Absolute risk vs relative measures US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Absolute risk vs relative measures. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
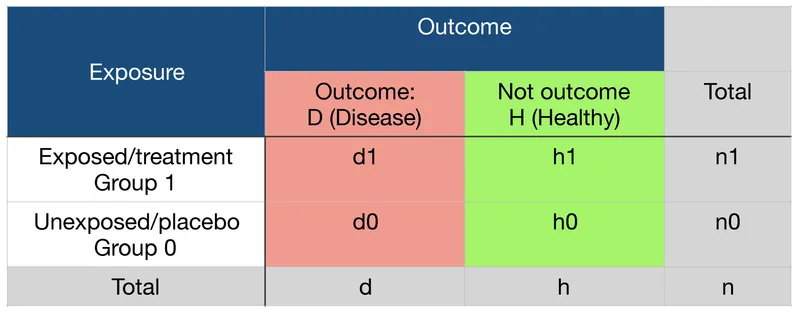
Absolute risk vs relative measures US Medical PG Question 1: You have been asked to quantify the relative risk of developing bacterial meningitis following exposure to a patient with active disease. You analyze 200 patients in total, half of which are controls. In the trial arm, 30% of exposed patients ultimately contracted bacterial meningitis. In the unexposed group, only 1% contracted the disease. Which of the following is the relative risk due to disease exposure?
- A. (30 * 99) / (70 * 1)
- B. [30 / (30 + 70)] / [1 / (1 + 99)] (Correct Answer)
- C. [70 / (30 + 70)] / [99 / (1 + 99)]
- D. [[1 / (1 + 99)] / [30 / (30 + 70)]]
- E. (70 * 1) / (30 * 99)
Absolute risk vs relative measures Explanation: ***[30 / (30 + 70)] / [1 / (1 + 99)]***
- This formula correctly calculates the **relative risk (RR)**. The numerator represents the **incidence rate in the exposed group** (30% of 100 exposed patients = 30 cases out of 100), and the denominator represents the **incidence rate in the unexposed group** (1% of 100 unexposed patients = 1 case out of 100).
- Relative risk is the ratio of the **risk of an event** in an **exposed group** to the **risk of an event** in an **unexposed group**.
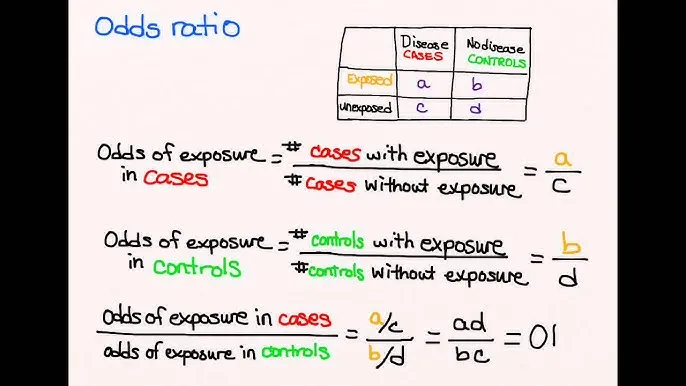
*[(30 * 99) / (70 * 1)]*
- This formula is for calculating the **odds ratio (OR)**, specifically using a 2x2 table setup where 30 represents exposed cases, 70 represents exposed non-cases, 1 represents unexposed cases, and 99 represents unexposed non-cases.
- The odds ratio is a measure of association between an exposure and an outcome, representing the **odds of an outcome** given exposure compared to the odds of the outcome without exposure.
*[70 / (30 + 70)] / [99 / (1 + 99)]*
- This formula calculates the **relative risk of *not* developing the disease**, which is the inverse of what the question asks for.
- It compares the proportion of exposed individuals who *do not* contract the disease to the proportion of unexposed individuals who *do not* contract the disease.
*[[1 / (1 + 99)] / [30 / (30 + 70)]]*
- This formula calculates the **inverse of the relative risk**, which is not what the question asks for.
- It would represent the ratio of the incidence in the unexposed group to the incidence in the exposed group.
*[(70 * 1) / (30 * 99)]*
- This is an **incorrect variation** of the odds ratio calculation, with the terms in the numerator and denominator swapped compared to the standard formula.
- Therefore, it does not represent the relative risk or a correctly calculated odds ratio.
Absolute risk vs relative measures US Medical PG Question 2: A research team develops a new monoclonal antibody checkpoint inhibitor for advanced melanoma that has shown promise in animal studies as well as high efficacy and low toxicity in early phase human clinical trials. The research team would now like to compare this drug to existing standard of care immunotherapy for advanced melanoma. The research team decides to conduct a non-randomized study where the novel drug will be offered to patients who are deemed to be at risk for toxicity with the current standard of care immunotherapy, while patients without such risk factors will receive the standard treatment. Which of the following best describes the level of evidence that this study can offer?
- A. Level 1
- B. Level 3 (Correct Answer)
- C. Level 5
- D. Level 4
- E. Level 2
Absolute risk vs relative measures Explanation: ***Level 3***
- A **non-randomized controlled trial** like the one described, where patient assignment to treatment groups is based on specific characteristics (risk of toxicity), falls into Level 3 evidence.
- This level typically includes **non-randomized controlled trials** and **well-designed cohort studies** with comparison groups, which are prone to selection bias and confounding.
- The study compares two treatments but lacks randomization, making it Level 3 evidence.
*Level 1*
- Level 1 evidence is the **highest level of evidence**, derived from **systematic reviews and meta-analyses** of multiple well-designed randomized controlled trials or large, high-quality randomized controlled trials.
- The described study is explicitly stated as non-randomized, ruling out Level 1.
*Level 2*
- Level 2 evidence involves at least one **well-designed randomized controlled trial** (RCT) or **systematic reviews** of randomized trials.
- The current study is *non-randomized*, which means it cannot be classified as Level 2 evidence, as randomization is a key criterion for this level.
*Level 4*
- Level 4 evidence includes **case series**, **case-control studies**, and **poorly designed cohort or case-control studies**.
- While the study is non-randomized, it is a controlled comparative trial rather than a case series or retrospective case-control study, placing it at Level 3.
*Level 5*
- Level 5 evidence is the **lowest level of evidence**, typically consisting of **expert opinion** without explicit critical appraisal, or based on physiology, bench research, or animal studies.
- While the drug was initially tested in animal studies, the current human comparative study offers a higher level of evidence than expert opinion or preclinical data.
Absolute risk vs relative measures US Medical PG Question 3: A researcher is trying to determine whether a newly discovered substance X can be useful in promoting wound healing after surgery. She conducts this study by enrolling the next 100 patients that will be undergoing this surgery and separating them into 2 groups. She decides which patient will be in which group by using a random number generator. Subsequently, she prepares 1 set of syringes with the novel substance X and 1 set of syringes with a saline control. Both of these sets of syringes are unlabeled and the substances inside cannot be distinguished. She gives the surgeon performing the surgery 1 of the syringes and does not inform him nor the patient which syringe was used. After the study is complete, she analyzes all the data that was collected and performs statistical analysis. This study most likely provides which level of evidence for use of substance X?
- A. Level 3
- B. Level 1 (Correct Answer)
- C. Level 4
- D. Level 5
- E. Level 2
Absolute risk vs relative measures Explanation: ***Level 1***
- The study design described is a **randomized controlled trial (RCT)**, which is considered the **highest level of evidence (Level 1)** in the hierarchy of medical evidence.
- Key features like **randomization**, **control group**, and **blinding (double-blind)** help minimize bias and strengthen the validity of the findings.
*Level 2*
- Level 2 evidence typically comprises **well-designed controlled trials without randomization** (non-randomized controlled trials) or **high-quality cohort studies**.
- While strong, they do not possess the same level of internal validity as randomized controlled trials.
*Level 3*
- Level 3 evidence typically includes **case-control studies** or **cohort studies**, which are observational designs and carry a higher risk of bias compared to RCTs.
- These studies generally do not involve randomization or intervention assignment by the researchers.
*Level 4*
- Level 4 evidence is usually derived from **case series** or **poor quality cohort and case-control studies**.
- These studies provide descriptive information or investigate associations without strong control for confounding factors.
*Level 5*
- Level 5 evidence is the **lowest level of evidence**, consisting of **expert opinion** or **animal research/bench research**.
- This level lacks human clinical data or systematic investigative rigor needed for higher evidence levels.
Absolute risk vs relative measures US Medical PG Question 4: A physician attempts to study cirrhosis in his state. Using a registry of admitted patients over the last 10 years at the local hospital, he isolates all patients who have been diagnosed with cirrhosis. Subsequently, he contacts this group of patients, asking them to complete a survey assessing their prior exposure to alcohol use, intravenous drug abuse, blood transfusions, personal history of cancer, and other medical comorbidities. An identical survey is given to an equal number of patients in the registry who do not carry a prior diagnosis of cirrhosis. Which of the following is the study design utilized by this physician?
- A. Randomized controlled trial
- B. Case-control study (Correct Answer)
- C. Cross-sectional study
- D. Cohort study
- E. Meta-analysis
Absolute risk vs relative measures Explanation: ***Case-control study***
- This study design **identifies subjects based on their outcome (cases with cirrhosis, controls without cirrhosis)** and then retrospectively investigates their past exposures.
- The physician selected patients with cirrhosis (cases) and patients without cirrhosis (controls), then assessed their prior exposures to risk factors like alcohol use and intravenous drug abuse.
*Randomized controlled trial*
- This design involves randomly assigning participants to an **intervention group** or a **control group** to assess the effect of an intervention.
- There is no intervention being tested or randomization occurring in this study; it is observational.
*Cross-sectional study*
- A cross-sectional study measures the **prevalence of disease and exposure at a single point in time** in a defined population.
- This study collects retrospective exposure data and compares two distinct groups (cases and controls), rather than assessing prevalence at one time point.
*Cohort study*
- A cohort study **follows a group of individuals over time** to see if their exposure to a risk factor is associated with the development of a disease.
- This study starts with the outcome (cirrhosis) and looks backward at exposures, which is the opposite direction of a cohort study.
*Meta-analysis*
- A meta-analysis is a statistical method that **combines the results of multiple independent studies** to produce a single, more powerful estimate of treatment effect or association.
- This is an original research study collecting new data, not a systematic review or synthesis of existing studies.
Absolute risk vs relative measures US Medical PG Question 5: You submit a paper to a prestigious journal about the effects of coffee consumption on mesothelioma risk. The first reviewer lauds your clinical and scientific acumen, but expresses concern that your study does not have adequate statistical power. Statistical power refers to which of the following?
- A. The probability of detecting an association when no association exists.
- B. The probability of not detecting an association when an association does exist.
- C. The probability of detecting an association when an association does exist. (Correct Answer)
- D. The first derivative of work.
- E. The square root of the variance.
Absolute risk vs relative measures Explanation: ***The probability of detecting an association when an association does exist.***
- **Statistical power** is defined as the probability that a study will correctly reject a false null hypothesis, meaning it will detect a true effect or association if one exists.
- A study with **adequate statistical power** is less likely to miss a real effect.
*The probability of detecting an association when no association exists.*
- This describes a **Type I error** or **false positive**, often represented by **alpha (α)**.
- It is the probability of incorrectly concluding an effect or association exists when, in reality, there is none.
*The probability of not detecting an association when an association does exist.*
- This refers to a **Type II error** or **false negative**, represented by **beta (β)**.
- **Statistical power** is calculated as **1 - β**, so this option describes the complement of power.
*The first derivative of work.*
- The first derivative of work with respect to time represents **power** in physics, which is the rate at which work is done.
- This option is a **distractor** from physics and is unrelated to statistical power in research.
*The square root of the variance.*
- The **square root of the variance** is the **standard deviation**, a measure of the dispersion or spread of data.
- This is a statistical concept but is not the definition of statistical power.
Absolute risk vs relative measures US Medical PG Question 6: A survey was conducted in a US midwestern town in an effort to assess maternal mortality over the past year. The data from the survey are given in the table below:
Women of childbearing age 250,000
Maternal deaths 2,500
Number of live births 100, 000
Number of deaths of women of childbearing age 7,500
Maternal death is defined as the death of a woman while pregnant or within 42 days of termination of pregnancy from any cause related to or aggravated by, the pregnancy. Which of the following is the maternal mortality rate in this midwestern town?
- A. 1,000 per 100,000 live births
- B. 33 per 100,000 live births
- C. 3,000 per 100,000 live births
- D. 33,300 per 100,000 live births
- E. 2,500 per 100,000 live births (Correct Answer)
Absolute risk vs relative measures Explanation: ***2,500 per 100,000 live births***
- The maternal mortality rate is calculated as the number of **maternal deaths** per 100,000 **live births**. The given data directly provide these values.
- Calculation: (2,500 maternal deaths / 100,000 live births) × 100,000 = **2,500 per 100,000 live births**.
*1,000 per 100,000 live births*
- This value is incorrect as it does not align with the provided numbers for maternal deaths and live births in the calculation.
- It might result from a miscalculation or using incorrect numerator/denominator values from the dataset.
*33 per 100,000 live births*
- This value is significantly lower than the correct rate and suggests a substantial error in calculation or an incorrect understanding of how the maternal mortality rate is derived.
- It could potentially result from dividing the number of live births by maternal deaths, which is the inverse of the correct formula.
*3,000 per 100,000 live births*
- This option is close to the correct answer but slightly higher, indicating a possible calculation error, for instance, including non-maternal deaths or other causes of deaths in the numerator.
- The definition of maternal death is specific to pregnancy-related or aggravated causes, so extraneous deaths would inflate the rate.
*33,300 per 100,000 live births*
- This figure results from incorrectly calculating the proportion of maternal deaths among all deaths of women of childbearing age: (2,500 / 7,500) × 100,000 = 33,333.
- This is a conceptual error as the maternal mortality rate should use live births as the denominator, not total deaths of women of childbearing age.
Absolute risk vs relative measures US Medical PG Question 7: A randomized controlled trial was initiated to evaluate a novel DPP-4 inhibitor for blood glucose management in diabetic patients. The study used a commonly prescribed sulfonylurea as the standard of care treatment. 2,000 patients were enrolled in the study with 1,000 patients in each arm. One of the primary outcomes was the development of diabetic nephropathy during treatment. This outcome occurred in 68 patients on the DPP-4 inhibitor and 134 patients on the sulfonylurea. What is the relative risk reduction (RRR) for patients using the DPP-4 inhibitor compared with the sulfonylurea?
- A. 23%
- B. 49% (Correct Answer)
- C. 33%
- D. 59%
- E. 43%
Absolute risk vs relative measures Explanation: ***49%***
- To calculate **relative risk reduction (RRR)**, first determine the **event rate (ER)** for each group.
- ER (DPP-4 inhibitor) = 68/1000 = 0.068. ER (Sulfonylurea) = 134/1000 = 0.134.
- Next, calculate the **absolute risk reduction (ARR)**: ARR = ER (Sulfonylurea) - ER (DPP-4 inhibitor) = 0.134 - 0.068 = 0.066.
- Finally, calculate RRR: RRR = ARR / ER (Sulfonylurea) = 0.066 / 0.134 ≈ 0.4925 or **49%**.
*23%*
- This value is incorrect and does not result from the proper application of the **relative risk reduction (RRR)** formula.
- A common mistake is to reverse the subtrahend and minuend in the numerator or denominator.
*33%*
- This value is incorrect and does not result from the proper application of the **relative risk reduction (RRR)** formula.
- Incorrect calculations in either the numerator or denominator of the **RRR formula** would lead to this incorrect result.
*59%*
- This value is incorrect and is likely the result of an error in calculating either the **absolute risk reduction (ARR)** or dividing it by the wrong **event rate**.
- Always ensure the correct event rates are used for the control group and the intervention group.
*43%*
- This value is incorrect and does not align with the correct calculation of **relative risk reduction (RRR)**.
- Errors in setting up the formula or executing the division could lead to this result.
Absolute risk vs relative measures US Medical PG Question 8: A medical research study is evaluating an investigational novel drug (medication 1) as compared with standard therapy (medication 2) in patients presenting to the emergency department with myocardial infarction (MI). The study enrolled a total of 3,000 subjects, 1,500 in each study arm. Follow-up was conducted at 45 days post-MI. The following are the results of the trial:
Endpoints Medication 1 Medication 2 P-Value
Primary: death from cardiac causes 134 210 0.03
Secondary: hyperkalemia 57 70 0.4
What is the relative risk of death from a cardiac cause, expressed as a percentage? (Round to the nearest whole number.)
- A. 64% (Correct Answer)
- B. 42%
- C. 72%
- D. 36%
- E. 57%
Absolute risk vs relative measures Explanation: ***64%***
- The **relative risk (RR)** is calculated as the event rate in the exposed group divided by the event rate in the unexposed (control) group.
- For cardiac death, the event rate for Medication 1 is 134/1500 = 0.0893, and for Medication 2 is 210/1500 = 0.14. Therefore, RR = 0.0893 / 0.14 = 0.6378.
- Expressing as a percentage: 0.6378 × 100 = 63.78%, which rounds to **64%**.
- This indicates that Medication 1 has 64% of the risk of cardiac death compared to Medication 2, representing a **36% relative risk reduction**.
*42%*
- This option is incorrect as it does not reflect the accurate calculation of **relative risk** using the provided event rates.
- A calculation error or conceptual misunderstanding of the relative risk formula would lead to this value.
*72%*
- This percentage is higher than the calculated relative risk, suggesting an incorrect application of the formula or a misinterpretation of the event rates.
- It does not represent the ratio of risk between the two medication groups for cardiac death.
*36%*
- This value represents the **relative risk reduction** (100% - 64% = 36%), not the relative risk itself.
- This is a common error where students confuse relative risk with relative risk reduction.
*57%*
- While closer to the correct answer, this value is not the precise result when rounding to the nearest whole number.
- Small calculation discrepancies or rounding at intermediate steps could lead to this slightly different percentage.
Absolute risk vs relative measures US Medical PG Question 9: A 42-year-old woman presents to the physician because of an abnormal breast biopsy report following suspicious findings on breast imaging. Other than being concerned about her report, she feels well. She has no history of any serious illnesses and takes no medications. She does not smoke. She consumes wine 1–2 times per week with dinner. There is no significant family history of breast or ovarian cancer. Vital signs are within normal limits. Physical examination shows no abnormal findings. The biopsy shows lobular carcinoma in situ (LCIS) in the left breast. Which of the following is the most appropriate next step in management?
- A. Careful observation + routine mammography (Correct Answer)
- B. Left mastectomy + axillary dissection + local irradiation
- C. Lumpectomy + routine screening
- D. Lumpectomy + breast irradiation
- E. Breast irradiation + tamoxifen
Absolute risk vs relative measures Explanation: ***Careful observation + routine mammography***
- **Lobular carcinoma in situ (LCIS)** is considered a **non-obligate precursor** to invasive carcinoma, meaning it indicates an increased risk for developing invasive breast cancer in either breast (approximately 1-2% per year), but it is not itself invasive.
- Management typically involves **careful surveillance** with routine clinical exams and **mammography**, as this is the most appropriate initial approach for classic LCIS.
- Surgical excision is often unnecessary due to LCIS's diffuse nature and the fact that it serves as a risk marker rather than a direct precancerous lesion requiring removal.
*Left mastectomy + axillary dissection + local irradiation*
- This aggressive approach is reserved for **invasive breast cancer** and would be excessive for LCIS, which is a non-invasive lesion and a marker of increased risk rather than an immediate threat.
- **Axillary dissection** is performed to stage nodal involvement in invasive cancer, which is not applicable here as LCIS does not metastasize.
*Lumpectomy + routine screening*
- While a **lumpectomy (excision)** may be considered for **pleomorphic LCIS** or when there is diagnostic uncertainty, it is not the standard initial management for classic LCIS.
- Classic LCIS is often multifocal and bilateral, making localized excision less effective as a risk-reduction strategy.
*Lumpectomy + breast irradiation*
- **Radiation therapy** is typically used to reduce local recurrence risk after **lumpectomy for invasive breast cancer** or **ductal carcinoma in situ (DCIS)**.
- For LCIS, irradiation is generally not recommended as it is non-invasive and does not benefit from local radiation treatment.
*Breast irradiation + tamoxifen*
- **Tamoxifen** is a selective estrogen receptor modulator (SERM) that can be **offered for risk reduction** in women with LCIS, potentially reducing the risk of invasive breast cancer by approximately 50%.
- However, tamoxifen is typically discussed as an **additional preventive option** after initial diagnosis and counseling, not as the immediate next step.
- **Breast irradiation** is not indicated for LCIS, as it is non-invasive and does not require local radiation treatment, making this combination inappropriate.
Absolute risk vs relative measures US Medical PG Question 10: A research fellow proposes a nested case-control study within an existing cohort examining antibiotic exposure and C. difficile infection. The mentor suggests this design wastes the cohort structure and that relative risk should be calculated instead. The fellow argues that odds ratios from nested case-control studies approximate relative risk while being more efficient. Evaluate the validity of each position and synthesize the optimal approach.
- A. Calculate RR from full cohort since all data are available and RR is more interpretable
- B. Use nested case-control only if computational resources are limited, otherwise use full cohort
- C. Use nested case-control with OR since it's mathematically equivalent to cohort RR with proper sampling (Correct Answer)
- D. Perform both analyses and compare results to validate the nested case-control approach
- E. Calculate hazard ratios using Cox regression as a compromise between efficiency and accuracy
Absolute risk vs relative measures Explanation: ***Use nested case-control with OR since it's mathematically equivalent to cohort RR with proper sampling***
- In a **nested case-control study** using **incidence density sampling** (risk-set sampling), the **Odds Ratio (OR)** provides an unbiased estimate of the **Rate Ratio** or **Relative Risk (RR)** without requiring the rare disease assumption.
- This design is highly efficient as it preserves the **temporal sequence** of the cohort while significantly reducing the need to process exposure data for the entire **at-risk population**.
*Calculate RR from full cohort since all data are available and RR is more interpretable*
- While **Relative Risk** is intuitive, calculating it requires data for the **entire denominator**, which may be prohibitively expensive or time-consuming if additional exposure processing (e.g., biomarker testing) is needed.
- The mentor's insistence ignores the **efficiency gains** of the nested design, which provides the same statistical inference with a fraction of the data processing.
*Perform both analyses and compare results to validate the nested case-control approach*
- Performing both analyses is redundant and contradicts the primary goal of using a **nested case-control** design, which is to **save resources** by not analyzing the full cohort.
- Validation is unnecessary because the **mathematical validity** of the nested case-control method is already well-established in epidemiological theory.
*Use nested case-control only if computational resources are limited, otherwise use full cohort*
- The primary limitation addressed by nested designs is usually **resource-intensive exposure assessment** (e.g., expensive lab assays) rather than mere **computational power**.
- Even if resources are available, the nested approach is often preferred in large cohorts to maintain a manageable **sub-sample** while achieving nearly identical statistical power.
*Calculate hazard ratios using Cox regression as a compromise between efficiency and accuracy*
- **Cox regression** is typically performed on the **full cohort** data, whereas the fellow is specifically proposing a sampling method to reduce the data set size.
- While a **Hazard Ratio** is a valid measure of effect, it does not solve the resource issue unless used in conjunction with a **case-cohort** or nested design, which leads back to the fellow's original point.
More Absolute risk vs relative measures US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.