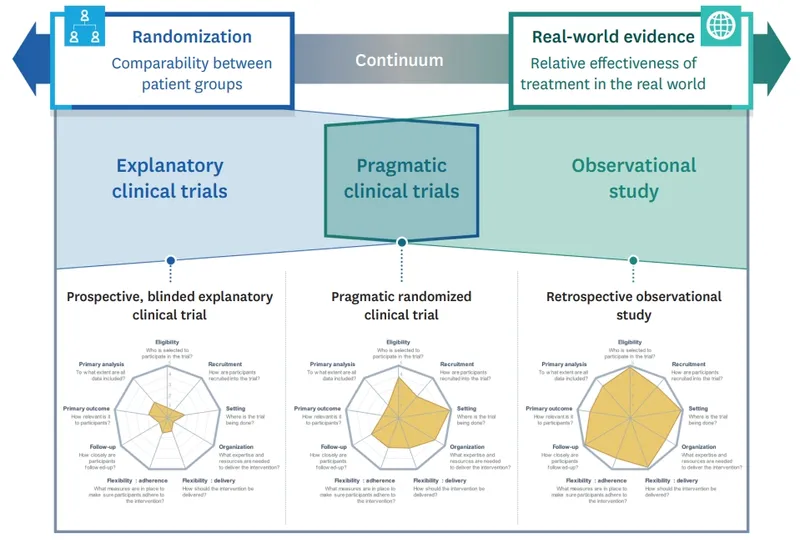
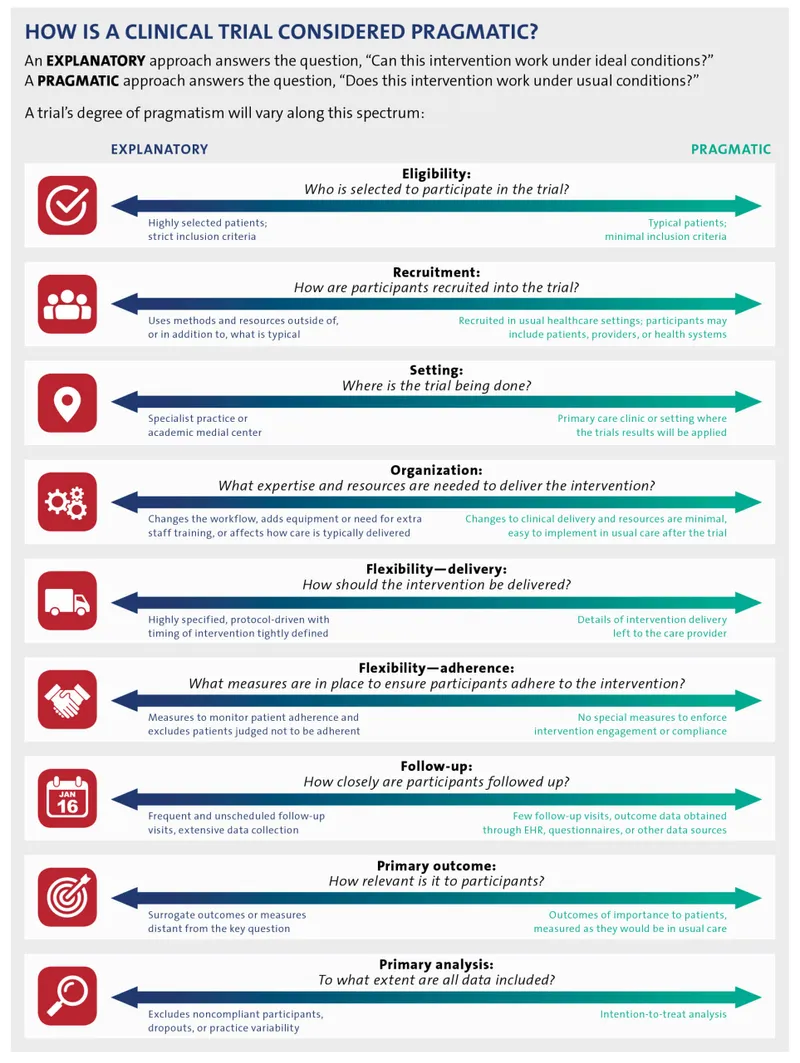
Pragmatic vs explanatory trials US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Pragmatic vs explanatory trials. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Pragmatic vs explanatory trials US Medical PG Question 1: A researcher is trying to determine whether a newly discovered substance X can be useful in promoting wound healing after surgery. She conducts this study by enrolling the next 100 patients that will be undergoing this surgery and separating them into 2 groups. She decides which patient will be in which group by using a random number generator. Subsequently, she prepares 1 set of syringes with the novel substance X and 1 set of syringes with a saline control. Both of these sets of syringes are unlabeled and the substances inside cannot be distinguished. She gives the surgeon performing the surgery 1 of the syringes and does not inform him nor the patient which syringe was used. After the study is complete, she analyzes all the data that was collected and performs statistical analysis. This study most likely provides which level of evidence for use of substance X?
- A. Level 3
- B. Level 1 (Correct Answer)
- C. Level 4
- D. Level 5
- E. Level 2
Pragmatic vs explanatory trials Explanation: ***Level 1***
- The study design described is a **randomized controlled trial (RCT)**, which is considered the **highest level of evidence (Level 1)** in the hierarchy of medical evidence.
- Key features like **randomization**, **control group**, and **blinding (double-blind)** help minimize bias and strengthen the validity of the findings.
*Level 2*
- Level 2 evidence typically comprises **well-designed controlled trials without randomization** (non-randomized controlled trials) or **high-quality cohort studies**.
- While strong, they do not possess the same level of internal validity as randomized controlled trials.
*Level 3*
- Level 3 evidence typically includes **case-control studies** or **cohort studies**, which are observational designs and carry a higher risk of bias compared to RCTs.
- These studies generally do not involve randomization or intervention assignment by the researchers.
*Level 4*
- Level 4 evidence is usually derived from **case series** or **poor quality cohort and case-control studies**.
- These studies provide descriptive information or investigate associations without strong control for confounding factors.
*Level 5*
- Level 5 evidence is the **lowest level of evidence**, consisting of **expert opinion** or **animal research/bench research**.
- This level lacks human clinical data or systematic investigative rigor needed for higher evidence levels.
Pragmatic vs explanatory trials US Medical PG Question 2: An investigator is measuring the blood calcium level in a sample of female cross country runners and a control group of sedentary females. If she would like to compare the means of the two groups, which statistical test should she use?
- A. Chi-square test
- B. Linear regression
- C. t-test (Correct Answer)
- D. ANOVA (Analysis of Variance)
- E. F-test
Pragmatic vs explanatory trials Explanation: ***t-test***
- A **t-test** is appropriate for comparing the means of two independent groups, such as the blood calcium levels between runners and sedentary females.
- It assesses whether the observed difference between the two sample means is statistically significant or occurred by chance.
*Chi-square test*
- The **chi-square test** is used to analyze categorical data to determine if there is a significant association between two variables.
- It is not suitable for comparing continuous variables like blood calcium levels.
*Linear regression*
- **Linear regression** is used to model the relationship between a dependent variable (outcome) and one or more independent variables (predictors).
- It aims to predict the value of a variable based on the value of another, rather than comparing means between groups.
*ANOVA (Analysis of Variance)*
- **ANOVA** is used to compare the means of **three or more independent groups**.
- Since there are only two groups being compared in this scenario, a t-test is more specific and appropriate.
*F-test*
- The **F-test** is primarily used to compare the variances of two populations or to assess the overall significance of a regression model.
- While it is the basis for ANOVA, it is not the direct test for comparing the means of two groups.
Pragmatic vs explanatory trials US Medical PG Question 3: A physician attempts to study cirrhosis in his state. Using a registry of admitted patients over the last 10 years at the local hospital, he isolates all patients who have been diagnosed with cirrhosis. Subsequently, he contacts this group of patients, asking them to complete a survey assessing their prior exposure to alcohol use, intravenous drug abuse, blood transfusions, personal history of cancer, and other medical comorbidities. An identical survey is given to an equal number of patients in the registry who do not carry a prior diagnosis of cirrhosis. Which of the following is the study design utilized by this physician?
- A. Randomized controlled trial
- B. Case-control study (Correct Answer)
- C. Cross-sectional study
- D. Cohort study
- E. Meta-analysis
Pragmatic vs explanatory trials Explanation: ***Case-control study***
- This study design **identifies subjects based on their outcome (cases with cirrhosis, controls without cirrhosis)** and then retrospectively investigates their past exposures.
- The physician selected patients with cirrhosis (cases) and patients without cirrhosis (controls), then assessed their prior exposures to risk factors like alcohol use and intravenous drug abuse.
*Randomized controlled trial*
- This design involves randomly assigning participants to an **intervention group** or a **control group** to assess the effect of an intervention.
- There is no intervention being tested or randomization occurring in this study; it is observational.
*Cross-sectional study*
- A cross-sectional study measures the **prevalence of disease and exposure at a single point in time** in a defined population.
- This study collects retrospective exposure data and compares two distinct groups (cases and controls), rather than assessing prevalence at one time point.
*Cohort study*
- A cohort study **follows a group of individuals over time** to see if their exposure to a risk factor is associated with the development of a disease.
- This study starts with the outcome (cirrhosis) and looks backward at exposures, which is the opposite direction of a cohort study.
*Meta-analysis*
- A meta-analysis is a statistical method that **combines the results of multiple independent studies** to produce a single, more powerful estimate of treatment effect or association.
- This is an original research study collecting new data, not a systematic review or synthesis of existing studies.
Pragmatic vs explanatory trials US Medical PG Question 4: You are currently employed as a clinical researcher working on clinical trials of a new drug to be used for the treatment of Parkinson's disease. Currently, you have already determined the safe clinical dose of the drug in a healthy patient. You are in the phase of drug development where the drug is studied in patients with the target disease to determine its efficacy. Which of the following phases is this new drug currently in?
- A. Phase 4
- B. Phase 1
- C. Phase 2 (Correct Answer)
- D. Phase 0
- E. Phase 3
Pragmatic vs explanatory trials Explanation: ***Phase 2***
- **Phase 2 trials** involve studying the drug in patients with the target disease to assess its **efficacy** and further evaluate safety, typically involving a few hundred patients.
- The question describes a stage after safe dosing in healthy patients (Phase 1) and before large-scale efficacy confirmation (Phase 3), focusing on efficacy in the target population.
*Phase 4*
- **Phase 4 trials** occur **after a drug has been approved** and marketed, monitoring long-term effects, optimal use, and rare side effects in a diverse patient population.
- This phase is conducted post-market approval, whereas the question describes a drug still in development prior to approval.
*Phase 1*
- **Phase 1 trials** primarily focus on determining the **safety and dosage** of a new drug in a **small group of healthy volunteers** (or sometimes patients with advanced disease if the drug is highly toxic).
- The question states that the safe clinical dose in a healthy patient has already been determined, indicating that Phase 1 has been completed.
*Phase 0*
- **Phase 0 trials** are exploratory, very early-stage studies designed to confirm that the drug reaches the target and acts as intended, typically involving a very small number of doses and participants.
- These trials are conducted much earlier in the development process, preceding the determination of safe clinical doses and large-scale efficacy studies.
*Phase 3*
- **Phase 3 trials** are large-scale studies involving hundreds to thousands of patients to confirm **efficacy**, monitor side effects, compare it to commonly used treatments, and collect information that will allow the drug to be used safely.
- While Phase 3 does assess efficacy, it follows Phase 2 and is typically conducted on a much larger scale before submitting for regulatory approval.
Pragmatic vs explanatory trials US Medical PG Question 5: A pharmaceutical company conducts a randomized clinical trial in an attempt to show that their new anticoagulant drug prevents more thrombotic events following total knee arthroplasty than the current standard of care. However, a significant number of patients are lost to follow-up or fail to complete treatment according to the study arm to which they were assigned. Several patients in the novel drug arm are also switched at a later time to a novel anticoagulant or warfarin per their primary care physician. All patients enrolled in the study are subsequently analyzed based on the initial group they were assigned to and there is a significant improvement in outcome of the new drug. What analysis most appropriately describes this trial?
- A. Per protocol
- B. As treated
- C. Non-inferiority
- D. Intention to treat (Correct Answer)
- E. Modified intention to treat
Pragmatic vs explanatory trials Explanation: ***Intention to treat***
- **Intention-to-treat (ITT)** analysis includes all participants randomized to a treatment arm, regardless of whether they completed the intervention or switched treatments, reflecting a real-world scenario and preserving randomization benefits.
- This approach minimizes bias from **loss to follow-up** or **treatment crossovers** and provides a more conservative estimate of treatment effect.
*Per protocol*
- **Per-protocol analysis** only includes participants who completed the study exactly as planned without any deviations.
- This method is susceptible to **selection bias** because it excludes patients who may have experienced adverse events or treatment failures, potentially overestimating treatment efficacy.
*As treated*
- **As-treated analysis** analyzes patients based on the actual treatment received, rather than the treatment to which they were randomized.
- This approach can introduce **confounding** and selection bias, as patients who switch treatments may do so for reasons related to their prognosis or treatment response.
*Non-inferiority*
- A **non-inferiority trial** design aims to show that a new treatment is not appreciably worse than an active control, rather than proving superiority.
- This describes a **type of study design** or hypothesis, not an analysis method for handling patient data after randomization with non-adherence.
*Modified intention to treat*
- A **modified intention-to-treat (mITT)** analysis typically excludes a small, predefined group of patients from the ITT population, such as those who never received any study drug or were found to be ineligible after randomization.
- While similar to ITT, it involves specific exclusions that are not described in this scenario, where all randomized patients were analyzed **based on initial assignment**.
Pragmatic vs explanatory trials US Medical PG Question 6: A scientist is designing a study to determine whether eating a new diet is able to lower blood pressure in a group of patients. In particular, he believes that starting the diet may help decrease peak blood pressures throughout the day. Therefore, he will equip study participants with blood pressure monitors and follow pressure trends over a 24-hour period. He decides that after recruiting subjects, he will start them on either the new diet or a control diet and follow them for 1 month. After this time, he will switch patients onto the other diet and follow them for an additional month. He will analyze the results from the first month against the results from the second month for each patient. This type of study design is best at controlling for which of the following problems with studies?
- A. Hawthorne effect
- B. Recall bias
- C. Confounding (Correct Answer)
- D. Selection bias
- E. Pygmalion effect
Pragmatic vs explanatory trials Explanation: ***Confounding***
- This **crossover design** (switching patients to the other diet) effectively controls for **confounding variables** by making each patient their own control, ensuring that inherent patient characteristics do not bias the comparison between diets.
- By comparing the effects of both diets within the same individual, individual variability in factors such as genetics, lifestyle, and other co-morbidities are accounted for, reducing their potential as confounders.
*Hawthorne effect*
- The **Hawthorne effect** refers to subjects modifying their behavior in response to being observed, which this study design does not specifically address or eliminate.
- While patients are being monitored, the design aims to compare the diets' effects, not to prevent behavioral changes due to observation itself.
*Recall bias*
- **Recall bias** occurs when participants' memories of past events are inaccurate, often influenced by their current health status or beliefs.
- This study measures **real-time blood pressure** data, not relying on recollection of past exposures or outcomes, thereby mitigating recall bias.
*Selection bias*
- **Selection bias** arises from non-random selection of participants into study groups, leading to systematic differences between groups.
- While patient recruitment could introduce selection bias into the overall study population, the **crossover design** itself helps control for differences between treatment arms because all participants eventually receive both treatments.
*Pygmalion effect*
- The **Pygmalion effect** (or observer-expectancy effect) describes phenomena where higher expectations lead to increased performance, usually from a researcher influencing a subject.
- This effect is not directly addressed by the crossover design; the design focuses on controlling for patient-specific confounders rather than investigator bias in expectations.
Pragmatic vs explanatory trials US Medical PG Question 7: You are reading through a recent article that reports significant decreases in all-cause mortality for patients with malignant melanoma following treatment with a novel biological infusion. Which of the following choices refers to the probability that a study will find a statistically significant difference when one truly does exist?
- A. Type II error
- B. Type I error
- C. Confidence interval
- D. p-value
- E. Power (Correct Answer)
Pragmatic vs explanatory trials Explanation: ***Power***
- **Power** is the probability that a study will correctly reject the null hypothesis when it is, in fact, false (i.e., will find a statistically significant difference when one truly exists).
- A study with high power minimizes the risk of a **Type II error** (failing to detect a real effect).
*Type II error*
- A **Type II error** (or **beta error**) occurs when a study fails to reject a false null hypothesis, meaning it concludes there is no significant difference when one actually exists.
- This is the **opposite** of what the question describes, which asks for the probability of *finding* a difference.
*Type I error*
- A **Type I error** (or **alpha error**) occurs when a study incorrectly rejects a true null hypothesis, concluding there is a significant difference when one does not actually exist.
- This relates to the **p-value** and the level of statistical significance (e.g., p < 0.05).
*Confidence interval*
- A **confidence interval** provides a range of values within which the true population parameter is likely to lie with a certain degree of confidence (e.g., 95%).
- It does not directly represent the probability of finding a statistically significant difference when one truly exists.
*p-value*
- The **p-value** is the probability of observing data as extreme as, or more extreme than, that obtained in the study, assuming the null hypothesis is true.
- It is used to determine statistical significance, but it is not the probability of detecting a true effect.
Pragmatic vs explanatory trials US Medical PG Question 8: A study seeks to investigate the therapeutic efficacy of treating asymptomatic subclinical hypothyroidism in preventing symptoms of hypothyroidism. The investigators found 300 asymptomatic patients with subclinical hypothyroidism, defined as serum thyroid-stimulating hormone (TSH) of 5 to 10 μU/mL with normal serum thyroxine (T4) levels. The patients were randomized to either thyroxine 75 μg daily or placebo. Both investigators and study subjects were blinded. Baseline patient characteristics were distributed similarly in the treatment and control group (p > 0.05). Participants' serum T4 and TSH levels and subjective quality of life were evaluated at a 3-week follow-up. No difference was found between the treatment and placebo groups. Which of the following is the most likely explanation for the results of this study?
- A. Observer effect
- B. Berkson bias
- C. Latency period (Correct Answer)
- D. Confounding bias
- E. Lead-time bias
Pragmatic vs explanatory trials Explanation: ***Latency period***
- A **latency period** refers to the time between exposure to a cause (e.g., treatment) and the manifestation of its effects (e.g., symptom improvement). The study's **3-week follow-up is too short** to observe the therapeutic benefits of thyroxine in subclinical hypothyroidism.
- Levothyroxine (T4) has a **half-life of approximately 7 days**, and it typically takes **6-8 weeks or longer** for steady-state levels to be achieved and for clinical symptoms to improve. The slow onset of action for thyroid hormone replacement and the gradual nature of symptom resolution mean a longer observation period (typically 3-6 months) is needed to assess efficacy in hypothyroidism.
- The null results likely reflect insufficient follow-up time rather than lack of treatment effect.
*Observer effect*
- The **observer effect**, or Hawthorne effect, occurs when subjects change their behavior because they know they are being observed. This study used **double-blinding** (both investigators and subjects), which effectively minimizes the observer effect.
- The primary issue here is the lack of observed therapeutic effect due to timing, not a change in behavior due to observation.
*Berkson bias*
- **Berkson bias** is a form of selection bias that arises in case-control studies conducted in hospitals, where the probability of being admitted to the hospital can be affected by both exposure and disease.
- This study is a **randomized controlled trial**, not a case-control study, and the selection of participants does not illustrate this specific bias.
*Confounding bias*
- **Confounding bias** occurs when an extraneous variable is associated with both the exposure and the outcome, distorting the observed relationship. The study states that **baseline patient characteristics were similarly distributed (p > 0.05)**, indicating successful randomization and minimization of confounding.
- While confounding is a common concern in observational studies, the RCT design and reported baseline similarities make it unlikely to be the primary explanation for the null results compared to an insufficient follow-up period.
*Lead-time bias*
- **Lead-time bias** is a form of detection bias where early detection of a disease through screening appears to prolong survival, even if the treatment does not change the course of the disease.
- This study is evaluating the **efficacy of treatment** in asymptomatic individuals with subclinical hypothyroidism, not the effect of screening on survival, making lead-time bias irrelevant to these results.
Pragmatic vs explanatory trials US Medical PG Question 9: In the study, all participants who were enrolled and randomly assigned to treatment with pulmharkimab were analyzed in the pulmharkimab group regardless of medication nonadherence or refusal of allocated treatment. A medical student reading the abstract is confused about why some participants assigned to pulmharkimab who did not adhere to the regimen were still analyzed as part of the pulmharkimab group. Which of the following best reflects the purpose of such an analysis strategy?
- A. To minimize type 2 errors
- B. To assess treatment efficacy more accurately
- C. To reduce selection bias (Correct Answer)
- D. To increase internal validity of study
- E. To increase sample size
Pragmatic vs explanatory trials Explanation: ***To reduce selection bias***
- Analyzing participants in their originally assigned groups, regardless of adherence, is known as **intention-to-treat (ITT) analysis**.
- This method helps **preserve randomization** and minimizes **selection bias** that could arise if participants who did not adhere to treatment were excluded or re-assigned.
- **This is the most direct and specific purpose** of ITT analysis - preventing systematic differences between groups caused by post-randomization exclusions.
*To minimize type 2 errors*
- While ITT analysis affects statistical power, its primary purpose is not specifically to minimize **type 2 errors** (false negatives).
- ITT analysis may sometimes *increase* the likelihood of a type 2 error by diluting the treatment effect due to non-adherence.
*To assess treatment efficacy more accurately*
- ITT analysis assesses the **effectiveness** of *assigning* a treatment in a real-world setting, rather than the pure biological **efficacy** of the treatment itself.
- Efficacy is better assessed by a **per-protocol analysis**, which only includes compliant participants.
- ITT provides a more **conservative** and **pragmatic** estimate of treatment effect.
*To increase internal validity of study*
- While ITT analysis does contribute to **internal validity** by maintaining randomization, this is a **broader, secondary benefit** rather than the primary purpose.
- Internal validity encompasses many aspects of study design; ITT specifically addresses **post-randomization bias prevention**.
- The more precise answer is that ITT reduces **selection bias**, which is one specific threat to internal validity.
- Many other design features also contribute to internal validity (blinding, standardized protocols, etc.), making this option less specific.
*To increase sample size*
- ITT analysis includes all randomized participants, so it maintains the initial **sample size** that was randomized.
- However, the primary purpose is to preserve the integrity of randomization and prevent bias, not simply to increase the number of participants in the final analysis.
Pragmatic vs explanatory trials US Medical PG Question 10: A randomized control double-blind study is conducted on the efficacy of 2 sulfonylureas. The study concluded that medication 1 was more efficacious in lowering fasting blood glucose than medication 2 (p ≤ 0.05; 95% CI: 14 [10-21]). Which of the following is true regarding a 95% confidence interval (CI)?
- A. If the same study were repeated multiple times, approximately 95% of the calculated confidence intervals would contain the true population parameter. (Correct Answer)
- B. The 95% confidence interval is the probability chosen by the researcher to be the threshold of statistical significance.
- C. When a 95% CI for the estimated difference between groups contains the value ‘0’, the results are significant.
- D. It represents the probability that chance would not produce the difference shown, 95% of the time.
- E. The study is adequately powered at the 95% confidence interval.
Pragmatic vs explanatory trials Explanation: ***If the same study were repeated multiple times, approximately 95% of the calculated confidence intervals would contain the true population parameter.***
- This statement accurately defines the **frequentist interpretation** of a confidence interval (CI). It reflects the long-run behavior of the CI over hypothetical repetitions of the study.
- A 95% CI means that if you were to repeat the experiment many times, 95% of the CIs calculated from those experiments would capture the **true underlying population parameter**.
*The 95% confidence interval is the probability chosen by the researcher to be the threshold of statistical significance.*
- The **alpha level (α)**, typically set at 0.05 (or 5%), is the threshold for statistical significance (p ≤ 0.05), representing the probability of a Type I error.
- The 95% confidence level (1-α) is related to statistical significance, but it is not the *threshold* itself; rather, it indicates the **reliability** of the interval estimate.
*When a 95% CI for the estimated difference between groups contains the value ‘0’, the results are significant.*
- If a 95% CI for the difference between groups **contains 0**, it implies that there is **no statistically significant difference** between the groups at the 0.05 alpha level.
- A statistically significant difference (p ≤ 0.05) would be indicated if the 95% CI **does NOT contain 0**, suggesting that the intervention had a real effect.
*It represents the probability that chance would not produce the difference shown, 95% of the time.*
- This statement misinterprets the meaning of a CI and probability. The chance of not producing the observed difference is typically addressed by the **p-value**, not directly by the CI in this manner.
- A CI provides a **range of plausible values** for the population parameter, not a probability about the role of chance in producing the observed difference.
*The study is adequately powered at the 95% confidence interval.*
- **Statistical power** is the probability of correctly rejecting a false null hypothesis, typically set at 80% or 90%. It is primarily determined by sample size, effect size, and alpha level.
- A 95% CI is a measure of the **precision** of an estimate, while power refers to the **ability of a study to detect an effect** if one exists. They are related but distinct concepts.
More Pragmatic vs explanatory trials US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.