Likelihood ratios US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Likelihood ratios. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Likelihood ratios US Medical PG Question 1: A scientist in Chicago is studying a new blood test to detect Ab to EBV with increased sensitivity and specificity. So far, her best attempt at creating such an exam reached 82% sensitivity and 88% specificity. She is hoping to increase these numbers by at least 2 percent for each value. After several years of work, she believes that she has actually managed to reach a sensitivity and specificity much greater than what she had originally hoped for. She travels to China to begin testing her newest blood test. She finds 2,000 patients who are willing to participate in her study. Of the 2,000 patients, 1,200 of them are known to be infected with EBV. The scientist tests these 1,200 patients' blood and finds that only 120 of them tested negative with her new exam. Of the patients who are known to be EBV-free, only 20 of them tested positive. Given these results, which of the following correlates with the exam's specificity?
- A. 82%
- B. 90%
- C. 84%
- D. 86%
- E. 98% (Correct Answer)
Likelihood ratios Explanation: ***98%***
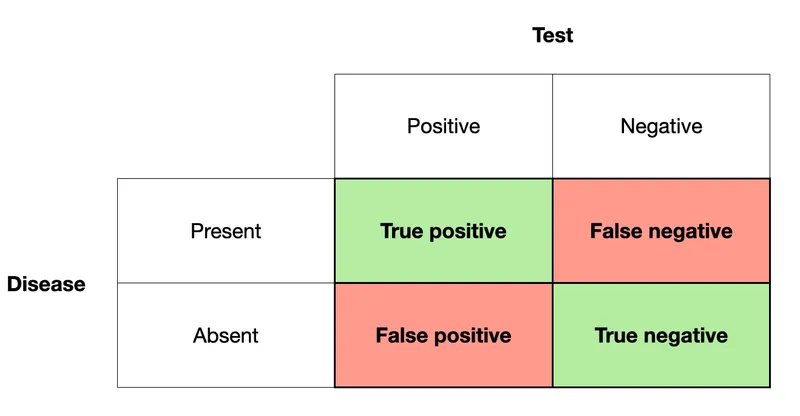
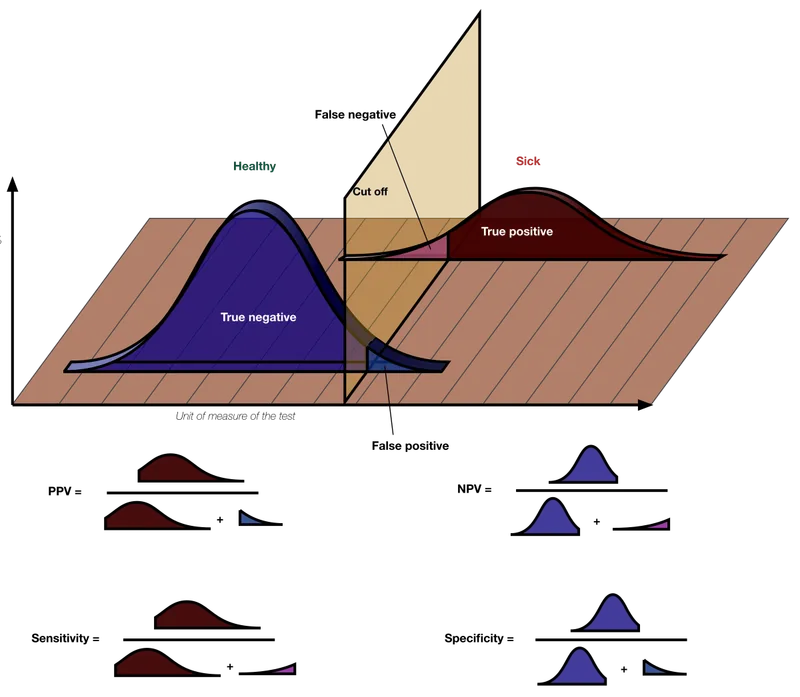
- **Specificity** measures the proportion of **true negatives** among all actual negatives.
- In this case, 800 patients are known to be EBV-free (actual negatives), and 20 of them tested positive (false positives). This means 800 - 20 = 780 tested negative (true negatives). Specificity = (780 / 800) * 100% = **98%**.
*82%*
- This value represents the *original sensitivity* before the scientist’s new attempts to improve the test.
- It does not reflect the *newly calculated specificity* based on the provided data.
*90%*
- This value represents the *newly calculated sensitivity* of the test, not the specificity.
- Out of 1200 EBV-infected patients, 120 tested negative (false negatives), meaning 1080 tested positive (true positives). Sensitivity = (1080 / 1200) * 100% = 90%.
*84%*
- This percentage is not directly derived from the information given for either sensitivity or specificity after the new test results.
- It does not correspond to any of the calculated values for the new test's performance.
*86%*
- This percentage is not directly derived from the information given for either sensitivity or specificity after the new test results.
- It does not correspond to any of the calculated values for the new test's performance.
Likelihood ratios US Medical PG Question 2: Group of 100 medical students took an end of the year exam. The mean score on the exam was 70%, with a standard deviation of 25%. The professor states that a student's score must be within the 95% confidence interval of the mean to pass the exam. Which of the following is the minimum score a student can have to pass the exam?
- A. 45%
- B. 63.75%
- C. 67.5%
- D. 20%
- E. 65% (Correct Answer)
Likelihood ratios Explanation: ***65%***
- To find the **95% confidence interval (CI) of the mean**, we use the formula: Mean ± (Z-score × Standard Error). For a 95% CI, the Z-score is approximately **1.96**.
- The **Standard Error (SE)** is calculated as SD/√n, where n is the sample size (100 students). So, SE = 25%/√100 = 25%/10 = **2.5%**.
- The 95% CI is 70% ± (1.96 × 2.5%) = 70% ± 4.9%. The lower bound is 70% - 4.9% = **65.1%**, which rounds to **65%** as the minimum passing score.
*45%*
- This value is significantly lower than the calculated lower bound of the 95% confidence interval (approximately 65.1%).
- It would represent a score far outside the defined passing range.
*63.75%*
- This value falls below the calculated lower bound of the 95% confidence interval (approximately 65.1%).
- While close, this score would not meet the professor's criterion for passing.
*67.5%*
- This value is within the 95% confidence interval (65.1% to 74.9%) but is **not the minimum score**.
- Lower scores within the interval would still qualify as passing.
*20%*
- This score is extremely low and falls significantly outside the 95% confidence interval for a mean of 70%.
- It would indicate performance far below the defined passing threshold.
Likelihood ratios US Medical PG Question 3: A 36-year-old female presents to clinic inquiring about the meaning of a previous negative test result from a new HIV screening test. The efficacy of this new screening test for HIV has been assessed by comparison against existing gold standard detection of HIV RNA via PCR. The study includes 1000 patients, with 850 HIV-negative patients (by PCR) receiving a negative test result, 30 HIV-negative patients receiving a positive test result, 100 HIV positive patients receiving a positive test result, and 20 HIV positive patients receiving a negative test result. Which of the following is most likely to increase the negative predictive value for this test?
- A. Decreased prevalence of HIV in the tested population (Correct Answer)
- B. Increased prevalence of HIV in the tested population
- C. Increased number of false positive test results
- D. Increased number of false negative test results
- E. Decreased number of false positive test results
Likelihood ratios Explanation: ***Decreased prevalence of HIV in the tested population***
- A **lower prevalence** of a disease in the population means there are fewer actual cases, making a **negative test result** more reliable in ruling out the disease.
- This increases the probability that a person with a negative test truly does not have the disease, thus elevating the **negative predictive value (NPV)**.
*Increased prevalence of HIV in the tested population*
- A **higher prevalence** means there are more actual cases of HIV in the population.
- In this scenario, a negative test result is less reassuring, as there's a greater chance of missing a true positive case, leading to a **decreased NPV**.
*Increased number of false positive test results*
- **False positives** are instances where a test indicates disease when it's not present; they do not directly impact the ability of a negative test to predict absence of disease.
- While they affect the **positive predictive value (PPV)**, they do not directly alter the reliability of a negative result to exclude disease, so the NPV is not increased.
*Increased number of false negative test results*
- **False negatives** occur when a test indicates no disease, but the disease is actually present.
- An increase in false negatives directly implies that a negative test result is less trustworthy, leading to a **decrease in the NPV**.
*Decreased number of false positive test results*
- A decrease in false positive results primarily improves the **positive predictive value (PPV)**.
- While it indicates a more accurate test overall, it does not directly affect NPV, which measures the reliability of a negative test result in ruling out disease.
Likelihood ratios US Medical PG Question 4: You are reading through a recent article that reports significant decreases in all-cause mortality for patients with malignant melanoma following treatment with a novel biological infusion. Which of the following choices refers to the probability that a study will find a statistically significant difference when one truly does exist?
- A. Type II error
- B. Type I error
- C. Confidence interval
- D. p-value
- E. Power (Correct Answer)
Likelihood ratios Explanation: ***Power***
- **Power** is the probability that a study will correctly reject the null hypothesis when it is, in fact, false (i.e., will find a statistically significant difference when one truly exists).
- A study with high power minimizes the risk of a **Type II error** (failing to detect a real effect).
*Type II error*
- A **Type II error** (or **beta error**) occurs when a study fails to reject a false null hypothesis, meaning it concludes there is no significant difference when one actually exists.
- This is the **opposite** of what the question describes, which asks for the probability of *finding* a difference.
*Type I error*
- A **Type I error** (or **alpha error**) occurs when a study incorrectly rejects a true null hypothesis, concluding there is a significant difference when one does not actually exist.
- This relates to the **p-value** and the level of statistical significance (e.g., p < 0.05).
*Confidence interval*
- A **confidence interval** provides a range of values within which the true population parameter is likely to lie with a certain degree of confidence (e.g., 95%).
- It does not directly represent the probability of finding a statistically significant difference when one truly exists.
*p-value*
- The **p-value** is the probability of observing data as extreme as, or more extreme than, that obtained in the study, assuming the null hypothesis is true.
- It is used to determine statistical significance, but it is not the probability of detecting a true effect.
Likelihood ratios US Medical PG Question 5: A research group wants to assess the safety and toxicity profile of a new drug. A clinical trial is conducted with 20 volunteers to estimate the maximum tolerated dose and monitor the apparent toxicity of the drug. The study design is best described as which of the following phases of a clinical trial?
- A. Phase 0
- B. Phase III
- C. Phase V
- D. Phase II
- E. Phase I (Correct Answer)
Likelihood ratios Explanation: ***Phase I***
- **Phase I clinical trials** involve a small group of healthy volunteers (typically 20-100) to primarily assess **drug safety**, determine a safe dosage range, and identify side effects.
- The main goal is to establish the **maximum tolerated dose (MTD)** and evaluate the drug's pharmacokinetic and pharmacodynamic profiles.
*Phase 0*
- **Phase 0 trials** are exploratory studies conducted in a very small number of subjects (10-15) to gather preliminary data on a drug's **pharmacodynamics and pharmacokinetics** in humans.
- They involve microdoses, not intended to have therapeutic effects, and thus cannot determine toxicity or MTD.
*Phase III*
- **Phase III trials** are large-scale studies involving hundreds to thousands of patients to confirm the drug's **efficacy**, monitor side effects, compare it to standard treatments, and collect information that will allow the drug to be used safely.
- These trials are conducted after safety and initial efficacy have been established in earlier phases.
*Phase V*
- "Phase V" is not a standard, recognized phase in the traditional clinical trial classification (Phase 0, I, II, III, IV).
- This term might be used in some non-standard research contexts or for post-marketing studies that go beyond Phase IV surveillance, but it is not a formal phase for initial drug development.
*Phase II*
- **Phase II trials** involve several hundred patients with the condition the drug is intended to treat, focusing on **drug efficacy** and further evaluating safety.
- While safety is still monitored, the primary objective shifts to determining if the drug works for its intended purpose and at what dose.
Likelihood ratios US Medical PG Question 6: A student health coordinator plans on leading a campus-wide HIV screening program that will be free for the entire undergraduate student body. The goal is to capture as many correct HIV diagnoses as possible with the fewest false positives. The coordinator consults with the hospital to see which tests are available to use for this program. Test A has a sensitivity of 0.92 and a specificity of 0.99. Test B has a sensitivity of 0.95 and a specificity of 0.96. Test C has a sensitivity of 0.98 and a specificity of 0.93. Which of the following testing schemes should the coordinator pursue?
- A. Test A on the entire student body followed by Test B on those who are positive
- B. Test A on the entire student body followed by Test C on those who are positive
- C. Test C on the entire student body followed by Test B on those who are positive
- D. Test C on the entire student body followed by Test A on those who are positive (Correct Answer)
- E. Test B on the entire student body followed by Test A on those who are positive
Likelihood ratios Explanation: ***Test C on the entire student body followed by Test A on those who are positive***
- To "capture as many correct HIV diagnoses as possible" (maximize true positives), the initial screening test should have the **highest sensitivity**. Test C has the highest sensitivity (0.98).
- To "capture as few false positives as possible" (maximize true negatives and confirm diagnoses), the confirmatory test should have the **highest specificity**. Test A has the highest specificity (0.99).
*Test A on the entire student body followed by Test B on those who are positive*
- Starting with Test A (sensitivity 0.92) would miss more true positive cases than starting with Test C (sensitivity 0.98), failing the goal of **capturing as many cases as possible**.
- Following with Test B (specificity 0.96) would result in more false positives than following with Test A (specificity 0.99).
*Test A on the entire student body followed by Test C on those who are positive*
- This scheme would miss many true positive cases initially due to Test A's lower sensitivity compared to Test C.
- Following with Test C would introduce more false positives than necessary, as it has a lower specificity (0.93) than Test A (0.99).
*Test C on the entire student body followed by Test B on those who are positive*
- While Test C is a good initial screen for its high sensitivity, following it with Test B (specificity 0.96) is less optimal than Test A (specificity 0.99) for minimizing false positives in the confirmation step.
- This combination would therefore yield more false positives in the confirmatory stage than using Test A.
*Test B on the entire student body followed by Test A on those who are positive*
- Test B has a sensitivity of 0.95, which is lower than Test C's sensitivity of 0.98, meaning it would miss more true positive cases at the initial screening stage.
- While Test A provides excellent specificity for confirmation, the initial screening step is suboptimal for the goal of capturing as many diagnoses as possible.
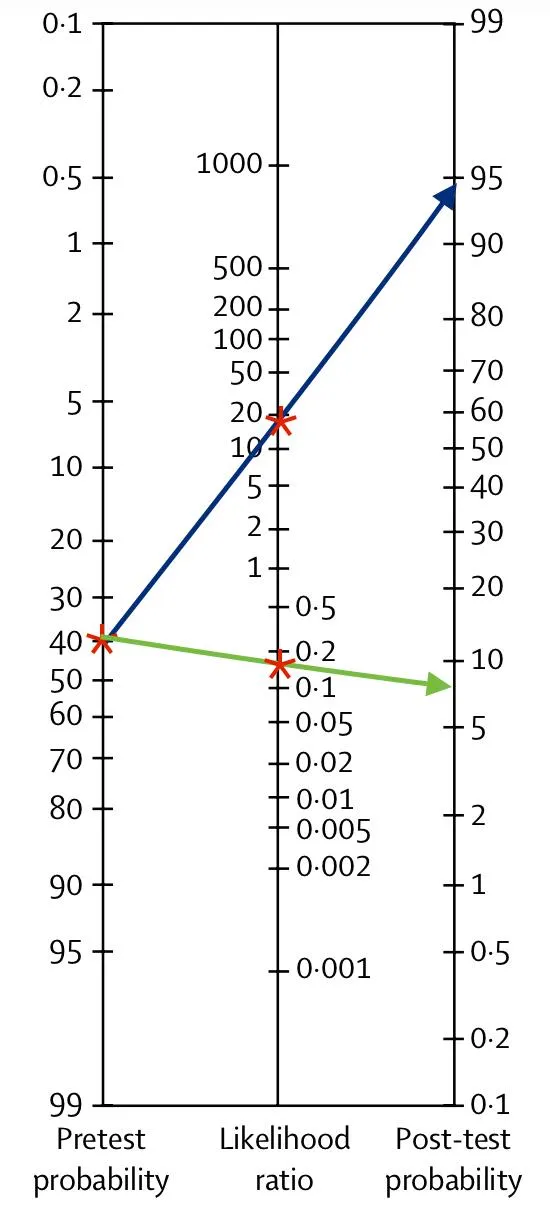
Likelihood ratios US Medical PG Question 7: A 14-month-old boy is brought in by his parents with an 8-month history of diarrhea, abdominal tenderness and concomitant failure to thrive. The pediatric attending physician believes that Crohn’s disease is the best explanation of this patient’s symptoms. Based on the pediatric attending physician’s experience, the pretest probability of this diagnosis is estimated at 40%. According to Fagan nomogram (see image). If the likelihood ratio of a negative test result (LR-) for Crohn’s disease is 0.04, what is the chance that this is the correct diagnosis in this patient with a negative test result?
- A. 40%
- B. 75%
- C. 97.5%
- D. 25%
- E. 2.5% (Correct Answer)
Likelihood ratios Explanation: ***2.5%***
- Begin by locating the **pretest probability of 40%** on the left-hand scale of the **Fagan nomogram**
- Draw a line from this point through the **likelihood ratio negative (LR-) of 0.04** on the middle scale, extending it to the right-hand scale to find the **posttest probability of approximately 2.5%**
- This can be verified mathematically: pretest odds = 0.40/0.60 = 0.667; posttest odds = 0.667 × 0.04 = 0.027; posttest probability = 0.027/1.027 ≈ 2.6% (rounds to 2.5%)
*40%*
- This represents the **initial pretest probability** before incorporating the test result
- It does not account for the impact of the **negative test result** with an LR- of 0.04
- The Fagan nomogram is used to **update this probability** based on the test outcome
*75%*
- This value does not align with a **negative test result** and an **LR- of 0.04**
- A posttest probability higher than the pretest probability would require a positive test with an LR+ greater than 1
- With a negative test and LR- = 0.04, the probability must decrease significantly
*97.5%*
- This extremely high posttest probability would require a **positive test** with a very high **likelihood ratio positive (LR+)**
- It is completely inconsistent with a **negative test result** and a low LR- of 0.04
- An LR- of 0.04 indicates strong evidence against the disease, not for it
*25%*
- While this represents a decrease from the pretest probability of 40%, it underestimates the impact of the test result
- An **LR- of 0.04** means the odds of having the disease are reduced by a factor of 25 (multiplied by 0.04)
- This should yield a much lower posttest probability than 25%
Likelihood ratios US Medical PG Question 8: You are reviewing raw data from a research study performed at your medical center examining the effectiveness of a novel AIDS screening examination. The study enrolled 250 patients with confirmed AIDS, and 240 of these patients demonstrated a positive screening examination. The control arm of the study enrolled 250 patients who do not have AIDS, and only 5 of these patients tested positive on the novel screening examination. What is the NPV of this novel test?
- A. 240 / (240 + 15)
- B. 240 / (240 + 5)
- C. 240 / (240 + 10)
- D. 245 / (245 + 10) (Correct Answer)
- E. 245 / (245 + 5)
Likelihood ratios Explanation: ***245 / (245 + 10)***
- The **negative predictive value (NPV)** is calculated as **true negatives (TN)** divided by the sum of **true negatives (TN)** and **false negatives (FN)**.
- In this study, there are 250 patients with AIDS; 240 tested positive (true positives, TP), meaning 10 tested negative (false negatives, FN = 250 - 240). There are 250 patients without AIDS; 5 tested positive (false positives, FP), meaning 245 tested negative (true negatives, TN = 250 - 5). Therefore, NPV = 245 / (245 + 10).
*240 / (240 + 15)*
- This calculation incorrectly uses the number of **true positives** (240) in the numerator and denominator, which is relevant for **positive predictive value (PPV)**, not NPV.
- The denominator `(240 + 15)` does not correspond to a valid sum for calculating NPV from the given data.
*240 / (240 + 5)*
- This calculation incorrectly uses **true positives** (240) in the numerator, which is not part of the NPV formula.
- The denominator `(240 + 5)` mixes true positives and false positives, which is incorrect for NPV.
*240 / (240 + 10)*
- This incorrectly places **true positives** (240) in the numerator instead of **true negatives**.
- The denominator `(240+10)` represents **true positives + false negatives**, which is related to sensitivity, not NPV.
*245 / (245 + 5)*
- This calculation correctly identifies **true negatives** (245) in the numerator but incorrectly uses **false positives** (5) in the denominator instead of **false negatives**.
- The denominator for NPV should be **true negatives + false negatives**, which is 245 + 10.
Likelihood ratios US Medical PG Question 9: A medical research study is beginning to evaluate the positive predictive value of a novel blood test for non-Hodgkin’s lymphoma. The diagnostic arm contains 700 patients with NHL, of which 400 tested positive for the novel blood test. In the control arm, 700 age-matched control patients are enrolled and 0 are found positive for the novel test. What is the PPV of this test?
- A. 400 / (400 + 0) (Correct Answer)
- B. 700 / (700 + 300)
- C. 400 / (400 + 300)
- D. 700 / (700 + 0)
- E. 700 / (400 + 400)
Likelihood ratios Explanation: ***400 / (400 + 0) = 1.0 or 100%***
- The **positive predictive value (PPV)** is calculated as **True Positives / (True Positives + False Positives)**.
- In this scenario, **True Positives (TP)** are the 400 patients with NHL who tested positive, and **False Positives (FP)** are 0, as no control patients tested positive.
- This gives a PPV of 400/400 = **1.0 or 100%**, indicating that all patients who tested positive actually had the disease.
*700 / (700 + 300)*
- This calculation does not align with the formula for PPV based on the given data.
- The denominator `(700+300)` suggests an incorrect combination of various patient groups.
*400 / (400 + 300)*
- The denominator `(400+300)` incorrectly includes 300, which is the number of **False Negatives** (patients with NHL who tested negative), not False Positives.
- PPV focuses on the proportion of true positives among all positive tests, not all diseased individuals.
*700 / (700 + 0)*
- This calculation incorrectly uses the total number of patients with NHL (700) as the numerator, rather than the number of positive test results in that group.
- The numerator should be the **True Positives** (400), not the total number of diseased individuals.
*700 / (400 + 400)*
- This calculation uses incorrect values for both the numerator and denominator, not corresponding to the PPV formula.
- The numerator 700 represents the total number of patients with the disease, not those who tested positive, and the denominator incorrectly sums up values that don't represent the proper PPV calculation.
Likelihood ratios US Medical PG Question 10: You are tasked with analyzing the negative predictive value of an experimental serum marker for ovarian cancer. You choose to enroll 2,000 patients across multiple clinical sites, including both 1,000 patients with ovarian cancer and 1,000 age-matched controls. From the disease and control subgroups, 700 and 100 are found positive for this novel serum marker, respectively. Which of the following represents the NPV for this test?
- A. 700 / (700 + 300)
- B. 700 / (300 + 900)
- C. 700 / (700 + 100)
- D. 900 / (900 + 100)
- E. 900 / (900 + 300) (Correct Answer)
Likelihood ratios Explanation: ***900 / (900 + 300)***
- The **Negative Predictive Value (NPV)** is the probability that a person with a **negative test result** does not have the disease. It is calculated as **true negatives (TN)** divided by the sum of true negatives and **false negatives (FN)**, i.e., TN / (TN + FN).
- In this scenario: there are 1,000 ovarian cancer patients, and 700 tested positive, meaning **300 tested negative (false negatives)**. There are 1,000 controls, and 100 tested positive, meaning **900 tested negative (true negatives)**. Therefore, NPV = 900 / (900 + 300).
*700 / (700 + 300)*
- This calculation represents the sensitivity of the test, which is the proportion of true positives among all individuals with the disease (700 true positives / 1000 diseased individuals).
- It does not account for the true negatives or false positives, which are crucial for determining predictive values.
*700 / (300 + 900)*
- This formula mixes elements and does not correspond to a standard measure of test validity.
- The numerator (700) is the number of true positives, and the denominator incorrectly combines false negatives (300) and true negatives (900).
*700 / (700 + 100)*
- This calculation represents the **Positive Predictive Value (PPV)**, which is the probability that a person with a **positive test result** actually has the disease (700 true positives / (700 true positives + 100 false positives)).
- It does not assess the negative predictive power of the test.
*900 / (900 + 100)*
- This calculation represents the **specificity** of the test, which is the proportion of true negatives among all individuals without the disease (900 true negatives / 1000 controls).
- While this involves true negatives, it does not account for false negatives, which are essential for calculating NPV.
More Likelihood ratios US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.