Cost-effectiveness and NNT US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Cost-effectiveness and NNT. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Cost-effectiveness and NNT US Medical PG Question 1: A 25-year-old man with a genetic disorder presents for genetic counseling because he is concerned about the risk that any children he has will have the same disease as himself. Specifically, since childhood he has had difficulty breathing requiring bronchodilators, inhaled corticosteroids, and chest physiotherapy. He has also had diarrhea and malabsorption requiring enzyme replacement therapy. If his wife comes from a population where 1 in 10,000 people are affected by this same disorder, which of the following best represents the likelihood a child would be affected as well?
- A. 0.01%
- B. 2%
- C. 0.5%
- D. 1% (Correct Answer)
- E. 50%
Cost-effectiveness and NNT Explanation: ***Correct Option: 1%***
- The patient's symptoms (difficulty breathing requiring bronchodilators, inhaled corticosteroids, and chest physiotherapy; diarrhea and malabsorption requiring enzyme replacement therapy) are classic for **cystic fibrosis (CF)**, an **autosomal recessive disorder**.
- For an autosomal recessive disorder with a prevalence of 1 in 10,000 in the general population, **q² = 1/10,000**, so **q = 1/100 = 0.01**. The carrier frequency **(2pq)** is approximately **2q = 2 × (1/100) = 1/50 = 0.02**.
- The affected man is **homozygous recessive (aa)** and will always pass on the recessive allele. His wife has a **1/50 chance of being a carrier (Aa)**. If she is a carrier, she has a **1/2 chance of passing on the recessive allele**.
- Therefore, the probability of an affected child = **(Probability wife is a carrier) × (Probability wife passes recessive allele) = 1/50 × 1/2 = 1/100 = 1%**.
*Incorrect Option: 0.01%*
- This percentage is too low and does not correctly account for the carrier frequency in the population and the probability of transmission from a carrier mother.
*Incorrect Option: 2%*
- This represents approximately the carrier frequency (1/50 ≈ 2%), but does not account for the additional 1/2 probability that a carrier mother would pass on the recessive allele.
*Incorrect Option: 0.5%*
- This value would be correct if the carrier frequency were 1/100 instead of 1/50, which does not match the given population prevalence.
*Incorrect Option: 50%*
- **50%** would be the risk if both parents were carriers of an autosomal recessive disorder (1/4 chance = 25% for affected, but if we know one parent passes the allele, conditional probability changes). More accurately, 50% would apply if the disorder were **autosomal dominant** with one affected parent, which is not the case here.
Cost-effectiveness and NNT US Medical PG Question 2: A 68-year-old man is being evaluated in your radiation oncology clinic for treatment of a solid tumor. Your hospital has just purchased a new proton beam purported to deliver targeted radiation with fewer side effects than traditional radiation therapy. The patient expresses strong interest in receiving proton beam therapy, and you feel that he may have a better outcome with this new treatment modality. Later that day, an executive from the patient's insurance company calls to tell you that proton beam therapy will cost the company (but not the patient) a much larger amount of money than traditional therapy. They are willing to pay for proton beam therapy, but request that you convince the patient to undergo traditional therapy instead. You have a longstanding relationship with this insurance company as well as this particular executive. How should you proceed?
- A. Tell the patient that proton beam therapy will not be covered by his insurance company, so you will need to proceed with traditional radiation therapy
- B. Discuss the issue of cost to the insurer with your patient, relaying the company's request to him without making further commentary or recommendation
- C. Call your hospital's ethics committee for a formal consultation
- D. Proceed with proton beam therapy as discussed at your patient's appointment (Correct Answer)
- E. Discuss the issue of cost to the insurer with your patient, pointing out that keeping his insurance company happy may make them more likely to cover additional treatments in the future
Cost-effectiveness and NNT Explanation: ***Proceed with proton beam therapy as discussed at your patient's appointment***
- The physician's primary **fiduciary duty** is to the patient's best interest, not the insurance company's financial concerns or their own relationship with the company.
- The patient has expressed interest, and the physician believes proton beam therapy offers a **better outcome with fewer side effects**, which constitutes optimal medical care in this scenario.
*Tell the patient that proton beam therapy will not be covered by his insurance company, so you will need to proceed with traditional radiation therapy*
- This is a deceptive act, as the insurance company has stated they **are willing to pay** for proton beam therapy.
- Misleading the patient about coverage status to benefit an insurance company is a breach of **medical ethics** and the physician's duty to the patient.
*Discuss the issue of cost to the insurer with your patient, relaying the company's request to him without making further commentary or recommendation*
- While seemingly transparent, introducing the insurance company's financial request to the patient can create **undue pressure** and influence their medical decisions based on external factors rather than their health needs.
- This can undermine the **trust** in the physician-patient relationship by involving the patient in the financial negotiations of third parties.
*Call your hospital's ethics committee for a formal consultation*
- While seeking ethical advice is generally good practice, the ethical obligation to prioritize the patient's best interest is **clear and immediate** in this situation.
- Delaying treatment or involving a committee for a scenario where the physician already believes a specific treatment is superior and available could unnecessarily **complicate the process** for the patient.
*Discuss the issue of cost to the insurer with your patient, pointing out that keeping his insurance company happy may make them more likely to cover additional treatments in the future*
- This suggestion subtly pressures the patient to choose a less optimal treatment based on future hypothetical benefits to the insurance company, which is a clear **conflict of interest**.
- It prioritizes the financial interests of the insurer and the physician's relationship with them over the patient's immediate medical needs and reinforces the concept of **undue influence**.
Cost-effectiveness and NNT US Medical PG Question 3: A new treatment for hemorrhagic stroke, which is a life-threatening clinical condition that occurs when a diseased blood vessel in the brain ruptures or leaks, was evaluated as soon as it hit the market by an international group of neurology specialists. In those treated with the new drug, a good outcome was achieved in 30%, while those treated with the current standard of care had a good outcome in just 10% of cases. The clinicians involved in this cohort study concluded that the newer drug is more effective and prompted for urgent changes in the guidelines addressing hemorrhagic stroke incidents. According to the aforementioned percentages, how many patients must be treated with the new drug to see 1 additional good outcome?
- A. 5 (Correct Answer)
- B. 30
- C. 20
- D. 15
- E. 10
Cost-effectiveness and NNT Explanation: ***Correct: 5***
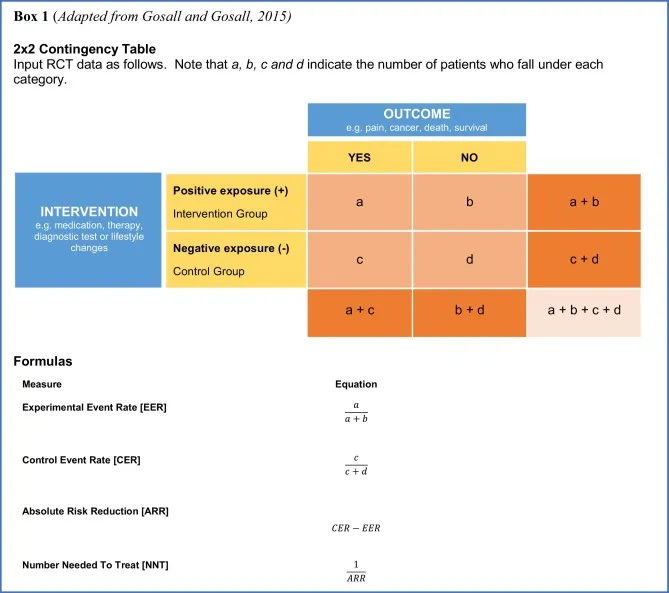
- This is calculated using the concept of **Number Needed to Treat (NNT)**, which tells us how many patients need to receive the new treatment to see one additional good outcome compared to standard care.
- The **Absolute Risk Reduction (ARR)** is the difference in good outcome rates: 30% - 10% = 20% (or 0.20 as a proportion).
- **NNT = 1 / ARR = 1 / 0.20 = 5**
- Therefore, treating 5 patients with the new drug will result in 1 additional patient with a good outcome compared to standard care.
*Incorrect: 30*
- This represents the **percentage of patients** who achieved a good outcome with the new drug, not the number needed to treat.
- It does not account for the baseline effectiveness of standard treatment, which is essential for calculating the marginal benefit.
- This is the absolute event rate in the treatment group, not a comparative measure.
*Incorrect: 20*
- This is the **Absolute Risk Reduction (ARR)** expressed as a percentage (30% - 10% = 20%).
- While this is a key component in calculating NNT, it is not the NNT itself.
- The NNT requires taking the reciprocal of the ARR when expressed as a proportion: 1/0.20 = 5.
*Incorrect: 15*
- This number does not correspond to any standard epidemiological or biostatistical measure in this context.
- It is neither the ARR, NNT, relative risk, nor any other interpretable value from the given data.
- This is an arbitrary distractor with no mathematical basis.
*Incorrect: 10*
- This represents the **percentage of patients** who achieved a good outcome with standard care (the control group).
- It is the baseline event rate, not a measure of treatment effect or comparative effectiveness.
- Like option 30, it does not reflect the additional benefit from the new treatment.
Cost-effectiveness and NNT US Medical PG Question 4: You are currently employed as a clinical researcher working on clinical trials of a new drug to be used for the treatment of Parkinson's disease. Currently, you have already determined the safe clinical dose of the drug in a healthy patient. You are in the phase of drug development where the drug is studied in patients with the target disease to determine its efficacy. Which of the following phases is this new drug currently in?
- A. Phase 4
- B. Phase 1
- C. Phase 2 (Correct Answer)
- D. Phase 0
- E. Phase 3
Cost-effectiveness and NNT Explanation: ***Phase 2***
- **Phase 2 trials** involve studying the drug in patients with the target disease to assess its **efficacy** and further evaluate safety, typically involving a few hundred patients.
- The question describes a stage after safe dosing in healthy patients (Phase 1) and before large-scale efficacy confirmation (Phase 3), focusing on efficacy in the target population.
*Phase 4*
- **Phase 4 trials** occur **after a drug has been approved** and marketed, monitoring long-term effects, optimal use, and rare side effects in a diverse patient population.
- This phase is conducted post-market approval, whereas the question describes a drug still in development prior to approval.
*Phase 1*
- **Phase 1 trials** primarily focus on determining the **safety and dosage** of a new drug in a **small group of healthy volunteers** (or sometimes patients with advanced disease if the drug is highly toxic).
- The question states that the safe clinical dose in a healthy patient has already been determined, indicating that Phase 1 has been completed.
*Phase 0*
- **Phase 0 trials** are exploratory, very early-stage studies designed to confirm that the drug reaches the target and acts as intended, typically involving a very small number of doses and participants.
- These trials are conducted much earlier in the development process, preceding the determination of safe clinical doses and large-scale efficacy studies.
*Phase 3*
- **Phase 3 trials** are large-scale studies involving hundreds to thousands of patients to confirm **efficacy**, monitor side effects, compare it to commonly used treatments, and collect information that will allow the drug to be used safely.
- While Phase 3 does assess efficacy, it follows Phase 2 and is typically conducted on a much larger scale before submitting for regulatory approval.
Cost-effectiveness and NNT US Medical PG Question 5: In a randomized controlled trial studying a new treatment, the primary endpoint (mortality) occurred in 14.4% of the treatment group and 16.7% of the control group. Which of the following represents the number of patients needed to treat to save one life, based on the primary endpoint?
- A. 1/(0.144 - 0.167)
- B. 1/(0.167 - 0.144) (Correct Answer)
- C. 1/(0.300 - 0.267)
- D. 1/(0.267 - 0.300)
- E. 1/(0.136 - 0.118)
Cost-effectiveness and NNT Explanation: ***1/(0.167 - 0.144)***
- The **Number Needed to Treat (NNT)** is calculated as **1 / Absolute Risk Reduction (ARR)**.
- The **Absolute Risk Reduction (ARR)** is the difference between the event rate in the control group (16.7%) and the event rate in the treatment group (14.4%), which is **0.167 - 0.144**.
*1/(0.144 - 0.167)*
- This calculation represents 1 divided by the **Absolute Risk Increase**, which would be relevant if the treatment increased mortality.
- The **NNT should always be a positive value**, indicating the number of patients to treat to prevent one adverse event.
*1/(0.300 - 0.267)*
- This option uses arbitrary numbers (0.300 and 0.267) that do not correspond to the given **mortality rates** in the problem.
- It does not reflect the correct calculation for **absolute risk reduction** based on the provided data.
*1/(0.267 - 0.300)*
- This option also uses arbitrary numbers not derived from the problem's data, and it would result in a **negative value** for the denominator.
- The difference between event rates of 0.267 and 0.300 is not present in the given information for this study.
*1/(0.136 - 0.118)*
- This calculation uses arbitrary numbers (0.136 and 0.118) that are not consistent with the reported **mortality rates** of 14.4% and 16.7%.
- These values do not represent the **Absolute Risk Reduction** required for calculating NNT in this specific scenario.
Cost-effectiveness and NNT US Medical PG Question 6: A 28-year-old male presents to his primary care physician with complaints of intermittent abdominal pain and alternating bouts of constipation and diarrhea. His medical chart is not significant for any past medical problems or prior surgeries. He is not prescribed any current medications. Which of the following questions would be the most useful next question in eliciting further history from this patient?
- A. "Does the diarrhea typically precede the constipation, or vice-versa?"
- B. "Is the diarrhea foul-smelling?"
- C. "Please rate your abdominal pain on a scale of 1-10, with 10 being the worst pain of your life"
- D. "Are the symptoms worse in the morning or at night?"
- E. "Can you tell me more about the symptoms you have been experiencing?" (Correct Answer)
Cost-effectiveness and NNT Explanation: ***Can you tell me more about the symptoms you have been experiencing?***
- This **open-ended question** encourages the patient to provide a **comprehensive narrative** of their symptoms, including details about onset, frequency, duration, alleviating/aggravating factors, and associated symptoms, which is crucial for diagnosis.
- In a patient presenting with vague, intermittent symptoms like alternating constipation and diarrhea, allowing them to elaborate freely can reveal important clues that might not be captured by more targeted questions.
*Does the diarrhea typically precede the constipation, or vice-versa?*
- While knowing the sequence of symptoms can be helpful in understanding the **pattern of bowel dysfunction**, it is a very specific question that might overlook other important aspects of the patient's experience.
- It prematurely narrows the focus without first obtaining a broad understanding of the patient's overall symptomatic picture.
*Is the diarrhea foul-smelling?*
- Foul-smelling diarrhea can indicate **malabsorption** or **bacterial overgrowth**, which are important to consider in some gastrointestinal conditions.
- However, this is a **specific symptom inquiry** that should follow a more general exploration of the patient's symptoms, as it may not be relevant if other crucial details are missed.
*Please rate your abdominal pain on a scale of 1-10, with 10 being the worst pain of your life*
- Quantifying pain intensity is useful for assessing the **severity of discomfort** and monitoring changes over time.
- However, for a patient with intermittent rather than acute, severe pain, understanding the **character, location, and triggers** of the pain is often more diagnostically valuable than just a numerical rating initially.
*Are the symptoms worse in the morning or at night?*
- Diurnal variation can be relevant in certain conditions, such as inflammatory bowel diseases where nocturnal symptoms might be more concerning, or functional disorders whose symptoms might be stress-related.
- This is another **specific question** that should come after gathering a more complete initial picture of the patient's symptoms to ensure no key information is overlooked.
Cost-effectiveness and NNT US Medical PG Question 7: A research team is working on a new assay meant to increase the sensitivity of testing in cervical cancer. Current sensitivity is listed at 77%. If this research team's latest work culminates in the following results (listed in the table), has the sensitivity improved, and, if so, then by what percentage?
Research team's latest results:
| | Patients with cervical cancer | Patients without cervical cancer |
|--------------------------|-------------------------------|----------------------------------|
| Test is Positive (+) | 47 | 4 |
| Test is Negative (-) | 9 | 44 |
- A. No, the research team has seen a decrease in sensitivity according to the new results listed.
- B. No, the research team has not seen any improvement in sensitivity according to the new results listed.
- C. Yes, the research team has seen an improvement in sensitivity of almost 7% according to the new results listed. (Correct Answer)
- D. Yes, the research team has seen an improvement in sensitivity of more than 10% according to the new results listed.
- E. Yes, the research team has seen an improvement in sensitivity of less than 2% according to new results listed; this improvement is negligible and should be improved upon for significant contribution to the field.
Cost-effectiveness and NNT Explanation: ***Yes, the research team has seen an improvement in sensitivity of almost 7% according to the new results listed.***
- **Sensitivity** is calculated as **True Positives / (True Positives + False Negatives)**. From the table: True Positives = 47, False Negatives = 9.
- New sensitivity = 47 / (47 + 9) = 47 / 56 $\approx$ **83.9%**. Compared to the current sensitivity of 77%, this is an improvement of 83.9% - 77% = **6.9%**, which is almost 7%.
*No, the research team has not seen any improvement in sensitivity according to the new results listed.*
- The new sensitivity calculated is **83.9%**, which is indeed higher than the current sensitivity of **77%**.
- This option incorrectly states there is no improvement, as a clear increase of nearly 7% is observed.
*No, the research team has seen a decrease in sensitivity according to the new results listed.*
- The calculated new sensitivity of **83.9%** is higher than the original 77%, indicating an **increase**, not a decrease.
- This statement is factually incorrect based on the provided data.
*Yes, the research team has seen an improvement in sensitivity of more than 10% according to the new results listed.*
- The improvement is approximately **6.9%** (83.9% - 77%), which is less than 10%.
- This option overstates the degree of improvement observed.
*Yes, the research team has seen an improvement in sensitivity of less than 2% according to new results listed; this improvement is negligible and should be improved upon for significant contribution to the field.*
- The calculated improvement is approximately **6.9%**, not less than 2%.
- While clinical significance can be debated, the mathematical calculation of improvement is not accurately reflected by "less than 2%".
Cost-effectiveness and NNT US Medical PG Question 8: A pharmaceutical company reports a new antihypertensive drug reduces cardiovascular events with an NNT of 50 over 5 years based on a trial of 10,000 patients. An independent analysis reveals the benefit was driven entirely by a subgroup with resistant hypertension (20% of participants, NNT=15), while the remaining 80% showed no benefit over standard therapy (NNT approaching infinity). Evaluate the ethical and regulatory implications of reporting the overall NNT.
- A. Conduct a new trial in the general hypertensive population to validate efficacy before broader approval
- B. The subgroup analysis represents data dredging; only the overall NNT should be used for clinical decisions
- C. The overall NNT of 50 is statistically valid and appropriate for regulatory approval and marketing
- D. Report both overall and subgroup NNTs; allow clinicians to determine appropriate use based on patient characteristics
- E. The overall NNT is misleading; approval should be restricted to resistant hypertension population where benefit is demonstrated (Correct Answer)
Cost-effectiveness and NNT Explanation: ***The overall NNT is misleading; approval should be restricted to resistant hypertension population where benefit is demonstrated***
- Reporting an **aggregate NNT** when the clinical benefit is confined to a specific **subgroup** obscures the fact that the drug is ineffective for 80% of the study population.
- Regulatory and ethical standards dictate that **indication for use** must be limited to populations where a **favorable benefit-risk ratio** has been proven, preventing unnecessary exposure to side effects in non-responders.
*The overall NNT of 50 is statistically valid and appropriate for regulatory approval and marketing*
- While the math is accurate for the trial population as a whole, it ignores **heterogeneity of treatment effect**, which is critical for making safe **clinical recommendations**.
- Marketing a drug based on an **averaged NNT** when the majority of patients derive zero benefit is considered **clinically misleading** and ethically questionable.
*Report both overall and subgroup NNTs; allow clinicians to determine appropriate use based on patient characteristics*
- This approach puts the burden of identifying the correct population on the clinician rather than setting **clear regulatory boundaries** through specific labelling.
- Merely reporting the **overall NNT** may lead to **off-label use** in populations where the NNT is effectively **infinity**, representing a failure in evidence-based guidance.
*Conduct a new trial in the general hypertensive population to validate efficacy before broader approval*
- The existing data already demonstrates that the **general population** (the 80% non-resistant group) showed no benefit over standard therapy.
- Conducting a new trial for the general population would be **unethical and redundant**, as the lack of efficacy in that specific group has already been established by the **independent analysis**.
*The subgroup analysis represents data dredging; only the overall NNT should be used for clinical decisions*
- **Data dredging** refers to finding random patterns; however, identifying a lack of benefit in 80% of a population is a critical **safety and efficacy finding** that cannot be ignored.
- Dismissing the **subgroup effect** would result in potentially treating millions of patients with an **ineffective medication**, violating the principle of **non-maleficence**.
Cost-effectiveness and NNT US Medical PG Question 9: A public health agency must allocate a fixed budget between two interventions for diabetes prevention. Program A (intensive lifestyle modification): NNT=7, cost $3,500/person. Program B (metformin): NNT=14, cost $1,000/person. Both prevent one case of diabetes over 3 years. The budget allows treating 1,000 people with Program A or 3,500 people with Program B. Evaluate the optimal allocation strategy to maximize population health impact.
- A. Choose based on cost per case prevented: Program A ($24,500) vs Program B ($14,000), favoring Program B (Correct Answer)
- B. Choose Program B exclusively: higher population reach (3,500 vs 1,000) and lower cost per person treated maximizes prevention (250 cases) despite higher NNT
- C. Allocate budget equally between programs: provides both high-efficacy and broad-reach approaches
- D. Choose Program A for high-risk individuals and Program B for moderate-risk: risk-stratified approach optimizes NNT
- E. Choose Program A exclusively: lower NNT means superior efficacy, preventing 143 cases versus 250 with Program B
Cost-effectiveness and NNT Explanation: ***Choose based on cost per case prevented: Program A ($24,500) vs Program B ($14,000), favoring Program B***
- To maximize **population health impact** under a fixed budget, one must calculate the **cost per case prevented**, which is found by multiplying the **NNT** by the **cost per person** ($1,000 x 14 = $14,000 for Program B).
- Program B allows for a much higher total number of cases prevented (**250 cases**) compared to Program A (**142 cases**) because the **lower unit cost** outweighs the higher NNT.
*Choose Program A exclusively: lower NNT means superior efficacy, preventing 143 cases versus 250 with Program B*
- While Program A has a **lower NNT**, indicating it is more effective for an individual, it is significantly less **cost-effective** for a population due to its high cost.
- Exclusive use of Program A would result in fewer total cases prevented (142) compared to the 250 cases prevented by Program B, wasting **allocated resources**.
*Choose Program B exclusively: higher population reach (3,500 vs 1,000) and lower cost per person treated maximizes prevention (250 cases) despite higher NNT*
- This option correctly identifies the outcome but lacks the precise **economic justification** (cost per outcome) required for optimal health allocation decisions.
- Public health decisions are fundamentally based on **incremental cost-effectiveness ratios** or cost per case prevented rather than reach alone.
*Allocate budget equally between programs: provides both high-efficacy and broad-reach approaches*
- Managing a fixed budget by splitting it equally results in **196 total cases prevented**, which is mathematically inferior to the 250 cases prevented by prioritizing the more cost-efficient program.
- This approach fails to address the **opportunity cost** of not spending the entire budget on the more efficient intervention.
*Choose Program A for high-risk individuals and Program B for moderate-risk: risk-stratified approach optimizes NNT*
- While **risk stratification** is clinically sound, the prompt asks to maximize impact based on the provided fixed costs and NNTs for the general intervention group.
- Adding complexity to the delivery model can further increase **administrative costs**, which are not accounted for in this basic **cost-effectiveness analysis**.
Cost-effectiveness and NNT US Medical PG Question 10: A 45-year-old woman with a strong family history of breast cancer (lifetime risk 25%) is considering chemoprevention with tamoxifen. A trial shows tamoxifen reduces breast cancer incidence from 5% to 3% over 5 years in high-risk women, but increases endometrial cancer from 0.2% to 0.6% and thromboembolic events from 0.5% to 1.5%. Evaluate whether she should be recommended this therapy based on comprehensive risk-benefit analysis.
- A. Recommend alternative screening strategies as chemoprevention shows no net benefit when all outcomes are considered
- B. Recommend tamoxifen only if patient values breast cancer prevention significantly more than other risks, given similar absolute risk magnitudes (Correct Answer)
- C. Recommend tamoxifen: NNT=50 for breast cancer prevention outweighs combined NNH=250 for endometrial cancer and NNH=100 for thromboembolism
- D. Insufficient data to make recommendation without knowing patient's personal values and quality-of-life preferences
- E. Recommend against tamoxifen: The harms (2 complications per 100 treated) outweigh benefits (2 cancers prevented per 100 treated)
Cost-effectiveness and NNT Explanation: ***Recommend tamoxifen only if patient values breast cancer prevention significantly more than other risks, given similar absolute risk magnitudes***
- The **Absolute Risk Reduction (ARR)** for breast cancer is 2% (5% minus 3%), while the cumulative **Absolute Risk Increase (ARI)** for major harms is 1.4% (0.4% for endometrial cancer and 1.0% for thromboembolism).
- Because the magnitude of benefit (2 preventable cancers) is narrowly balanced against the magnitude of harm (1.4 serious complications), the decision relies on **patient preferences** and how they weigh the severity of different health outcomes.
*Recommend tamoxifen: NNT=50 for breast cancer prevention outweighs combined NNH=250 for endometrial cancer and NNH=100 for thromboembolism*
- While the **Number Needed to Treat (NNT)** is indeed 50 (1/0.02), the combined **Number Needed to Harm (NNH)** for any serious complication is approximately 71 (1/0.014), not 250 or 100 individually.
- This option oversimplifies the trade-off by suggesting a clear-cut advantage that does not exist when both serious adverse events are aggregated.
*Recommend against tamoxifen: The harms (2 complications per 100 treated) outweigh benefits (2 cancers prevented per 100 treated)*
- The calculation of harms is slightly inaccurate as the **ARI** is 1.4 per 100, which is numerically lower than the benefit of 2 cancers prevented per 100.
- A blanket recommendation against therapy ignores that a **2% ARR** in breast cancer may be clinically significant for a high-risk patient willing to accept the side-effect profile.
*Insufficient data to make recommendation without knowing patient's personal values and quality-of-life preferences*
- While patient values are crucial, the **clinical data** provided is sufficient to form a recommendation framework based on the **risk-benefit ratio**.
- The objective is to evaluate the therapy within the context of **evidence-based medicine**, which allows for a conditional recommendation rather than a claim of "insufficient data."
*Recommend alternative screening strategies as chemoprevention shows no net benefit when all outcomes are considered*
- This is incorrect because **chemoprevention** is a distinct primary prevention strategy that can be used in conjunction with, not just as a replacement for, high-risk screening.
- The data shows a **net numerical benefit** (2.0% reduction vs 1.4% increase), meaning it cannot be claimed there is "no net benefit" across all outcomes.
More Cost-effectiveness and NNT US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.