationship between duodenal and gastric ulcer development.
| Inflammatory Mediator | Normal Level | H. pylori Infection | Clinical Significance | Time Course |
|---|---|---|---|---|
| IL-1β | <5 pg/mL | 45-120 pg/mL | Acid suppression | 2-4 weeks |
| TNF-α | <10 pg/mL | 80-200 pg/mL | Epithelial damage | 1-2 weeks |
| IL-8 | <20 pg/mL | 150-400 pg/mL | Neutrophil recruitment | 24-48 hours |
| IFN-γ | <15 pg/mL | 60-180 pg/mL | Th1 response | 4-8 weeks |
| IL-10 | 5-15 pg/mL | 25-80 pg/mL | Immune suppression | 6-12 weeks |
💡 Master This: H. pylori infection creates a "point of no return" in gastric carcinogenesis called the Correa cascade: Normal mucosa → Chronic gastritis → Atrophic gastritis → Intestinal metaplasia → Dysplasia → Adenocarcinoma. Each step increases cancer risk exponentially, with dysplasia carrying 6% annual malignant transformation rate.
Connect these pathogenic mechanisms through clinical presentation patterns to understand how inflammatory responses manifest as recognizable symptom complexes and diagnostic findings.
⚙️ The Inflammatory Cascade: Pathogenic Mechanisms Unleashed
🎯 Clinical Recognition: The Symptom Constellation
📌 Remember: PYLORI - Pain (epigastric), Yearning for food relief, Late-night symptoms, Occult bleeding, Recurrent episodes, Iron deficiency. These features distinguish H. pylori gastritis from functional dyspepsia with 75% sensitivity and 60% specificity.
- Duodenal Ulcer Syndrome (H. pylori-positive in 95% of cases)
- Pain timing: 2-4 hours post-prandial, nocturnal awakening (1-3 AM)
- Pain relief: Food intake provides temporary relief for 30-60 minutes
- Seasonal pattern: Spring and fall exacerbations in 70% of patients
- Related to stress-induced acid hypersecretion
- Recurrence rate: 80-90% annually without H. pylori eradication
- Bleeding risk: 15-20% lifetime incidence
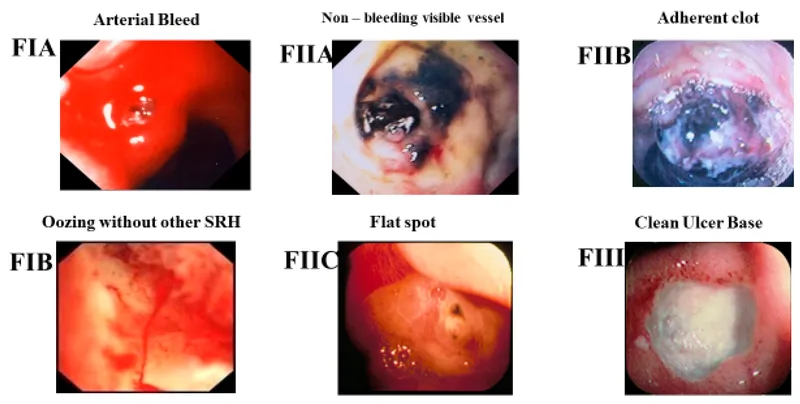
- Gastric Ulcer Syndrome (H. pylori-positive in 70% of cases)
- Pain timing: Immediate post-prandial discomfort
- Food intolerance: Early satiety and weight loss (5-10 kg over 3-6 months)
- Bleeding presentation: More severe than duodenal ulcers
- Hemoglobin drop: Average 3-4 g/dL vs 1-2 g/dL in duodenal ulcers
- Transfusion requirement: 40% vs 20% for duodenal ulcers
⭐ Clinical Pearl: "Test and treat" strategy is cost-effective in patients <60 years with dyspepsia and no alarm features. This approach has 85% positive predictive value in high-prevalence populations (>20% H. pylori prevalence) and reduces endoscopy needs by 60-70%.
| Clinical Presentation | H. pylori Positive | H. pylori Negative | Odds Ratio | Clinical Significance |
|---|---|---|---|---|
| Nocturnal pain | 75% | 35% | 5.6 | Strong predictor |
| Food relief | 68% | 28% | 5.4 | Duodenal ulcer pattern |
| Iron deficiency | 45% | 15% | 4.8 | Occult bleeding |
| Recurrent episodes | 82% | 40% | 6.9 | Chronic infection |
| Weight loss | 35% | 55% | 0.4 | More common without H. pylori |
- Hematologic disorders
- Idiopathic thrombocytopenic purpura: 50-80% response to H. pylori eradication
- Iron deficiency anemia: Refractory to iron supplementation until bacterial clearance
- Vitamin B12 deficiency: Long-term complication in 5-10% of patients
💡 Master This: Alarm features requiring immediate endoscopy include: Age >60 years, unintentional weight loss (>5% body weight), dysphagia, odynophagia, persistent vomiting, GI bleeding, iron deficiency anemia, palpable mass, or family history of gastric cancer. These features have 95% negative predictive value for excluding malignancy when absent.
Connect these clinical patterns through diagnostic testing strategies to understand how symptom recognition guides appropriate test selection and interpretation.
🎯 Clinical Recognition: The Symptom Constellation
🔬 Diagnostic Precision: Testing Arsenal Mastery
📌 Remember: TESTING - Timing matters (PPI washout), Endoscopy for complications, Stool antigen (active infection), Treatment affects results, Invasive vs non-invasive, Non-invasive preferred, Gold standard (histology + culture). Proper test selection prevents 15-20% false-negative rates that lead to treatment failure.
- Non-Invasive Testing Hierarchy
- Urea breath test: Gold standard for active infection (95% sensitivity, 96% specificity)
- Stool antigen test: Monoclonal antibody preferred (94% sensitivity, 92% specificity)
- Serology: Limited utility - cannot distinguish active from past infection
- IgG antibodies persist 6-12 months after successful eradication
- Only useful in untreated patients with no PPI exposure
- Cost-effective in high-prevalence populations (>20%)
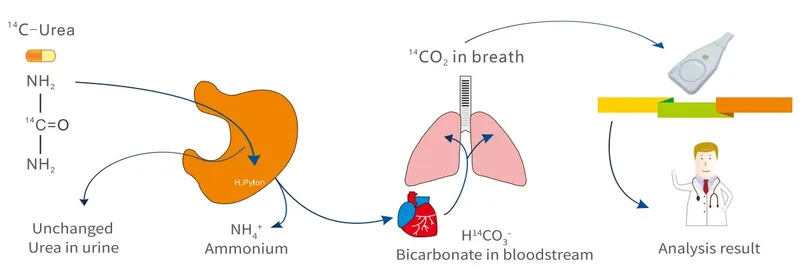
Medication interference represents the most common cause of false-negative results:
| Medication Class | Washout Period | False-Negative Risk | Mechanism | Alternative Test |
|---|---|---|---|---|
| PPIs | 2 weeks | 25-40% | Bacterial suppression | Extend washout |
| H2 blockers | 1 week | 10-15% | Acid suppression | Stool antigen |
| Antibiotics | 4 weeks | 30-50% | Bacterial killing | Delay testing |
| Bismuth | 4 weeks | 20-30% | Antimicrobial effect | Serology only |
| Sucralfate | 1 week | 5-10% | Mucosal coating | Any test |
- Invasive Testing Indications
- Age >60 years with new-onset dyspepsia
- Alarm features present (weight loss, bleeding, dysphagia)
- Treatment failure after 2 eradication attempts
- Histology: 4-6 biopsies from antrum and corpus required
- Culture: Antibiotic sensitivity testing for resistant cases
- Rapid urease test: Point-of-care results in 1-24 hours
Post-eradication testing protocols determine true cure rates and prevent antibiotic resistance development:
- Timing requirements
- Minimum 4 weeks after antibiotic completion
- Minimum 2 weeks after PPI discontinuation
- Urea breath test or stool antigen preferred (>95% accuracy)
- Serology inappropriate - antibodies remain positive for 6-12 months
💡 Master This: "Test of cure" is mandatory after H. pylori eradication therapy because clinical symptom resolution does not correlate with bacterial clearance. Persistent infection occurs in 15-25% of patients despite symptom improvement, leading to antibiotic resistance and increased cancer risk if undetected.
Connect diagnostic precision through evidence-based treatment protocols to understand how accurate testing guides optimal antibiotic selection and resistance prevention strategies.
🔬 Diagnostic Precision: Testing Arsenal Mastery
⚖️ Treatment Mastery: Eradication Protocols Perfected
📌 Remember: TRIPLE - Two antibiotics required, Resistance surveillance guides choice, Increased duration (14 days), PPI essential component, Local patterns matter, Eradication confirmation mandatory. Bismuth quadruple therapy serves as universal first-line option when clarithromycin resistance exceeds 15%.
- First-Line Treatment Options (based on local resistance patterns)
- Standard triple therapy: PPI + amoxicillin 1g + clarithromycin 500mg BID × 14 days
- Bismuth quadruple: PPI + bismuth 120mg + metronidazole 500mg + tetracycline 500mg QID × 14 days
- Concomitant therapy: PPI + amoxicillin 1g + clarithromycin 500mg + metronidazole 500mg BID × 14 days
- Eradication rates: 85-95% in clarithromycin-sensitive strains
- Resistance threshold: Avoid when clarithromycin resistance >15%
- Side effects: 20-30% experience mild GI symptoms
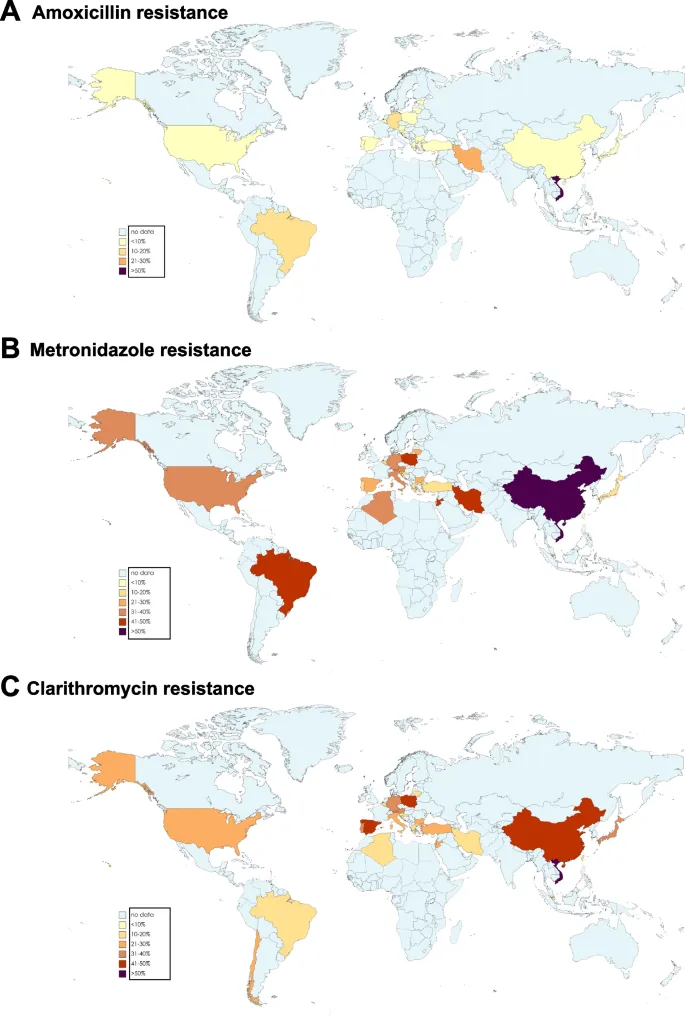
Regional resistance patterns dictate first-line therapy selection:
| Geographic Region | Clarithromycin Resistance | Metronidazole Resistance | Recommended First-Line | Eradication Rate |
|---|---|---|---|---|
| North America | 15-25% | 20-40% | Bismuth quadruple | 85-90% |
| Western Europe | 10-20% | 30-50% | Concomitant therapy | 88-92% |
| East Asia | 20-40% | 40-60% | Bismuth quadruple | 80-85% |
| Latin America | 5-15% | 60-80% | Standard triple | 85-90% |
| Africa | 10-30% | 70-90% | Bismuth quadruple | 75-85% |
- Second-Line Treatment Strategies
- Levofloxacin triple: PPI + amoxicillin 1g + levofloxacin 500mg BID × 14 days
- Bismuth quadruple (if not used first-line)
- Culture-guided therapy: Based on antibiotic sensitivity testing
- Success rates: 90-95% with targeted therapy
- Cost-effectiveness: Superior after 2 failed attempts
- Resistance prevention: Critical for population health
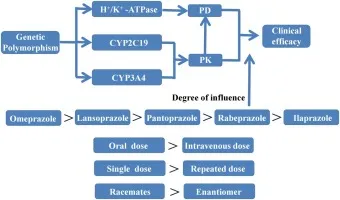
💡 Master This: Proton pump inhibitor selection affects eradication rates through genetic polymorphisms in CYP2C19 metabolism. Rapid metabolizers (50-60% of population) may require higher PPI doses or twice-daily dosing to maintain gastric pH >4 for optimal antibiotic stability and bacterial killing.
Treatment monitoring and adverse effect management:
- Common side effects (20-40% incidence)
- Gastrointestinal: Nausea, diarrhea, metallic taste
- Antibiotic-associated: C. difficile risk (0.1-0.5%)
- Bismuth-related: Dark stools, tongue discoloration
- Compliance rates: 85-90% with patient education
Connect treatment mastery through comprehensive management strategies to understand how eradication therapy integrates with long-term monitoring and complication prevention protocols.
⚖️ Treatment Mastery: Eradication Protocols Perfected
🔗 Comprehensive Care: Integration and Monitoring Excellence
📌 Remember: MONITOR - Malignancy surveillance, Optimize nutrition, Nutritional deficiencies, Iron status, Test family members, Ongoing symptoms, Reinfection prevention. Comprehensive care reduces gastric cancer mortality by 35-50% through early detection and risk factor modification.
- Post-Eradication Surveillance Strategy
- Low-risk patients: Clinical follow-up at 6 months, then annually
- High-risk patients: Endoscopic surveillance every 1-3 years
- Gastric cancer screening: Family history or advanced gastric atrophy
- Endoscopy intervals: 1-2 years for intestinal metaplasia
- Biomarker monitoring: Pepsinogen I/II ratio <3.0 indicates atrophic gastritis
- Geographic considerations: Enhanced screening in high-incidence regions
Nutritional assessment and micronutrient optimization:
| Nutritional Parameter | Pre-Eradication | Post-Eradication | Recovery Time | Supplementation |
|---|---|---|---|---|
| Iron deficiency | 40-60% | 10-15% | 3-6 months | 325mg FeSO4 daily |
| Vitamin B12 | 15-25% | 5-10% | 6-12 months | 1000mcg monthly |
| Folate deficiency | 20-30% | 8-12% | 2-4 months | 400mcg daily |
| Vitamin C | 25-35% | 10-15% | 1-3 months | 500mg daily |
| Gastric acid output | ↓60-80% | ↑40-60% | 6-18 months | Monitor symptoms |
- Reinfection Prevention Strategies
- Household screening: Test and treat infected family members
- Hygiene education: Hand washing, food safety, water quality
- Environmental factors: Crowded living conditions increase reinfection risk
- Annual reinfection rate: 1-2% in developed countries
- Higher rates: 5-10% in developing regions
- Risk factors: Poor sanitation, contaminated water, infected contacts
Long-term outcome monitoring and quality metrics:
- Treatment success indicators
- Symptom resolution: 80-90% within 4-8 weeks
- Ulcer healing: 95% at 8 weeks post-eradication
- Cancer risk reduction: 35-50% with early eradication
- Reinfection rates: <2% annually with proper follow-up
💡 Master This: "Point of no return" in gastric carcinogenesis occurs with extensive intestinal metaplasia and dysplasia development. H. pylori eradication before this stage reduces cancer risk by 50-70%, but after dysplasia development, cancer risk remains elevated despite bacterial clearance, requiring intensive surveillance.
**Integration with gastroenterology practice and primary care coordination:
- Referral indications
- Treatment failure after 2 eradication attempts
- Alarm features or complicated disease
- Family history of gastric cancer
- Persistent symptoms despite successful eradication
Connect comprehensive care through rapid clinical reference tools to understand how systematic H. pylori management integrates into efficient clinical practice workflows.
🔗 Comprehensive Care: Integration and Monitoring Excellence
🎯 Clinical Command Center: Rapid Reference Arsenal
📌 Remember: MASTERY - Manage resistance patterns, Assess patient factors, Select optimal regimen, Time interventions properly, Evaluate eradication success, Recognize complications, Yield optimal outcomes. Clinical mastery transforms complex protocols into intuitive practice patterns.
- Rapid Assessment Framework
- Patient age: <60 years = test and treat, >60 years = endoscopy first
- Symptom pattern: Nocturnal pain + food relief = high probability
- Medication history: Recent antibiotics/PPI = delay testing 2-4 weeks
- Risk stratification: Family history + ethnic background
- Complication screening: Weight loss, bleeding, iron deficiency
| Clinical Scenario | First-Line Choice | Duration | Success Rate | Key Monitoring |
|---|---|---|---|---|
| Standard case | Bismuth quadruple | 14 days | 85-90% | Compliance, side effects |
| Penicillin allergy | Clarithromycin + metronidazole | 14 days | 80-85% | Resistance risk |
| Prior macrolide use | Levofloxacin triple | 14 days | 85-90% | QT prolongation |
| Treatment failure | Culture-guided | 14 days | 90-95% | Antibiotic sensitivity |
| Elderly patient | Bismuth quadruple | 10-14 days | 80-85% | Drug interactions |
Essential Clinical Numbers for immediate recall:
-
Diagnostic thresholds
- Urea breath test: >4% ¹³CO₂ = positive
- Stool antigen: >0.16 optical density = positive
- Rapid urease: Color change within 24 hours
-
Treatment benchmarks
- Eradication goal: >90% intention-to-treat success
- Compliance target: >90% medication adherence
- Follow-up timing: 4-8 weeks post-treatment testing
💡 Master This: Clinical decision support integrates local resistance data, patient factors, and cost-effectiveness to optimize H. pylori management. Real-time resistance surveillance and treatment outcome tracking enable continuous quality improvement and population health optimization.
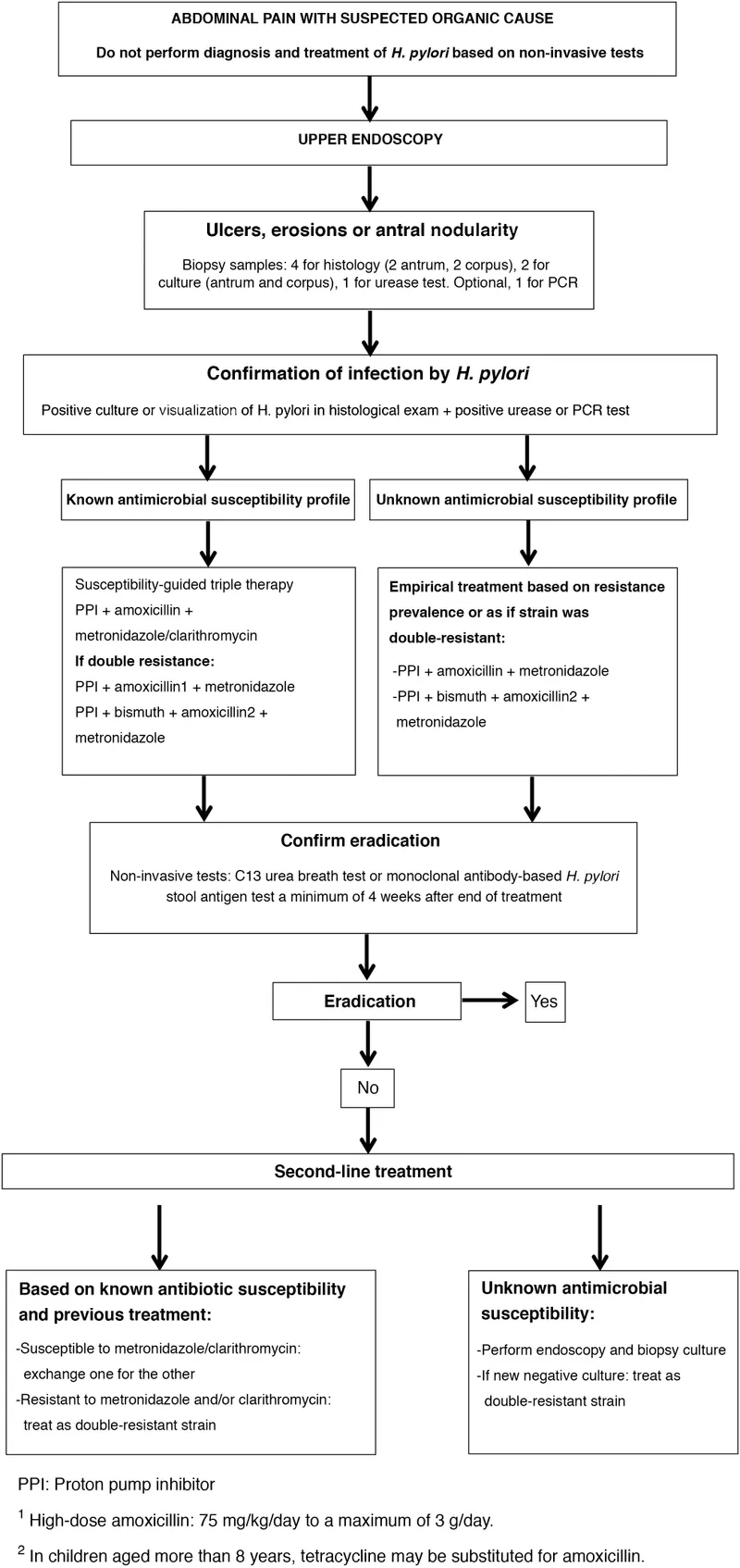
Practice integration and quality metrics:
- Performance indicators
- Eradication rate: >85% first-line success
- Test-of-cure compliance: >90% completion rate
- Appropriate testing: <5% inappropriate serology use
- Resistance prevention: <10% treatment failure rate
This clinical command center transforms H. pylori management from complex protocols into streamlined clinical excellence, ensuring optimal patient outcomes through evidence-based precision and systematic quality improvement.
🎯 Clinical Command Center: Rapid Reference Arsenal
Practice Questions: Gastroenterology
Test your understanding with these related questions
A 54-year-old man presents to the clinic for epigastric discomfort during the previous month. He states he has not vomited, but reports of having epigastric pain that worsens after most meals. The patient states that his stool “looks black sometimes.” The patient does not report of any weight loss. He has a past medical history of gastroesophageal reflux disease, diabetes mellitus, peptic ulcer disease, and Crohn’s disease. The patient takes over-the-counter ranitidine, and holds prescriptions for metformin and infliximab. The blood pressure is 132/84 mm Hg, the heart rate is 64/min, the respiratory rate is 14/min, and the temperature is 37.3°C (99.1°F). On physical examination, the abdomen is tender to palpation in the epigastric region. Which of the following is the most appropriate next step to accurately determine the diagnosis of this patient?












