Endocrinology (diabetes, thyroid disorders)

On this page
🏭 The Endocrine Command Center: Diabetes and Thyroid Mastery
Hormones orchestrate metabolism, growth, and homeostasis across every organ system, yet their dysregulation in diabetes and thyroid disorders creates some of medicine's most prevalent and complex challenges. You'll master how insulin resistance cascades into multi-organ damage, why subtle thyroid dysfunction mimics dozens of other conditions, and how to integrate clinical patterns with laboratory data to optimize treatment. This lesson builds your diagnostic precision through metabolic networks, equipping you to recognize endocrine emergencies and manage chronic disease with confidence.
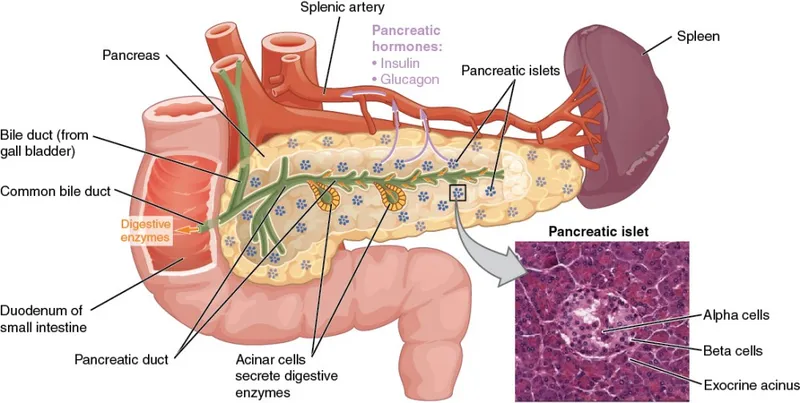
📌 Remember: PANCREAS - Pulses of insulin, Alpha cells (glucagon), Normal glucose 80-100, Cells need constant fuel, Regulation every 5 minutes, Endocrine and exocrine, Autoimmune destruction in T1DM, Second messenger systems
The thyroid-pituitary axis demonstrates elegant feedback control: TSH stimulates thyroid hormone production, while T3/T4 provide negative feedback. This system maintains metabolic homeostasis with 99.97% of thyroid hormones bound to proteins, leaving only 0.03% free and active.
| Parameter | Normal Range | T1DM | T2DM | Hyperthyroid | Hypothyroid |
|---|---|---|---|---|---|
| Glucose (mg/dL) | 80-100 | >126 | >126 | Normal | Normal |
| HbA1c (%) | <5.7 | >6.5 | >6.5 | Normal | Normal |
| TSH (mIU/L) | 0.4-4.0 | Normal | Normal | <0.1 | >10 |
| Free T4 (ng/dL) | 0.8-1.8 | Normal | Normal | >1.8 | <0.8 |
| Insulin Level | 5-25 μU/mL | <5 | >25 | Normal | Normal |
- Glucose Homeostasis Architecture
- Fasting state: Liver produces 150-200g glucose daily
- Fed state: Insulin suppresses hepatic glucose by 90%
- Muscle uptake increases 20-fold with insulin
- Adipose tissue switches from lipolysis to lipogenesis
- Thyroid Hormone Synthesis Pathway
- Iodine uptake: 20-40mg daily requirement
- T4 production: 80-100 μg daily
- Peripheral conversion: 80% of T3 from T4
- Nuclear receptor binding: 2-4 hour delay to effect
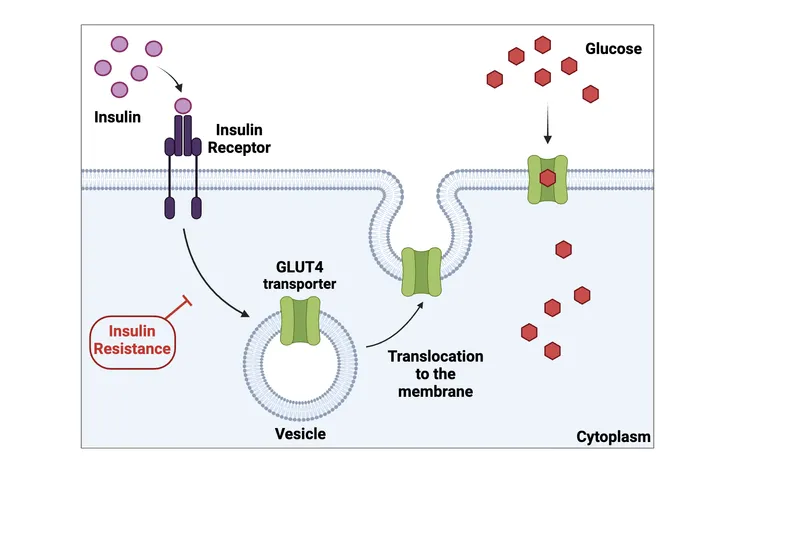
💡 Master This: Insulin resistance precedes hyperglycemia by years - muscle and liver become resistant first (fasting glucose >100), then pancreatic beta cells fail (postprandial glucose >140), finally resulting in overt diabetes (fasting >126).
Understanding endocrine feedback loops reveals why metformin targets hepatic glucose production (reducing output by 30%), while sulfonylureas stimulate remaining beta cell function. Similarly, thyroid replacement requires 6-8 weeks for steady state due to T4's long half-life, explaining why TSH monitoring occurs at this interval.
Connect these foundational principles through metabolic integration patterns to understand how diabetes and thyroid disorders create cascading effects throughout multiple organ systems.
🏭 The Endocrine Command Center: Diabetes and Thyroid Mastery
⚡ Metabolic Integration Networks: System Crosstalk Mastery
📌 Remember: THYROID-DIABETES - T3 increases glucose absorption, Hyperthyroid worsens diabetes, Yield higher insulin needs, Reduced clearance in hypothyroid, Oscillating glucose patterns, Insulin resistance increases, Dosing changes needed
The hypothalamic-pituitary-adrenal axis demonstrates how stress hormones disrupt glucose homeostasis. Cortisol increases hepatic gluconeogenesis by 300% and reduces peripheral insulin sensitivity by 50%, explaining why diabetics experience hyperglycemia during illness.
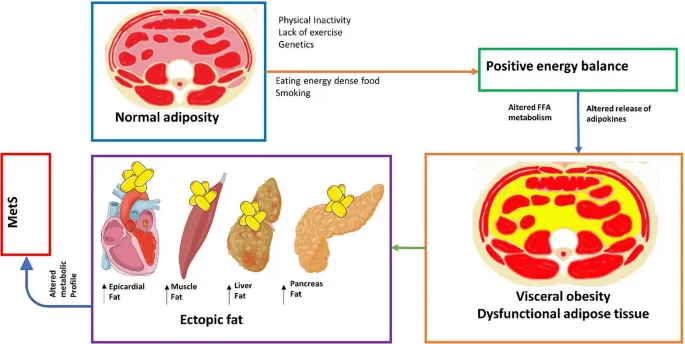
- Metabolic Syndrome Integration
- Central obesity: Visceral fat produces 50+ inflammatory cytokines
- Insulin resistance: Affects 85% of metabolic syndrome patients
- TNF-alpha blocks insulin signaling by 60%
- Adiponectin levels decrease by 40%
- Dyslipidemia: 70% have elevated triglycerides >150 mg/dL
- HDL typically <40 mg/dL in men, <50 mg/dL in women
- Small dense LDL particles increase 3-fold
| Hormone | Normal Function | Diabetes Effect | Thyroid Effect | Clinical Impact |
|---|---|---|---|---|
| Cortisol | Stress response | ↑ Glucose by 200% | ↑ T4 to T3 conversion | Morning hyperglycemia |
| Growth Hormone | Anabolic effects | ↑ Insulin resistance | ↑ IGF-1 production | Dawn phenomenon |
| Catecholamines | Fight/flight | ↑ Glycogenolysis | ↑ T4 to T3 conversion | Stress hyperglycemia |
| Glucagon | Counter-regulation | ↑ Hepatic glucose | Normal response | Postprandial spikes |
| Incretin | Glucose-dependent | ↓ GLP-1 by 50% | Normal levels | Lost meal response |
- Autoimmune Clustering Patterns
- Type 1 diabetes associations: 20% thyroid disease, 5% celiac disease
- Polyglandular syndrome type 2: Diabetes + thyroid + adrenal
- HLA-DR3/DR4 genetic markers in 95% of cases
- Anti-GAD antibodies persist for decades
- Hashimoto's thyroiditis: 8x higher diabetes risk
- TPO antibodies in 90% of cases
- Goiter present in 50% at diagnosis
💡 Master This: The "diabetic triad" of polyuria, polydipsia, and polyphagia results from glucose osmotic diuresis (>180 mg/dL renal threshold), while the "thyrotoxic triad" of weight loss, tachycardia, and heat intolerance reflects increased metabolic rate (20-30% above normal).
Incretin hormones reveal sophisticated glucose regulation: GLP-1 enhances insulin secretion only when glucose >100 mg/dL, suppresses glucagon by 50%, and slows gastric emptying by 70%. This glucose-dependent mechanism explains why GLP-1 agonists rarely cause hypoglycemia.
Connect metabolic integration through pattern recognition frameworks to understand how clinical presentations overlap and how treatment of one condition affects others.
⚡ Metabolic Integration Networks: System Crosstalk Mastery
🎯 Pattern Recognition Arsenal: Clinical Correlation Mastery
📌 Remember: DKA TRIAD - Dehydration (10-15% body weight), Ketones >3 mmol/L, Acidosis pH <7.3; THYROID STORM - Temperature >104°F, Heart rate >140, Yellow sclera (rare), Restlessness, Organ dysfunction, Increased reflexes, Diaphoresis
- Hyperglycemic Emergency Patterns
- DKA presentation: 24-48 hours symptom progression
- Fruity breath odor in 70% of cases
- Abdominal pain mimics surgical abdomen in 30%
- Kussmaul respirations: Deep, rapid breathing pattern
- HHS presentation: Days to weeks gradual onset
- Glucose typically >600 mg/dL (vs >250 in DKA)
- Osmolality >320 mOsm/kg (vs <320 in DKA)
- Minimal ketosis: <3 mmol/L (vs >3 in DKA)
- DKA presentation: 24-48 hours symptom progression
| Clinical Feature | DKA | HHS | Thyroid Storm | Hypoglycemia |
|---|---|---|---|---|
| Onset | 24-48 hours | Days-weeks | Hours-days | Minutes-hours |
| Glucose | >250 mg/dL | >600 mg/dL | Normal | <70 mg/dL |
| Mental Status | Alert to coma | Stupor/coma | Agitated/psychotic | Confused/coma |
| Dehydration | Severe (10-15%) | Extreme (15-20%) | Mild | None |
| Temperature | Normal/low | Normal/high | >104°F | Normal/low |
- Thyroid Dysfunction Recognition Patterns
- Hyperthyroid presentations: Weight loss despite increased appetite in 90%
- Tremor: Fine, rapid hand tremor in 95%
- Heat intolerance: Excessive sweating in 85%
- Graves' ophthalmopathy: Lid lag, proptosis in 50%
- Hypothyroid presentations: Fatigue and cold intolerance in 95%
- Weight gain: 5-10 pounds average
- Bradycardia: <60 bpm in 70%
- Delayed reflexes: Prolonged relaxation phase in 80%
- Hyperthyroid presentations: Weight loss despite increased appetite in 90%
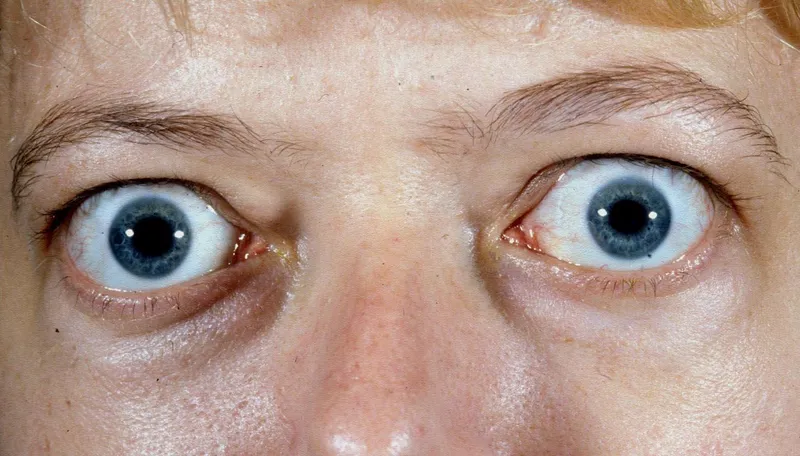
💡 Master This: The "dawn phenomenon" (morning glucose rise) affects 75% of Type 1 and 55% of Type 2 diabetics due to 4-8 AM growth hormone and cortisol surges, while the "Somogyi effect" (rebound hyperglycemia) follows nocturnal hypoglycemia in <5% of patients.
- Diagnostic Threshold Mastery
- Diabetes diagnosis: Fasting ≥126 or Random ≥200 or HbA1c ≥6.5%
- Prediabetes: Fasting 100-125 or HbA1c 5.7-6.4%
- Normal: Fasting <100 and HbA1c <5.7%
- Thyroid dysfunction: TSH is the primary screening test
- Subclinical hyper: TSH <0.4, normal T4/T3
- Overt hyper: TSH <0.1, elevated T4/T3
- Subclinical hypo: TSH 4-10, normal T4
- Overt hypo: TSH >10, low T4
- Diabetes diagnosis: Fasting ≥126 or Random ≥200 or HbA1c ≥6.5%
Connect pattern recognition through systematic discrimination frameworks to understand how laboratory values, timing patterns, and clinical presentations create diagnostic certainty.
🎯 Pattern Recognition Arsenal: Clinical Correlation Mastery
🔬 Laboratory Integration Matrix: Diagnostic Precision Mastery
📌 Remember: LAB TIMING - Lasting HbA1c (3 months), Acute glucose (current), Brief fructosamine (3 weeks), Thyroid TSH (6-8 weeks), Immediate ketones (hours), Monitoring C-peptide (beta cell function), Insulin levels (resistance), Normal ranges vary, Glucose variability matters
- Glucose Monitoring Hierarchy
- Continuous glucose monitoring: 288 readings daily (every 5 minutes)
- Time in range: 70-180 mg/dL target >70% of time
- Glucose variability: Coefficient of variation <36%
- Dawn phenomenon: 3-8 AM glucose rise >30 mg/dL
- Self-monitoring blood glucose: 4-8 times daily minimum
- Fasting: 80-130 mg/dL target
- Postprandial: <180 mg/dL at 2 hours
- Bedtime: 100-140 mg/dL target
- Continuous glucose monitoring: 288 readings daily (every 5 minutes)
| Test | Normal | Prediabetes | Diabetes | Clinical Significance |
|---|---|---|---|---|
| Fasting Glucose | <100 mg/dL | 100-125 mg/dL | ≥126 mg/dL | Hepatic glucose production |
| 2-hr OGTT | <140 mg/dL | 140-199 mg/dL | ≥200 mg/dL | Insulin sensitivity |
| HbA1c | <5.7% | 5.7-6.4% | ≥6.5% | 3-month average |
| Random Glucose | Variable | Variable | ≥200 mg/dL | Acute hyperglycemia |
| C-peptide | 0.8-3.1 ng/mL | Variable | Low in T1DM | Beta cell function |
- Thyroid Function Integration
- TSH patterns: Primary screening test with 95% sensitivity
- Normal: 0.4-4.0 mIU/L (varies by lab)
- Subclinical disease: TSH abnormal, normal T4/T3
- Central disease: Low/normal TSH with low T4
- Free T4 significance: Metabolically active hormone
- Normal: 0.8-1.8 ng/dL (10-23 pmol/L)
- Pregnancy: Increases 50% due to TBG elevation
- Medications: Phenytoin, carbamazepine decrease levels
- TSH patterns: Primary screening test with 95% sensitivity
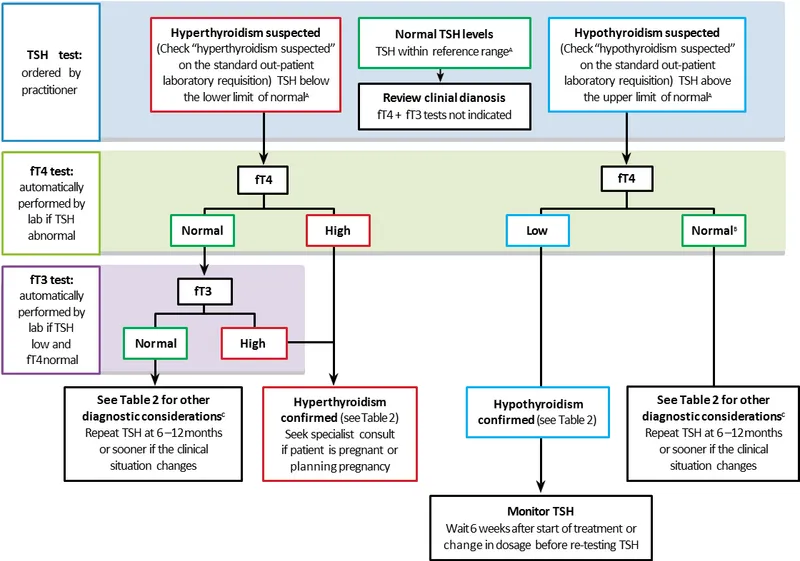
💡 Master This: Reverse T3 (rT3) increases during illness, creating "euthyroid sick syndrome" with low T3, normal/low T4, and normal/low TSH. This affects 60% of ICU patients and resolves with recovery - avoid thyroid replacement during acute illness.
- Advanced Endocrine Markers
- Autoantibody patterns: Predict disease progression years before symptoms
- Anti-GAD: Present in 80% Type 1 diabetes
- Anti-IA2: Indicates rapid progression to insulin dependence
- TPO antibodies: 90% of Hashimoto's thyroiditis
- TSI antibodies: 95% of Graves' disease
- Insulin resistance markers: HOMA-IR calculation
- Formula: (Fasting insulin × Fasting glucose) ÷ 405
- Normal: <2.5, Insulin resistant: >2.5
- Correlates with metabolic syndrome risk
- Autoantibody patterns: Predict disease progression years before symptoms
Connect laboratory precision through treatment optimization algorithms to understand how monitoring guides therapeutic decisions and prevents complications.
🔬 Laboratory Integration Matrix: Diagnostic Precision Mastery
💊 Treatment Optimization Algorithms: Therapeutic Precision Mastery
📌 Remember: INSULIN TYPES - Rapid (lispro, aspart) 15-minute onset, Short (regular) 30-minute onset, Intermediate (NPH) 2-hour onset, Long (glargine, detemir) 2-4 hour onset, Ultra-long (degludec) 42-hour duration
- Diabetes Medication Mechanisms
- Metformin: Activates AMPK pathway, reduces hepatic gluconeogenesis
- Dose: 500-2000mg daily, maximum 2550mg
- Benefits: Weight neutral, cardiovascular protection
- Contraindications: eGFR <30, acute illness
- GLP-1 agonists: Glucose-dependent insulin secretion
- Weight loss: 5-10% body weight reduction
- Cardiovascular benefits: 14% MACE reduction
- Side effects: Nausea 40%, pancreatitis risk <0.1%
- Metformin: Activates AMPK pathway, reduces hepatic gluconeogenesis
| Medication Class | Mechanism | HbA1c Reduction | Weight Effect | Hypoglycemia Risk |
|---|---|---|---|---|
| Metformin | ↓ Hepatic glucose | 1.0-1.5% | Neutral | Very low |
| Sulfonylureas | ↑ Insulin secretion | 1.0-1.5% | +2-5 kg | High |
| GLP-1 agonists | ↑ Insulin, ↓ glucagon | 1.0-1.8% | -3-8 kg | Low |
| SGLT-2 inhibitors | ↑ Glucose excretion | 0.7-1.0% | -2-4 kg | Very low |
| Insulin | Direct replacement | 1.5-3.0% | +2-6 kg | High |
- Thyroid Replacement Precision
- Levothyroxine dosing: 1.6 μg/kg/day for complete replacement
- Elderly: Start 25-50 μg daily, increase by 25 μg every 6-8 weeks
- Cardiac disease: Start 12.5-25 μg daily
- Pregnancy: Increase dose 30-50% in first trimester
- Monitoring strategy: TSH every 6-8 weeks until stable
- Target TSH: 0.4-2.5 mIU/L for most patients
- Pregnancy: <2.5 mIU/L first trimester, <3.0 second/third
- Elderly: 4-6 mIU/L acceptable to avoid overtreatment
- Levothyroxine dosing: 1.6 μg/kg/day for complete replacement
💡 Master This: Insulin-to-carbohydrate ratios typically start at 1:15 (1 unit per 15g carbs) but range from 1:5 to 1:30 based on insulin sensitivity. Correction factors usually begin at 1:50 (1 unit lowers glucose 50 mg/dL) but vary from 1:20 to 1:100.
- Advanced Insulin Strategies
- Basal-bolus regimen: 50% basal, 50% bolus insulin distribution
- Basal: Long-acting insulin once or twice daily
- Bolus: Rapid-acting before meals and corrections
- Total daily dose: 0.5-1.0 units/kg for Type 1, 0.3-0.5 for Type 2
- Continuous glucose monitoring integration: Time in range >70%
- Target range: 70-180 mg/dL for most adults
- Time below range: <4% at <70 mg/dL, <1% at <54 mg/dL
- Glucose variability: Coefficient of variation <36%
- Basal-bolus regimen: 50% basal, 50% bolus insulin distribution
Connect treatment optimization through multi-system integration to understand how endocrine therapies affect cardiovascular, renal, and neurological outcomes.
💊 Treatment Optimization Algorithms: Therapeutic Precision Mastery
🌐 Multi-System Integration Hub: Advanced Endocrine Networks
📌 Remember: DIABETIC COMPLICATIONS - Cardiovascular (68% of deaths), Arterial disease, Retinopathy (leading blindness cause), Diabetic nephropathy, Infections increased, Autonomic neuropathy, Cerebrovascular disease
- Cardiovascular-Endocrine Integration
- SGLT-2 inhibitor benefits: Cardiovascular outcomes independent of glucose
- Heart failure reduction: 35% in diabetics, 26% in non-diabetics
- Kidney protection: 39% reduction in ESRD progression
- Mechanism: Natriuresis, afterload reduction, metabolic effects
- GLP-1 agonist cardioprotection: 14% MACE reduction
- Weight loss: 5-15% body weight reduction
- Blood pressure: 2-6 mmHg systolic reduction
- Atherosclerosis: Plaque stabilization effects
- SGLT-2 inhibitor benefits: Cardiovascular outcomes independent of glucose
| Complication | Prevalence | Screening | Prevention Target | Treatment |
|---|---|---|---|---|
| Retinopathy | 35% at 10 years | Annual dilated exam | HbA1c <7%, BP <140/90 | Laser, anti-VEGF |
| Nephropathy | 40% lifetime | Annual ACR, eGFR | HbA1c <7%, BP <130/80 | ACE-I, ARB, SGLT-2 |
| Neuropathy | 50% at 10 years | Annual foot exam | HbA1c <7% | Pregabalin, duloxetine |
| CVD | 2-4x increased risk | Lipids, ECG | LDL <70, HbA1c <7% | Statin, aspirin |
| PAD | 20% prevalence | ABI if symptoms | Same as CVD | Antiplatelet, statin |
- Thyroid-Cardiac Integration
- Hyperthyroid cardiovascular effects: Increased cardiac output by 50-300%
- Atrial fibrillation: 15% of hyperthyroid patients
- Heart failure: 6% develop high-output failure
- Coronary disease: Increased oxygen demand may precipitate angina
- Hypothyroid cardiovascular effects: Decreased cardiac output by 30-50%
- Bradycardia: <60 bpm in 70% of patients
- Diastolic dysfunction: Impaired relaxation in 80%
- Lipid abnormalities: LDL increases 20-50 mg/dL
- Hyperthyroid cardiovascular effects: Increased cardiac output by 50-300%
💡 Master This: Diabetic autonomic neuropathy affects 25% of diabetics, causing gastroparesis (delayed gastric emptying), orthostatic hypotension (>20 mmHg drop), and cardiac denervation (loss of heart rate variability), requiring specialized management approaches.
-
Renal-Endocrine Networks
- Diabetic kidney disease progression: 5 stages based on eGFR
- Stage 1: eGFR >90 with albuminuria
- Stage 3: eGFR 30-59, moderate decrease
- Stage 5: eGFR <15, kidney failure
- Thyroid-kidney interactions: Hypothyroidism reduces GFR by 20%
- Drug clearance: Reduced metabolism of renally cleared drugs
- Fluid retention: Myxedema causes interstitial edema
- Electrolytes: Hyponatremia in severe hypothyroidism
- Diabetic kidney disease progression: 5 stages based on eGFR
-
Cutting-Edge Integration Concepts
- Continuous glucose monitoring with insulin pumps: Closed-loop systems
- Time in range improvement: 70% to 85% with automated insulin delivery
- Hypoglycemia reduction: 50-70% decrease in <70 mg/dL episodes
- HbA1c improvement: 0.3-0.5% additional reduction
- Precision medicine approaches: Genetic testing for drug selection
- TCF7L2 variants: Predict sulfonylurea response
- KCNJ11 mutations: Require specific insulin strategies
- Thyroid hormone resistance: RTH-beta mutations need higher doses
- Continuous glucose monitoring with insulin pumps: Closed-loop systems
Connect multi-system integration through rapid mastery frameworks to develop comprehensive clinical decision-making tools for complex endocrine patients.
🌐 Multi-System Integration Hub: Advanced Endocrine Networks
🚀 Clinical Command Arsenal: Rapid Mastery Framework
📌 Remember: EMERGENCY PRIORITIES - Airway/breathing, Blood pressure support, Circulation/IV access, Dextrose if hypoglycemic, Electrolyte correction, Fluids for dehydration, Glucose monitoring, Hormone replacement
- Essential Clinical Arsenal
- DKA Management Protocol: "Two-bag system" prevents hypoglycemia
- Bag 1: Normal saline with insulin
- Bag 2: D10W with insulin when glucose <250 mg/dL
- Insulin rate: 0.1 units/kg/hour until ketones clear
- Potassium: Replace aggressively (40-60 mEq/L) when >3.3 mEq/L
- Hypoglycemia Response: "15-15-15 Rule" for conscious patients
- 15g glucose tablets or juice
- Recheck in 15 minutes
- Repeat if <70 mg/dL
- Severe: Glucagon 1mg IM or D50W 25g IV
- DKA Management Protocol: "Two-bag system" prevents hypoglycemia
| Emergency | Recognition | Initial Treatment | Monitoring | Complications |
|---|---|---|---|---|
| DKA | Glucose >250, ketones >3, pH <7.3 | Fluids, insulin, K+ | Hourly glucose, q4h lytes | Cerebral edema <0.5% |
| HHS | Glucose >600, osmolality >320 | Fluids first, then insulin | Hourly glucose, neuro checks | Thrombosis 15% |
| Thyroid Storm | Temp >104°F, HR >140, altered mental | Beta-blockers, PTU, steroids | Continuous monitoring | Mortality 10-20% |
| Severe Hypoglycemia | Glucose <40, altered consciousness | Glucagon 1mg or D50W 25g | Q15min glucose checks | Seizures, coma |
- Rapid Assessment Tools
- Diabetes Control Quick Check: "ABC approach"
- A1c: Target <7% for most, <6.5% if low hypoglycemia risk
- Blood pressure: Target <130/80 for diabetics
- Cholesterol: LDL <70 mg/dL for high CVD risk
- Thyroid Function Interpretation: "TSH-first strategy"
- Normal TSH (0.4-4.0): Euthyroid in 95% of cases
- Low TSH (<0.4): Check free T4/T3 for hyperthyroidism
- High TSH (>4.0): Check free T4 for hypothyroidism
- Very high TSH (>10): Start levothyroxine regardless of T4
- Diabetes Control Quick Check: "ABC approach"
💡 Master This: Insulin dosing calculations - Total daily dose 0.5-1.0 units/kg, 50% basal/50% bolus split, carb ratio starts at 1:15, correction factor starts at 1:50, adjust based on pattern analysis over 3-5 days rather than single readings.
- Pattern Recognition Mastery
- Dawn phenomenon: 3-8 AM glucose rise >30 mg/dL
- Solution: Increase basal insulin or change timing
- Affects: 75% Type 1, 55% Type 2 diabetics
- Somogyi effect: Rebound hyperglycemia after nocturnal hypoglycemia
- Recognition: 3 AM glucose <70, 7 AM glucose >180
- Solution: Reduce evening insulin or bedtime snack
- Frequency: <5% of glucose variability cases
- Dawn phenomenon: 3-8 AM glucose rise >30 mg/dL
Connect rapid mastery frameworks through systematic clinical excellence to develop expertise that transforms patient outcomes through evidence-based endocrine care.
🚀 Clinical Command Arsenal: Rapid Mastery Framework
Practice Questions: Endocrinology (diabetes, thyroid disorders)
Test your understanding with these related questions
A 62-year-old man presents to the emergency department with confusion. The patient’s wife states that her husband has become more somnolent over the past several days and now is very confused. The patient has no complaints himself, but is answering questions inappropriately. The patient has a past medical history of diabetes and hypertension. His temperature is 98.3°F (36.8°C), blood pressure is 127/85 mmHg, pulse is 138/min, respirations are 14/min, and oxygen saturation is 99% on room air. Physical exam is notable for a confused man with dry mucous membranes. Initial laboratory studies are ordered as seen below. Serum: Na+: 135 mEq/L Cl-: 100 mEq/L K+: 3.0 mEq/L HCO3-: 23 mEq/L BUN: 30 mg/dL Glucose: 1,299 mg/dL Creatinine: 1.5 mg/dL Ca2+: 10.2 mg/dL Which of the following is the most appropriate initial treatment for this patient?













