RCTs

On this page
🎯 The RCT Arsenal: Clinical Evidence Mastery
Randomized controlled trials stand as the gold standard for determining what truly works in medicine, transforming clinical hunches into reliable evidence through rigorous design. You'll master how randomization eliminates bias, why concealment and blinding protect against distortion, and how proper outcome selection and analysis reveal genuine treatment effects. By understanding the architecture of RCTs-from allocation strategies to statistical interpretation-you'll critically evaluate published trials, identify fatal flaws that invalidate conclusions, and distinguish robust evidence from cleverly disguised noise.
📌 Remember: PICO-R - Population, Intervention, Comparison, Outcome, Randomization - The five pillars supporting every robust RCT design
The fundamental architecture of RCTs eliminates selection bias through random allocation, creating comparable groups that differ only by chance. This randomization process ensures that both known and unknown confounding variables are distributed equally between treatment arms, establishing the causal inference foundation that distinguishes RCTs from observational studies.
- Study Population Selection
- Inclusion criteria define target demographics with 85-90% specificity
- Exclusion criteria eliminate confounders affecting 15-20% of potential participants
- Safety exclusions: pregnancy, severe comorbidities
- Methodological exclusions: concurrent medications, compliance issues
- Randomization Implementation
- Simple randomization: 50% allocation probability per arm
- Block randomization: maintains balance within 4-8 participant blocks
- Stratified randomization: controls for 2-3 key prognostic factors
- Minimization algorithms: achieve >95% balance across multiple variables
| RCT Component | Purpose | Success Metric | Failure Rate | Clinical Impact |
|---|---|---|---|---|
| Randomization | Eliminate selection bias | Equal baseline characteristics | <5% imbalance | Causal inference validity |
| Blinding | Prevent performance bias | Successful masking >80% | 15-25% unblinding | Treatment effect accuracy |
| Allocation Concealment | Prevent selection bias | Zero prediction ability | 10-15% compromise | Internal validity |
| ITT Analysis | Preserve randomization | Include 100% randomized | 5-10% exclusions | Generalizability |
| Sample Size | Adequate statistical power | 80-90% power achieved | 20-30% underpowered | Clinical significance detection |
| %%{init: {'flowchart': {'htmlLabels': true}}}%% | ||||
| flowchart TD |
Pop["<b>👥 Study Population</b><br><span style='display:block; text-align:left; color:#555'>• Target subjects</span><span style='display:block; text-align:left; color:#555'>• Recruitment pool</span>"]
Eval{"<b>📋 Assessment</b><br><span style='display:block; text-align:left; color:#555'>• Check eligibility</span><span style='display:block; text-align:left; color:#555'>• Screen subjects</span>"}
Exclude["<b>❌ Document Reasons</b><br><span style='display:block; text-align:left; color:#555'>• Record exclusion</span><span style='display:block; text-align:left; color:#555'>• Ethics reporting</span>"]
Rand["<b>🎲 Randomization</b><br><span style='display:block; text-align:left; color:#555'>• Random allocation</span><span style='display:block; text-align:left; color:#555'>• Reduce bias</span>"]
Treat["<b>💊 Treatment Group</b><br><span style='display:block; text-align:left; color:#555'>• Active condition</span><span style='display:block; text-align:left; color:#555'>• Experimental arm</span>"]
Control["<b>⚖️ Control Group</b><br><span style='display:block; text-align:left; color:#555'>• Comparison arm</span><span style='display:block; text-align:left; color:#555'>• Standard of care</span>"]
IntDel["<b>💉 Intervention</b><br><span style='display:block; text-align:left; color:#555'>• Deliver therapy</span><span style='display:block; text-align:left; color:#555'>• Expert protocol</span>"]
ConDel["<b>🛡️ Control Delivery</b><br><span style='display:block; text-align:left; color:#555'>• Placebo/standard</span><span style='display:block; text-align:left; color:#555'>• Blinding used</span>"]
Outc["<b>🔬 Outcomes</b><br><span style='display:block; text-align:left; color:#555'>• Data collection</span><span style='display:block; text-align:left; color:#555'>• Primary endpoints</span>"]
Stats["<b>📊 Statistics</b><br><span style='display:block; text-align:left; color:#555'>• Data analysis</span><span style='display:block; text-align:left; color:#555'>• P-value testing</span>"]
Results["<b>✅ Interpretation</b><br><span style='display:block; text-align:left; color:#555'>• Draw conclusions</span><span style='display:block; text-align:left; color:#555'>• Study findings</span>"]
Pop --> Eval
Eval -->|Excluded| Exclude
Eval -->|Meets Criteria| Rand
Rand --> Treat
Rand --> Control
Treat --> IntDel
Control --> ConDel
IntDel --> Outc
ConDel --> Outc
Outc --> Stats
Stats --> Results
style Pop fill:#F6F5F5, stroke:#E7E6E6, stroke-width:1.5px, rx:12, ry:12, color:#525252
style Eval fill:#FEF8EC, stroke:#FBECCA, stroke-width:1.5px, rx:12, ry:12, color:#854D0E
style Exclude fill:#FDF4F3, stroke:#FCE6E4, stroke-width:1.5px, rx:12, ry:12, color:#B91C1C
style Rand fill:#F7F5FD, stroke:#F0EDFA, stroke-width:1.5px, rx:12, ry:12, color:#6B21A8
style Treat fill:#F1FCF5, stroke:#BEF4D8, stroke-width:1.5px, rx:12, ry:12, color:#166534
style Control fill:#EEFAFF, stroke:#DAF3FF, stroke-width:1.5px, rx:12, ry:12, color:#0369A1
style IntDel fill:#F1FCF5, stroke:#BEF4D8, stroke-width:1.5px, rx:12, ry:12, color:#166534
style ConDel fill:#EEFAFF, stroke:#DAF3FF, stroke-width:1.5px, rx:12, ry:12, color:#0369A1
style Outc fill:#FFF7ED, stroke:#FFEED5, stroke-width:1.5px, rx:12, ry:12, color:#C2410C
style Stats fill:#FFF7ED, stroke:#FFEED5, stroke-width:1.5px, rx:12, ry:12, color:#C2410C
style Results fill:#F6F5F5, stroke:#E7E6E6, stroke-width:1.5px, rx:12, ry:12, color:#525252
> ⭐ **Clinical Pearl**: RCTs with proper randomization and concealment show **25-30%** smaller treatment effects compared to inadequately randomized studies, reflecting the elimination of selection bias that artificially inflates intervention benefits.
> 💡 **Master This**: The hierarchy of evidence places well-designed RCTs above observational studies because randomization creates exchangeable groups - the only systematic difference between arms is the intervention itself, enabling causal conclusions about treatment efficacy.
Understanding RCT methodology provides the foundation for evaluating treatment evidence, but mastering the nuances of randomization techniques reveals how different approaches optimize study validity and efficiency.
🎯 The RCT Arsenal: Clinical Evidence Mastery
⚡ Randomization Mastery: The Bias-Breaking Engine
📌 Remember: SIMPLE-BLOCK-STRAT - Simple (basic coin flip), Imple Mentation, Permuted Lists, Equal allocation; Block (fixed sizes), Limited imbalance, Organized sequences, Consistent ratios, Known block sizes; Stratified (prognostic factors), Targeted balance, Risk-based groups, Adjusted allocation, Tailored randomization
- Simple Randomization Methods
- Coin flip allocation: 50% probability per assignment
- Random number generation: computer-based sequences with >99% unpredictability
- Advantages: complete unpredictability, simple implementation
- Disadvantages: potential imbalance in small studies (<100 participants)
- Block Randomization Strategies
- Fixed block sizes: 4, 6, 8 participants per block maintain balance
- Variable block sizes: 4-8 participant blocks prevent prediction
- Permuted block sequences: 24 possible arrangements for block size 4
- Stratified blocking: separate sequences for 2-3 prognostic subgroups
| Randomization Method | Balance Guarantee | Predictability Risk | Optimal Study Size | Implementation Complexity |
|---|---|---|---|---|
| Simple | No guarantee | Minimal | >200 participants | Very Low |
| Block (Fixed) | Perfect within blocks | High at block end | 50-500 participants | Low |
| Block (Variable) | Good overall | Moderate | 50-500 participants | Moderate |
| Stratified | Excellent by strata | Low | >100 participants | High |
| Minimization | Near-perfect | Very low | >50 participants | Very High |
- Stratified Randomization Applications
- Prognostic factor control: age groups (<65 vs ≥65 years)
- Disease severity stratification: mild, moderate, severe classifications
- Center-based stratification: multi-site studies with 3-20 participating centers
- Biomarker stratification: genetic variants affecting 20-40% of population
- Minimization Algorithm Features
- Dynamic probability adjustment: 0.6-0.8 allocation probability toward balancing
- Multiple factor consideration: simultaneously balance 4-6 prognostic variables
- Real-time imbalance calculation: weighted scoring across all stratification factors
- Biased coin allocation: 70-80% probability toward minority group assignment
⭐ Clinical Pearl: Studies using inadequate randomization methods show 40-50% larger treatment effects compared to properly randomized trials, primarily due to selection bias where investigators unconsciously allocate sicker patients to experimental treatments, creating artificial efficacy signals.
💡 Master This: The choice of randomization method depends on study size and prognostic factor importance - simple randomization suffices for large studies (>500 participants), while smaller studies require block or stratified methods to ensure baseline comparability essential for valid statistical inference.
Effective randomization creates the foundation for unbiased treatment comparison, but allocation concealment mechanisms ensure that this randomization process remains immune to manipulation and prediction.
⚡ Randomization Mastery: The Bias-Breaking Engine
🔒 Allocation Concealment: The Prediction-Proof Protocol
📌 Remember: SECURE-HIDE - Sealed envelopes (opaque, numbered), Electronic systems (web-based), Central randomization (phone/internet), Unbreakable sequences, Remote allocation, Encrypted systems; Hidden from investigators, Impenetrable methods, Decision-point revelation, Eliminate prediction
The distinction between randomization generation and allocation concealment often confuses researchers, yet both elements are essential for preventing selection bias. Randomization creates the sequence, while concealment ensures this sequence remains hidden until the moment of assignment.
- Concealment Method Categories
- Central randomization: 24/7 telephone or web-based systems
- Sealed envelope systems: opaque, sequentially numbered, tamper-evident
- Electronic concealment: password-protected, encrypted allocation systems
- Pharmacy-controlled: pre-packaged, coded medication containers
- Security Implementation Standards
- Envelope opacity: 100% light impermeability testing required
- Sequential numbering: prevents selective envelope opening
- Tamper-evident seals: immediate detection of unauthorized access
- Dual-person verification: 2-person envelope opening protocol
| Concealment Method | Security Level | Implementation Cost | Failure Rate | Audit Trail Quality |
|---|---|---|---|---|
| Central Phone | Very High | High | <1% | Excellent |
| Web-based System | Very High | Moderate | <2% | Excellent |
| Sealed Envelopes | Moderate | Low | 5-15% | Good |
| Pharmacy Control | High | High | <3% | Very Good |
| Open Lists | None | Very Low | 100% | None |
| %%{init: {'flowchart': {'htmlLabels': true}}}%% | ||||
| flowchart TD |
Start["👥 Eligible Participant
• Inclusion criteria• Recruitment phase"]
Contact["💻 Contact System
• Investigator login• Centralized portal"]
Verify{"📋 Data Verified
• Participant check• Details review"}
Return["🔄 Back to Verify
• Update info• Correct errors"]
Reveal["🎲 Reveal Allocation
• Randomized group• Concealment ends"]
Assign["💊 Treatment
• Active assignment• Protocol start"]
Audit["📜 Audit Trail
• Automated logging• GCP compliance"]
Start --> Contact Contact --> Verify Verify -->|Incomplete| Return Return --> Contact Verify -->|Confirmed| Reveal Reveal --> Assign Assign --> Audit
style Start fill:#F7F5FD, stroke:#F0EDFA, stroke-width:1.5px, rx:12, ry:12, color:#6B21A8 style Contact fill:#FEF8EC, stroke:#FBECCA, stroke-width:1.5px, rx:12, ry:12, color:#854D0E style Verify fill:#FEF8EC, stroke:#FBECCA, stroke-width:1.5px, rx:12, ry:12, color:#854D0E style Return fill:#EEFAFF, stroke:#DAF3FF, stroke-width:1.5px, rx:12, ry:12, color:#0369A1 style Reveal fill:#FFF7ED, stroke:#FFEED5, stroke-width:1.5px, rx:12, ry:12, color:#C2410C style Assign fill:#F1FCF5, stroke:#BEF4D8, stroke-width:1.5px, rx:12, ry:12, color:#166534 style Audit fill:#F6F5F5, stroke:#E7E6E6, stroke-width:1.5px, rx:12, ry:12, color:#525252
The effectiveness of allocation concealment depends on maintaining investigator blindness to upcoming assignments throughout the recruitment period. Even partial knowledge of allocation patterns can introduce systematic bias that compromises study validity.
* **Concealment Failure Mechanisms**
- Predictable allocation patterns: alternating assignments, date-based sequences
- Inadequate envelope security: translucent materials, poor sealing
+ Investigator manipulation: selective recruitment based on predicted assignments
+ Administrative breaches: unauthorized access to randomization lists
* **Quality Assurance Measures**
- Allocation sequence auditing: **100%** assignment verification
- Concealment integrity testing: random envelope inspection protocols
+ Investigator training: proper concealment procedure education
+ Monitoring visits: **quarterly** concealment compliance assessment
> ⭐ **Clinical Pearl**: Studies with inadequate allocation concealment demonstrate **30-40%** larger treatment effects compared to properly concealed trials, reflecting selection bias where investigators preferentially enroll patients with better prognoses when they predict assignment to experimental treatments.
> 💡 **Master This**: Allocation concealment differs from blinding - concealment prevents selection bias during recruitment, while blinding prevents performance and detection bias during treatment and assessment. Both are essential but address different sources of systematic error in clinical trials.
Robust allocation concealment preserves randomization integrity, but comprehensive blinding strategies extend bias prevention throughout the entire study conduct and outcome assessment process.
🔒 Allocation Concealment: The Prediction-Proof Protocol
👁️ Blinding Strategies: The Perception Protection System
📌 Remember: BLIND-MASK - Blind participants (prevent performance bias), Limit investigator knowledge, Identical placebos, Neutral appearance, Double-blind standard; Mask outcome assessors, Appearance matching, Similar side effects, Keep codes sealed
- Blinding Level Classifications
- Single-blind: participants unaware of assignment (participant blinding only)
- Double-blind: participants and investigators both blinded (standard approach)
- Triple-blind: adds outcome assessor blinding (optimal design)
- Quadruple-blind: includes data analyst blinding (maximum protection)
- Practical Blinding Challenges
- Distinctive side effect profiles: 60-80% of participants may guess assignment
- Route of administration differences: oral vs. injection delivery methods
- Dosing frequency variations: once daily vs. multiple daily doses
- Monitoring requirement differences: laboratory vs. clinical assessments
| Intervention Type | Blinding Feasibility | Typical Success Rate | Common Challenges | Alternative Strategies |
|---|---|---|---|---|
| Oral Medications | High | 80-90% | Taste, side effects | Matched placebos |
| Injectable Drugs | Moderate | 70-85% | Injection site reactions | Sham injections |
| Surgical Procedures | Low | 40-60% | Visible incisions | Sham surgery |
| Behavioral Interventions | Very Low | <30% | Participant awareness | Attention controls |
| Medical Devices | Variable | 50-80% | Device visibility | Sham devices |
- Pharmaceutical Blinding Methods
- Matched placebo design: identical appearance, taste, smell, texture
- Over-encapsulation: masking different capsule appearances
- Taste masking: flavoring agents to eliminate distinctive medication tastes
- Side effect simulation: adding agents to placebo causing similar adverse events
- Non-Pharmaceutical Blinding Approaches
- Sham procedures: surgical incisions without therapeutic intervention
- Attention control groups: equivalent contact time with different content
- Blinded outcome assessment: independent evaluators unaware of assignments
- Objective outcome measures: laboratory values, imaging, mortality
⭐ Clinical Pearl: Successful blinding assessment reveals that 15-25% of participants correctly guess their treatment assignment by chance alone, so blinding success rates below 60-70% indicate significant masking failure that may compromise study validity through differential behavior or reporting.
💡 Master This: When complete blinding is impossible, blinded outcome assessment becomes critical - independent evaluators unaware of treatment assignments can assess endpoints objectively, preventing detection bias even when participants and treating physicians know the intervention.
Effective blinding preserves treatment effect measurement integrity, but rigorous outcome selection and measurement strategies ensure that study endpoints accurately capture clinically meaningful intervention benefits.
👁️ Blinding Strategies: The Perception Protection System
🎯 Outcome Architecture: The Clinical Endpoint Framework
📌 Remember: PRIMARY-SECOND - Primary (single, pre-specified), Relevant to patients, Important clinically, Measurable objectively, Appropriate timing, Realistic to achieve, Yields adequate power; Secondary (multiple allowed), Exploratory analyses, Corroborating evidence, Other important outcomes, No power calculations, Descriptive primarily
- Primary Outcome Characteristics
- Single endpoint specification: one primary outcome prevents multiple testing issues
- Clinical relevance: mortality, morbidity, quality of life measures
- Objective measurement: laboratory values, imaging findings, standardized scales
- Appropriate timing: 6-month to 5-year follow-up depending on intervention
- Surrogate vs. Clinical Endpoints
- Surrogate markers: biomarkers predicting clinical benefit (HbA1c, blood pressure)
- Clinical endpoints: patient-important outcomes (death, stroke, heart attack)
- Validation requirements: surrogate-clinical correlation r>0.7 typically required
- Regulatory acceptance: FDA requires clinical endpoints for major approvals
| Outcome Type | Regulatory Weight | Sample Size Impact | Timeline | Clinical Relevance |
|---|---|---|---|---|
| Mortality | Highest | Large (1000s) | 2-5 years | Maximum |
| Major Morbidity | High | Moderate (100s) | 1-3 years | High |
| Surrogate Biomarker | Moderate | Small (50-200) | 3-12 months | Variable |
| Quality of Life | Moderate | Moderate (200-500) | 6-24 months | High |
| Composite Endpoints | Variable | Moderate (300-800) | 1-3 years | Moderate |
- Outcome Measurement Standardization
- Protocol specification: detailed measurement procedures, timing, personnel
- Assessor training: inter-rater reliability >0.8 for subjective measures
- Quality assurance: 10-20% of assessments undergo independent verification
- Missing data protocols: imputation strategies for incomplete outcome data
- Composite Endpoint Considerations
- Component weighting: mortality carries more weight than hospitalization
- Clinical coherence: components should represent similar clinical importance
- Statistical efficiency: composite endpoints increase event rates by 2-4 fold
- Interpretation challenges: individual component analysis required
⭐ Clinical Pearl: Studies using composite primary endpoints show 25-35% higher success rates compared to single clinical endpoints, but regulatory agencies increasingly scrutinize whether individual components demonstrate consistent treatment benefits, particularly for mortality and major morbidity outcomes.
💡 Master This: Primary outcome selection drives every aspect of study design - sample size, duration, cost, and regulatory pathway. Choosing surrogate endpoints enables smaller, shorter studies but may not translate to clinical benefit, while clinical endpoints provide definitive evidence but require larger, longer, more expensive trials.
Strategic outcome selection establishes the foundation for meaningful clinical evidence, but sophisticated analysis approaches ensure that study results accurately reflect intervention effects while preserving statistical validity.
🎯 Outcome Architecture: The Clinical Endpoint Framework
📊 Analysis Mastery: The Statistical Truth Engine
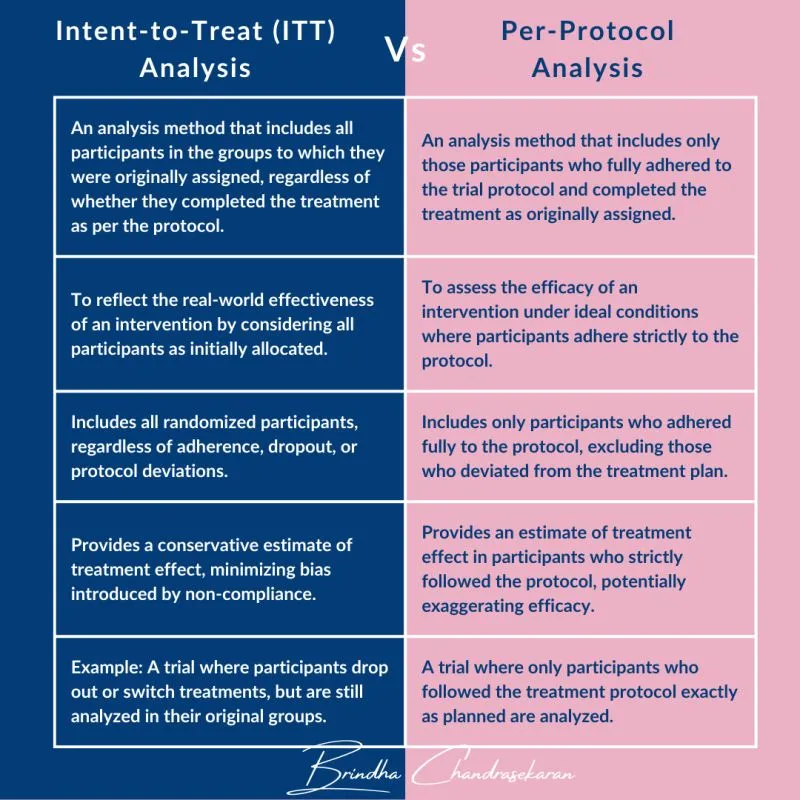
📌 Remember: ITT-PP-AS - Intention-to-treat (everyone randomized), True effectiveness, Treatment policy evaluation; Per-protocol (compliant only), Perfect adherence scenario; As-treated (actual treatment received), Safety analysis primary
- Intention-to-Treat Analysis Principles
- Include 100% of randomized participants in assigned groups
- Preserve randomization benefits: balanced confounders, unbiased comparison
- Conservative effect estimates: diluted by non-adherence, crossovers
- Regulatory preference: FDA/EMA require ITT for efficacy claims
- Per-Protocol Analysis Applications
- Exclude protocol violators: non-adherent, crossover, major deviations
- Estimate biological effect: intervention impact under ideal conditions
- Selection bias risk: exclusions may create imbalanced groups
- Supportive evidence: confirms ITT findings under optimal adherence
| Analysis Approach | Population Included | Bias Risk | Effect Size | Regulatory Use | Clinical Interpretation |
|---|---|---|---|---|---|
| Intention-to-Treat | 100% randomized | Low | Conservative | Primary | Real-world effectiveness |
| Per-Protocol | 70-90% compliant | Moderate | Liberal | Supportive | Biological efficacy |
| As-Treated | Variable | High | Variable | Safety only | Actual exposure effects |
| Modified ITT | 95-99% eligible | Low-Moderate | Intermediate | Alternative | Practical effectiveness |
- Missing Data Management Strategies
- Complete case analysis: exclude participants with missing primary outcomes
- Last observation carried forward: LOCF - outdated, potentially biased
- Multiple imputation: generate 5-10 complete datasets, pool results
- Sensitivity analysis: test robustness under different missing data assumptions
- Advanced Statistical Considerations
- Multiplicity adjustment: control family-wise error rate for multiple comparisons
- Interim analysis planning: alpha spending functions preserve overall Type I error
- Adaptive designs: modify study parameters based on accumulating data
- Bayesian approaches: incorporate prior evidence, update beliefs continuously
⭐ Clinical Pearl: When ITT and per-protocol analyses yield substantially different results (>20% relative difference), this suggests significant adherence issues that may limit real-world treatment effectiveness, requiring careful consideration of implementation strategies in clinical practice.
💡 Master This: ITT analysis answers "Does offering this treatment help patients?" while per-protocol analysis answers "Does receiving this treatment help patients?" Both questions are clinically relevant - ITT for policy decisions, per-protocol for understanding biological mechanisms and optimal adherence strategies.
Rigorous analysis approaches generate reliable evidence about intervention effects, but comprehensive quality assessment frameworks enable clinicians to evaluate study validity and applicability to their patient populations.
📊 Analysis Mastery: The Statistical Truth Engine
🏆 RCT Mastery Toolkit: The Evidence Evaluation Arsenal
📌 Remember: CONSORT-GRADE - Clear objectives, Outcome specification, Number adequate, Selection appropriate, Organization proper, Randomization valid, Treatment concealed; Grade evidence quality, Risk of bias, Applicability, Directness, Effect consistency
- Essential Quality Indicators
- Randomization method: adequate sequence generation and concealment
- Blinding implementation: participants, investigators, outcome assessors
- Sample size justification: power calculation with 80-90% power target
- Primary outcome pre-specification: single, clinically relevant endpoint
- Risk of Bias Assessment Domains
- Selection bias: randomization and allocation concealment adequacy
- Performance bias: blinding of participants and personnel
- Detection bias: blinding of outcome assessment
- Attrition bias: incomplete outcome data handling
- Reporting bias: selective outcome reporting
| Quality Domain | High Risk Indicators | Low Risk Indicators | Impact on Evidence | Assessment Priority |
|---|---|---|---|---|
| Randomization | Inadequate/unclear method | Computer-generated sequence | Major | Critical |
| Allocation Concealment | Open lists, unsealed envelopes | Central randomization | Major | Critical |
| Blinding | Open-label, obvious differences | Matched placebo, sham control | Moderate | Important |
| Incomplete Data | >20% missing, differential loss | <10% missing, balanced | Moderate | Important |
| Selective Reporting | Outcomes changed, data missing | Protocol matches publication | Major | Critical |
| %%{init: {'flowchart': {'htmlLabels': true}}}%% | ||||
| flowchart TD |
A["<b>📚 RCT Publication</b><br><span style='display:block; text-align:left; color:#555'>• Clinical trial</span><span style='display:block; text-align:left; color:#555'>• Primary source</span>"]
B["<b>📋 Quality Assessment</b><br><span style='display:block; text-align:left; color:#555'>• Critical appraisal</span><span style='display:block; text-align:left; color:#555'>• Study design</span>"]
C{"<b>🧐 Low Risk of Bias?</b><br><span style='display:block; text-align:left; color:#555'>• Systematic error</span><span style='display:block; text-align:left; color:#555'>• Study validity</span>"}
D["<b>✅ High Quality Evidence</b><br><span style='display:block; text-align:left; color:#555'>• Strong methodology</span><span style='display:block; text-align:left; color:#555'>• Robust findings</span>"]
E["<b>🏥 Clinical Applicability</b><br><span style='display:block; text-align:left; color:#555'>• Patient relevance</span><span style='display:block; text-align:left; color:#555'>• Generalizability</span>"]
F["<b>🩺 Treatment Recommendation</b><br><span style='display:block; text-align:left; color:#555'>• Guideline update</span><span style='display:block; text-align:left; color:#555'>• Clinical use</span>"]
G["<b>⚠️ Assess Bias Impact</b><br><span style='display:block; text-align:left; color:#555'>• Confounding factors</span><span style='display:block; text-align:left; color:#555'>• Error analysis</span>"]
H{"<b>🔍 Bias Direction Known?</b><br><span style='display:block; text-align:left; color:#555'>• Effect magnitude</span><span style='display:block; text-align:left; color:#555'>• Over/under estimate</span>"}
I["<b>🧮 Adjust Interpretation</b><br><span style='display:block; text-align:left; color:#555'>• Account for bias</span><span style='display:block; text-align:left; color:#555'>• Careful reading</span>"]
J["<b>⬇️ Downgrade Evidence</b><br><span style='display:block; text-align:left; color:#555'>• GRADE criteria</span><span style='display:block; text-align:left; color:#555'>• Weak evidence</span>"]
K["<b>🔭 Seek Better Evidence</b><br><span style='display:block; text-align:left; color:#555'>• Further research</span><span style='display:block; text-align:left; color:#555'>• Future trials</span>"]
A --> B
B --> C
C -->|Yes| D
D --> E
E --> F
C -->|No| G
G --> H
H -->|Yes| I
I --> F
H -->|No| J
J --> K
style A fill:#F6F5F5, stroke:#E7E6E6, stroke-width:1.5px, rx:12, ry:12, color:#525252
style B fill:#FEF8EC, stroke:#FBECCA, stroke-width:1.5px, rx:12, ry:12, color:#854D0E
style C fill:#FEF8EC, stroke:#FBECCA, stroke-width:1.5px, rx:12, ry:12, color:#854D0E
style D fill:#F7F5FD, stroke:#F0EDFA, stroke-width:1.5px, rx:12, ry:12, color:#6B21A8
style E fill:#FEF8EC, stroke:#FBECCA, stroke-width:1.5px, rx:12, ry:12, color:#854D0E
style F fill:#F1FCF5, stroke:#BEF4D8, stroke-width:1.5px, rx:12, ry:12, color:#166534
style G fill:#FEF8EC, stroke:#FBECCA, stroke-width:1.5px, rx:12, ry:12, color:#854D0E
style H fill:#FEF8EC, stroke:#FBECCA, stroke-width:1.5px, rx:12, ry:12, color:#854D0E
style I fill:#EEFAFF, stroke:#DAF3FF, stroke-width:1.5px, rx:12, ry:12, color:#0369A1
style J fill:#FDF4F3, stroke:#FCE6E4, stroke-width:1.5px, rx:12, ry:12, color:#B91C1C
style K fill:#EEFAFF, stroke:#DAF3FF, stroke-width:1.5px, rx:12, ry:12, color:#0369A1
> ⭐ **Clinical Pearl**: High-quality RCTs with low risk of bias demonstrate **15-25%** smaller treatment effects compared to studies with methodological limitations, reflecting the elimination of systematic biases that artificially inflate intervention benefits in lower-quality research.
> 💡 **Master This**: The GRADE approach provides a systematic framework for evidence evaluation, considering not only study quality but also consistency, directness, precision, and publication bias to generate overall confidence ratings that guide clinical practice recommendations and treatment guidelines.
🏆 RCT Mastery Toolkit: The Evidence Evaluation Arsenal
Practice Questions: RCTs
Test your understanding with these related questions
A 21-year-old man presents to the office for a follow-up visit. He was recently diagnosed with type 1 diabetes mellitus after being hospitalized for diabetic ketoacidosis following a respiratory infection. He is here today to discuss treatment options available for his condition. The doctor mentions a recent study in which researchers have developed a new version of the insulin pump that appears efficacious in type 1 diabetics. They are currently comparing it to insulin injection therapy. This new pump is not yet available, but it looks very promising. At what stage of clinical trials is this current treatment most likely at?













