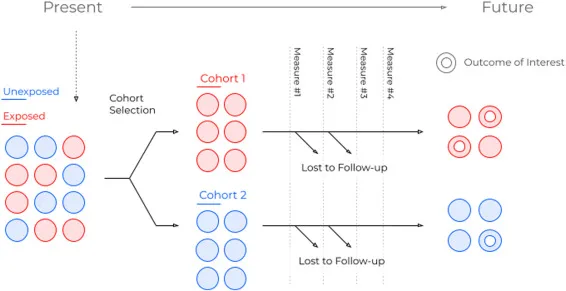
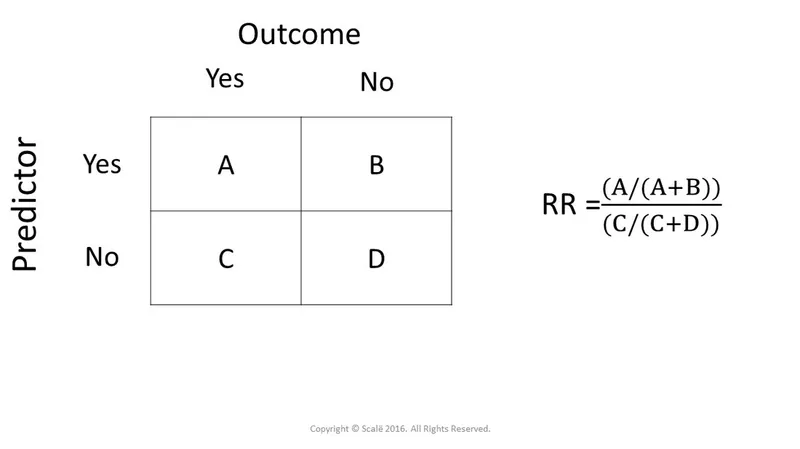
Prospective cohort design US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Prospective cohort design. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Prospective cohort design US Medical PG Question 1: A study is funded by the tobacco industry to examine the association between smoking and lung cancer. They design a study with a prospective cohort of 1,000 smokers between the ages of 20-30. The length of the study is five years. After the study period ends, they conclude that there is no relationship between smoking and lung cancer. Which of the following study features is the most likely reason for the failure of the study to note an association between tobacco use and cancer?
- A. Late-look bias
- B. Latency period (Correct Answer)
- C. Confounding
- D. Effect modification
- E. Pygmalion effect
Prospective cohort design Explanation: ***Latency period***
- **Lung cancer** typically has a **long latency period**, often **20-30+ years**, between initial exposure to tobacco carcinogens and the development of clinically detectable disease.
- A **five-year study duration** in young smokers (ages 20-30) is **far too short** to observe the development of lung cancer, which explains the false negative finding.
- This represents a **fundamental flaw in study design** rather than a bias—the biological timeline of disease development was not adequately considered.
*Late-look bias*
- **Late-look bias** occurs when a study enrolls participants who have already survived the early high-risk period of a disease, leading to **underestimation of true mortality or incidence**.
- Also called **survival bias**, it involves studying a population that has already been "selected" by survival.
- This is not applicable here, as the study simply ended before sufficient time elapsed for disease to develop.
*Confounding*
- **Confounding** occurs when a third variable is associated with both the exposure and outcome, distorting the apparent relationship between them.
- While confounding can affect study results, it would not completely eliminate the detection of a strong, well-established association like smoking and lung cancer in a properly conducted prospective cohort study.
- The issue here is temporal (insufficient follow-up time), not the presence of an unmeasured confounder.
*Effect modification*
- **Effect modification** (also called interaction) occurs when the magnitude of an association between exposure and outcome differs across levels of a third variable.
- This represents a **true biological phenomenon**, not a study design flaw or bias.
- It would not explain the complete failure to detect any association.
*Pygmalion effect*
- The **Pygmalion effect** (observer-expectancy effect) refers to a psychological phenomenon where higher expectations lead to improved performance in the observed subjects.
- This concept is relevant to **behavioral and educational research**, not to objective epidemiological studies of disease incidence.
- It has no relevance to the biological relationship between carcinogen exposure and cancer development.
Prospective cohort design US Medical PG Question 2: Researchers are studying the effects of a new medication for the treatment of type 2 diabetes. A randomized group of 100 subjects is given the new medication 1st for 2 months, followed by a washout period of 2 weeks, and then administration of the gold standard medication for 2 months. Another randomized group of 100 subjects is given the gold standard medication 1st for 2 months, followed by a washout period of 2 weeks, and then administration of the new medication for 2 months. What is the main disadvantage of this study design?
- A. Hawthorne effect
- B. Increasing selection bias
- C. Increasing confounding bias
- D. Decreasing power
- E. Carryover effect (Correct Answer)
Prospective cohort design Explanation: ***Carryover effect***
- The primary disadvantage here is the **carryover effect**, where the effects of the first treatment (new medication or gold standard) may persist into the period when the second treatment is administered, even after a washout period.
- This can **mask or alter the true effect** of the second treatment, making it difficult to accurately assess their individual efficacy.
*Hawthorne effect*
- The **Hawthorne effect** refers to subjects improving their behavior or performance in response to being observed or studied, not specifically an issue with sequential treatment administration.
- It would affect both groups equally and doesn't explain a disadvantage inherent to the crossover design itself.
*Increasing selection bias*
- **Selection bias** occurs when the randomization process fails to create comparable groups, but this study design involves **randomization** into two groups, and then a crossover, which typically aims to *reduce* selection bias by having each participant serve as their own control.
- The sequential administration within a randomized crossover design actually helps to mitigate selection bias between treatment arms.
*Increasing confounding bias*
- **Confounding bias** occurs when an unmeasured variable is associated with both the exposure and the outcome, distorting the observed relationship.
- This crossover design, where each participant receives both treatments, is intended to *reduce* confounding by inter-individual variability, as each subject acts as their own control, rather than increasing it.
*Decreasing power*
- **Power** is the ability of a study to detect a true effect if one exists. Crossover designs often *increase* statistical power compared to parallel designs because each participant receives both treatments, reducing inter-individual variability.
- This design typically requires a smaller sample size to achieve the same power as a parallel group study, so decreased power is not a disadvantage.
Prospective cohort design US Medical PG Question 3: A 25-year-old man with a genetic disorder presents for genetic counseling because he is concerned about the risk that any children he has will have the same disease as himself. Specifically, since childhood he has had difficulty breathing requiring bronchodilators, inhaled corticosteroids, and chest physiotherapy. He has also had diarrhea and malabsorption requiring enzyme replacement therapy. If his wife comes from a population where 1 in 10,000 people are affected by this same disorder, which of the following best represents the likelihood a child would be affected as well?
- A. 0.01%
- B. 2%
- C. 0.5%
- D. 1% (Correct Answer)
- E. 50%
Prospective cohort design Explanation: ***Correct Option: 1%***
- The patient's symptoms (difficulty breathing requiring bronchodilators, inhaled corticosteroids, and chest physiotherapy; diarrhea and malabsorption requiring enzyme replacement therapy) are classic for **cystic fibrosis (CF)**, an **autosomal recessive disorder**.
- For an autosomal recessive disorder with a prevalence of 1 in 10,000 in the general population, **q² = 1/10,000**, so **q = 1/100 = 0.01**. The carrier frequency **(2pq)** is approximately **2q = 2 × (1/100) = 1/50 = 0.02**.
- The affected man is **homozygous recessive (aa)** and will always pass on the recessive allele. His wife has a **1/50 chance of being a carrier (Aa)**. If she is a carrier, she has a **1/2 chance of passing on the recessive allele**.
- Therefore, the probability of an affected child = **(Probability wife is a carrier) × (Probability wife passes recessive allele) = 1/50 × 1/2 = 1/100 = 1%**.
*Incorrect Option: 0.01%*
- This percentage is too low and does not correctly account for the carrier frequency in the population and the probability of transmission from a carrier mother.
*Incorrect Option: 2%*
- This represents approximately the carrier frequency (1/50 ≈ 2%), but does not account for the additional 1/2 probability that a carrier mother would pass on the recessive allele.
*Incorrect Option: 0.5%*
- This value would be correct if the carrier frequency were 1/100 instead of 1/50, which does not match the given population prevalence.
*Incorrect Option: 50%*
- **50%** would be the risk if both parents were carriers of an autosomal recessive disorder (1/4 chance = 25% for affected, but if we know one parent passes the allele, conditional probability changes). More accurately, 50% would apply if the disorder were **autosomal dominant** with one affected parent, which is not the case here.
Prospective cohort design US Medical PG Question 4: A 45-year-old man comes to the clinic concerned about his recent exposure to radon. He heard from his co-worker that radon exposure can cause lung cancer. He brings in a study concerning the risks of radon exposure. In the study, there were 300 patients exposed to radon, and 18 developed lung cancer over a 10-year period. To compare, there were 500 patients without radon exposure and 11 developed lung cancer over the same 10-year period. If we know that 0.05% of the population has been exposed to radon, what is the attributable risk percent for developing lung cancer over a 10 year period after radon exposure?
- A. 3.8%
- B. 0.31%
- C. 2.2%
- D. 6.0%
- E. 63.3% (Correct Answer)
Prospective cohort design Explanation: ***63.3%***
- The **attributable risk percent (ARP)** quantifies the proportion of disease in the exposed group that is attributable to the exposure. It is calculated as [(Incidence in exposed - Incidence in unexposed) / Incidence in exposed] * 100.
- In this case, **Incidence in exposed (radon)** = 18/300 = 0.06 or 6%. **Incidence in unexposed** = 11/500 = 0.022 or 2.2%. Therefore, ARP = [(0.06 - 0.022) / 0.06] * 100 = (0.038 / 0.06) * 100 = **63.3%**.
*3.8%*
- This value represents the difference in the **absolute risk** or incidence between the exposed and unexposed groups (6% - 2.2% = 3.8%).
- It does not represent the proportion of disease in the exposed group that is due to the exposure.
*0.31%*
- This value is not derived from the given data using standard epidemiological formulas for attributable risk percent.
- It is possibly a miscalculation or an irrelevant measure in this context.
*2.2%*
- This value represents the **incidence of lung cancer in the unexposed group** (11/500 = 0.022 or 2.2%).
- It is a component of the ARP calculation but not the ARP itself.
*6.0%*
- This value represents the **incidence of lung cancer in the radon-exposed group** (18/300 = 0.06 or 6%).
- It is used in the numerator and denominator for calculating the attributable risk percent but is not the final ARP.
Prospective cohort design US Medical PG Question 5: Which of the following study designs would be most appropriate to investigate the association between electronic cigarette use and the subsequent development of lung cancer?
- A. Subjects with lung cancer who smoke and subjects with lung cancer who did not smoke
- B. Subjects who smoke electronic cigarettes and subjects who smoke normal cigarettes
- C. Subjects with lung cancer who smoke and subjects without lung cancer who smoke
- D. Subjects with lung cancer and subjects without lung cancer
- E. Subjects who smoke electronic cigarettes and subjects who do not smoke (Correct Answer)
Prospective cohort design Explanation: ***Subjects who smoke electronic cigarettes and subjects who do not smoke***
- This design represents a **cohort study**, which is ideal for investigating the **incidence** of a disease (lung cancer) in groups exposed and unexposed to a risk factor (electronic cigarette use).
- By following these two groups over time, researchers can directly compare the **risk of developing lung cancer** in e-cigarette users versus non-smokers.
*Subjects with lung cancer who smoke and subjects with lung cancer who did not smoke*
- This option incorrectly compares two groups both with lung cancer, where the exposure to smoking can either be **electronic or traditional cigarettes,** but does not provide a control group without lung cancer to assess the association.
- This design would not allow for the calculation of an **incidence rate** or a **relative risk** of lung cancer development specific to electronic cigarette use.
*Subjects who smoke electronic cigarettes and subjects who smoke normal cigarettes*
- This design compares two different types of smoking, which might be useful for comparing their relative risks but doesn't include a **non-smoking control group** to establish the absolute association with electronic cigarettes.
- While it could show if e-cigarettes are "safer" than traditional cigarettes, it wouldn't directly answer whether e-cigarettes themselves **cause lung cancer**.
*Subjects with lung cancer who smoke and subjects without lung cancer who smoke*
- This describes a **case-control study** but focuses on smoking in general rather than specifically electronic cigarettes, which is the independent variable of interest.
- While valuable for identifying risk factors, it would need to specifically differentiate between **electronic cigarette smokers** and other smokers to answer the question adequately.
*Subjects with lung cancer and subjects without lung cancer*
- This general description of a **case-control study** is too broad; it does not specify the exposure of interest, which is electronic cigarette use.
- To be relevant, the study would need to gather data on **electronic cigarette use** in both the lung cancer group and the non-lung cancer control group.
Prospective cohort design US Medical PG Question 6: A research group wants to assess the safety and toxicity profile of a new drug. A clinical trial is conducted with 20 volunteers to estimate the maximum tolerated dose and monitor the apparent toxicity of the drug. The study design is best described as which of the following phases of a clinical trial?
- A. Phase 0
- B. Phase III
- C. Phase V
- D. Phase II
- E. Phase I (Correct Answer)
Prospective cohort design Explanation: ***Phase I***
- **Phase I clinical trials** involve a small group of healthy volunteers (typically 20-100) to primarily assess **drug safety**, determine a safe dosage range, and identify side effects.
- The main goal is to establish the **maximum tolerated dose (MTD)** and evaluate the drug's pharmacokinetic and pharmacodynamic profiles.
*Phase 0*
- **Phase 0 trials** are exploratory studies conducted in a very small number of subjects (10-15) to gather preliminary data on a drug's **pharmacodynamics and pharmacokinetics** in humans.
- They involve microdoses, not intended to have therapeutic effects, and thus cannot determine toxicity or MTD.
*Phase III*
- **Phase III trials** are large-scale studies involving hundreds to thousands of patients to confirm the drug's **efficacy**, monitor side effects, compare it to standard treatments, and collect information that will allow the drug to be used safely.
- These trials are conducted after safety and initial efficacy have been established in earlier phases.
*Phase V*
- "Phase V" is not a standard, recognized phase in the traditional clinical trial classification (Phase 0, I, II, III, IV).
- This term might be used in some non-standard research contexts or for post-marketing studies that go beyond Phase IV surveillance, but it is not a formal phase for initial drug development.
*Phase II*
- **Phase II trials** involve several hundred patients with the condition the drug is intended to treat, focusing on **drug efficacy** and further evaluating safety.
- While safety is still monitored, the primary objective shifts to determining if the drug works for its intended purpose and at what dose.
Prospective cohort design US Medical PG Question 7: A study is conducted to find an association between serum cholesterol and ischemic heart disease. Data is collected, and patients are classified into either the "high cholesterol" or "normal cholesterol" group and also into groups whether or not the patient experiences stable angina. Which type of data analysis is most appropriate for this study?
- A. Attributable risk
- B. Analysis of variance
- C. Chi-squared (Correct Answer)
- D. T-test
- E. Pearson correlation
Prospective cohort design Explanation: ***Chi-squared***
- The **chi-squared test** is ideal for analyzing two **categorical variables**, such as cholesterol levels (high/normal) and the presence of stable angina (yes/no), to see if there's an association between them.
- It assesses whether the observed frequencies in each category differ significantly from the expected frequencies, under the assumption of no association.
*Attributable risk*
- **Attributable risk** quantifies the proportion of disease in an exposed group that is directly due to the exposure.
- While it might be calculated *after* establishing an association (e.g., using a chi-squared test), it's a measure of actual impact rather than a method for *finding the association* between two categorical variables.
*Analysis of variance*
- **Analysis of variance (ANOVA)** is used to compare the means of **three or more groups** for a continuous outcome variable.
- It works when you have a categorical independent variable with multiple levels and a continuous dependent variable, which is not the case here as both variables are categorical.
*T-test*
- A **t-test** is used to compare the means of **two groups** for a continuous outcome variable.
- It is not appropriate for analyzing the association between two categorical variables like cholesterol categories and angina presence.
*Pearson correlation*
- **Pearson correlation** measures the linear relationship between **two continuous variables**.
- It is unsuitable for this study as both cholesterol status and angina presence are categorical variables, not continuous.
Prospective cohort design US Medical PG Question 8: In recent years, psoriasis has been identified as a risk factor for cardiovascular disease. A researcher conducted a study in which he identified 200 patients with psoriasis and 200 patients without psoriasis. The patients were followed for 10 years. At the end of this period, participants' charts were reviewed for myocardial infarction during this time interval.
Myocardial infarction No myocardial infarction Total
Psoriasis 12 188 200
No psoriasis 4 196 200
Total 16 384 400
What is the 10-year risk of myocardial infarction in participants with psoriasis?
- A. 0.75
- B. 0.04
- C. 0.5
- D. 0.06 (Correct Answer)
- E. 0.02
Prospective cohort design Explanation: ***0.06***
- The **risk of myocardial infarction** in participants with psoriasis is calculated by dividing the number of psoriasis patients who had a myocardial infarction by the total number of psoriasis patients.
- This calculation is 12 (myocardial infarctions in psoriasis group) / 200 (total psoriasis patients) = **0.06 or 6%**.
- This represents the **cumulative incidence** or **absolute risk** in the exposed cohort over 10 years.
*0.75*
- This value represents the **proportion of all MI cases that occurred in the psoriasis group**: 12/16 = 0.75.
- This is not the same as risk, which requires the denominator to be the total at-risk population (all psoriasis patients), not just those with the outcome.
*0.04*
- This value represents the **risk of myocardial infarction in the control group** (no psoriasis): 4/200 = 0.02, not 0.04.
- However, 0.04 could represent 2 × 0.02, which has no meaningful epidemiological interpretation for this study.
*0.5*
- This value does not correspond to any standard epidemiological measure from the given data.
- It might represent a miscalculation or confusion with other statistical concepts.
*0.02*
- This value represents the **risk of myocardial infarction in the unexposed group** (no psoriasis): 4/200 = 0.02 or 2%.
- The question specifically asks for the risk in the psoriasis group, not the control group.
Prospective cohort design US Medical PG Question 9: A population is studied for risk factors associated with testicular cancer. Alcohol exposure, smoking, dietary factors, social support, and environmental exposure are all assessed. The researchers are interested in the incidence and prevalence of the disease in addition to other outcomes. Which pair of studies would best assess the 1. incidence and 2. prevalence?
- A. 1. Prospective cohort study 2. Cross sectional study (Correct Answer)
- B. 1. Prospective cohort study 2. Retrospective cohort study
- C. 1. Cross sectional study 2. Retrospective cohort study
- D. 1. Case-control study 2. Prospective cohort study
- E. 1. Clinical trial 2. Cross sectional study
Prospective cohort design Explanation: ***1. Prospective cohort study 2. Cross sectional study***
- A **prospective cohort study** is ideal for measuring **incidence** (new cases over time) because it follows a group of individuals forward in time to observe who develops the disease.
- A **cross-sectional study** is suitable for measuring **prevalence** (existing cases at a specific point in time) as it surveys a population at one moment to determine the proportion with the disease.
*1. Prospective cohort study 2. Retrospective cohort study*
- A **retrospective cohort study** assesses past exposures and outcomes and can measure incidence, but it is not the primary choice for prevalence.
- While a prospective cohort study is appropriate for incidence, a retrospective cohort study is less suited for determining current prevalence.
*1. Cross sectional study 2. Retrospective cohort study*
- A **cross-sectional study** measures prevalence, not incidence, as it captures disease status at a single point in time.
- A **retrospective cohort study** looks back in time to identify past exposures and subsequent outcomes, which is not the best method for current prevalence.
*1. Case-control study 2. Prospective cohort study*
- A **case-control study** compares exposures between individuals with a disease (cases) and those without (controls) and is best for studying rare diseases and estimating odds ratios, not incidence or prevalence directly.
- A **prospective cohort study** is suitable for incidence, but a case-control study is not for incidence or prevalence.
*1. Clinical trial 2. Cross sectional study*
- A **clinical trial** is an experimental study designed to test the efficacy of interventions and is not primarily used to measure disease incidence or prevalence in a general population.
- While a cross-sectional study is appropriate for prevalence, a clinical trial is not designed for incidence measurement.
Prospective cohort design US Medical PG Question 10: Many large clinics have noticed that the prevalence of primary biliary cholangitis (PBC) has increased significantly over the past 20 years. An epidemiologist is working to identify possible reasons for this. After analyzing a series of nationwide health surveillance databases, the epidemiologist finds that the incidence of PBC has remained stable over the past 20 years. Which of the following is the most plausible explanation for the increased prevalence of PBC?
- A. Improved quality of care for PBC (Correct Answer)
- B. Increased availability of diagnostic testing for PBC
- C. Increased exposure to environmental risk factors for PBC
- D. Increased awareness of PBC among clinicians
- E. Increased average age of the population at risk for PBC
Prospective cohort design Explanation: ***Improved quality of care for PBC***
- This leads to a **longer survival time** for patients with PBC. When incidence remains stable but patients live longer, the cumulative number of living cases (prevalence) naturally increases.
- An increase in prevalence with stable incidence is a classic indicator of **improved patient survival** due to better management or treatment.
*Increased availability of diagnostic testing for PBC*
- This would primarily impact the **incidence** of PBC by detecting more cases that were previously undiagnosed. The question states that the incidence has remained stable.
- While improved diagnostics might initially increase *reported* incidence, if the true incidence is stable, it wouldn't explain a sustained rise in prevalence without a corresponding change in incidence or survival.
*Increased exposure to environmental risk factors for PBC*
- This would directly lead to an **increase in the incidence** of PBC, as more people would be developing the disease.
- Since the incidence is stable, an increase in environmental risk factors is not the most plausible explanation for increased prevalence.
*Increased awareness of PBC among clinicians*
- Similar to increased diagnostic testing, increased awareness would likely lead to the diagnosis of more new cases, thus **increasing the incidence** of PBC.
- A stable incidence despite increased awareness means that the actual rate of new cases developing the disease has not changed, ruling this out as the primary cause of increased prevalence.
*Increased average age of the population at risk for PBC*
- An aging population could potentially increase the incidence of age-related diseases. However, if the **incidence has remained stable**, it implies that even with an older population, the rate of new diagnoses has not increased.
- While age is a risk factor for PBC, an increase in prevalence without a change in incidence suggests a factor influencing the duration of the disease rather than its onset.
More Prospective cohort design US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.