Historical cohorts US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Historical cohorts. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Historical cohorts US Medical PG Question 1: A case-control study with a focus on risk factors that may influence the development of depression was conducted among the elderly population in one tertiary hospital in Malaysia. The study involved 150 elderly patients diagnosed with depressive illness from the psychiatry ward, as well as another group of 150 elderly patients without any history of depressive illness (but hospitalized for other reasons) at the same ward. The data were collected through questionnaires, and 2 principal investigators (who were also the patients’ attending physicians) acted as interviewers after proper training for the purposes of this study. Multivariate analyses of logistic regression with independent variables were employed to determine the adjusted odds ratio for the risk of developing depression. The study results showed that a lower level of social support, lack of education, and the presence of chronic illnesses highly correlated with depression. In order to maximally avoid bias that may stem from this kind of study design, what should the researchers have done differently to increase the validity of their results?
- A. Used open-ended questions
- B. Blinded the investigators (Correct Answer)
- C. Included more interviewers
- D. Used closed testing procedures on the data
- E. Used Bonferroni correction on data
Historical cohorts Explanation: ***Blinded the investigators***
- Blinding the investigators (interviewers) would prevent them from knowing which patients were cases (depressed) and which were controls (non-depressed). This reduces the risk of **interviewer bias**, where their preconceptions or knowledge of participants' status might influence how they ask questions or interpret responses, thereby distorting the results.
- Given that the principal investigators were also the patients' attending physicians, they likely had prior knowledge of the patients' depressive status, which could lead to **detection bias** or information bias. Blinding would help standardize data collection.
*Used open-ended questions*
- While open-ended questions can provide rich qualitative data, they can introduce **variability and subjectivity** in responses and interpretation, potentially making comparisons more challenging and increasing the investigator's influence on data collection.
- For a case-control study focused on quantifiable risk factors, **structured questionnaires** are often preferred for consistency and easier statistical analysis, although a mix can be optimal.
*Included more interviewers*
- Simply including more interviewers does not inherently improve validity; it could even increase **inter-rater variability** if they are not adequately trained and standardized.
- The critical aspect is the **standardization of data collection** and the avoidance of bias, not merely the number of individuals collecting data.
*Used closed testing procedures on the data*
- "Closed testing procedures on the data" is not a standard term in research methodology in this context. Assuming it refers to using a **pre-defined set of statistical tests**, this does not directly address potential biases in data collection or patient selection.
- The issue here is related to **information bias** and **selection bias** stemming from the study design and interviewer role, not primarily the statistical analysis procedures.
*Used Bonferroni correction on data*
- **Bonferroni correction** is used to adjust the p-values when performing multiple statistical comparisons on the same data set to reduce the chance of making a **Type I error** (false positive).
- This correction addresses issues in **statistical analysis** (minimizing spurious findings due to multiple testing), not biases that arise during the design, data collection, or participant identification phases of a study.
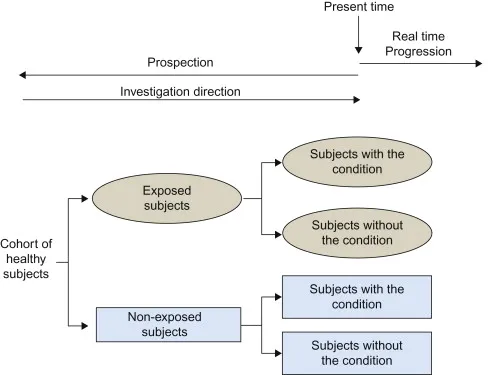
Historical cohorts US Medical PG Question 2: A recent study attempted to analyze whether increased "patient satisfaction" driven healthcare resulted in increased hospitalization. Using this patient population, the sociodemographics, health status, and hospital use were assessed. Next year, patient satisfaction with health care providers was assessed using 5 items from the Consumer Assessment of Health Plans Survey. Which of the following best describes this study design?
- A. Retrospective case-control
- B. Cross-sectional study
- C. Prospective case-control
- D. Retrospective cohort
- E. Prospective cohort (Correct Answer)
Historical cohorts Explanation: ***Prospective cohort***
- This study collects baseline data (sociodemographics, health status, hospital use) on a patient population and then follows them forward in time to assess patient satisfaction the following year. This forward-looking approach with follow-up over time defines a **prospective cohort study**.
- The study establishes a cohort at baseline, measures initial characteristics and hospital use, then prospectively assesses patient satisfaction and subsequent healthcare utilization, allowing analysis of associations between satisfaction and hospitalization patterns.
*Retrospective case-control*
- A **case-control study** identifies individuals with an outcome (cases) and without the outcome (controls) and then looks backward in time to determine past exposures.
- This study does not select participants based on outcome status; instead, it defines a cohort and follows them forward, which is characteristic of cohort design, not case-control.
*Cross-sectional study*
- A **cross-sectional study** measures both exposure and outcome at a single point in time, providing a snapshot of the population.
- This study involves follow-up over time, as patient satisfaction is assessed "next year" after baseline data collection, making it longitudinal rather than cross-sectional.
*Prospective case-control*
- **Case-control studies** inherently select participants based on their outcome status (cases vs. controls), whether prospective or retrospective.
- This study starts with a defined patient population before outcomes occur and follows them forward without outcome-based selection, which is characteristic of a cohort study, not a case-control design.
*Retrospective cohort*
- A **retrospective cohort study** uses existing data to define a cohort and then looks back in time to identify exposures and outcomes that have already occurred.
- This study involves collecting new data prospectively and following participants forward ("next year"), rather than analyzing past records, making it prospective rather than retrospective.
Historical cohorts US Medical PG Question 3: A 15-year-old female presents to her family physician for an annual school physical exam and check-up. She is accompanied by her mother to the visit and is present in the exam room. The patient has no complaints, and she does not have any past medical problems. She takes no medications. The patient reports that she remains active, exercising 5 times a week, and eats a healthy and varied diet. Which of the following would be the best way for the physician to obtain a more in-depth social history, including sexual history and use of alcohol, tobacco, or recreational drugs?
- A. Disallow the mother to be present in the examination room throughout the entirety of the visit
- B. Give the patient a social history questionnaire to fill out in the exam room
- C. Ask the mother to step outside into the hall for a portion of the visit (Correct Answer)
- D. Ask the patient the questions directly, with her mother still in the exam room
- E. Speak softly to the patient so that the mother does not hear and the patient is not embarrassed
Historical cohorts Explanation: ***Ask the mother to step outside into the hall for a portion of the visit***
- This approach allows the physician to speak with the adolescent **privately and confidentially**, which is crucial for obtaining sensitive information such as sexual history, drug use, and mental health concerns.
- Adolescents are more likely to disclose personal information when their parents are not present, fostering trust and ensuring **comprehensive history-taking** vital for their well-being.
*Disallow the mother to be present in the examination room throughout the entirety of the visit*
- This is an **overly restrictive** approach that might create tension or distrust between the physician, patient, and parent, especially at the start of the visit.
- While privacy is essential for sensitive topics, parental presence can be valuable for discussing general health, family history, and **treatment plans**, especially for younger adolescents.
*Give the patient a social history questionnaire to fill out in the exam room*
- While questionnaires can be useful for gathering basic information, they often **lack the nuance** of a direct conversation and may not prompt the patient to elaborate on sensitive issues.
- Furthermore, having the mother present while the patient fills out a questionnaire on sensitive topics still **compromises confidentiality** and may lead to incomplete or dishonest answers.
*Ask the patient the questions directly, with her mother still in the exam room*
- Asking sensitive questions with a parent present is **unlikely to yield truthful and complete answers**, as adolescents may feel embarrassed, judged, or fear parental disapproval.
- This approach compromises the **confidentiality** that is fundamental to building trust with adolescent patients.
*Speak softly to the patient so that the mother does not hear and the patient is not embarrassed*
- Speaking softly is **unprofessional** and still does not guarantee privacy, as the mother might still overhear parts of the conversation.
- This method also **fails to establish true confidentiality**, which is central to building rapport and encouraging open communication with adolescent patients about sensitive topics.
Historical cohorts US Medical PG Question 4: A resident in the department of obstetrics and gynecology is reading about a randomized clinical trial from the late 1990s that was conducted to compare breast cancer mortality risk, disease localization, and tumor size in women who were randomized to groups receiving either annual mammograms starting at age 40 or annual mammograms starting at age 50. One of the tables in the study compares the two experimental groups with regard to socioeconomic demographics (e.g., age, income), medical conditions at the time of recruitment, and family history of breast cancer. The purpose of this table is most likely to evaluate which of the following?
- A. Observer bias
- B. Statistical power
- C. Confounding
- D. Randomization (Correct Answer)
- E. Effect modification
Historical cohorts Explanation: ***Randomization***
- In a randomized clinical trial, the purpose of comparing baseline characteristics between experimental groups is to assess if **randomization successfully distributed potential confounders** evenly.
- An even distribution of baseline characteristics suggests that any observed differences in outcomes are more likely due to the intervention rather than **pre-existing differences** between the groups.
*Observer bias*
- **Observer bias** occurs when researchers' expectations influence their observations or interpretation of results, which is not evaluated by comparing baseline demographics.
- This type of bias is typically mitigated through **blinding** of researchers or participants, rather than checking baseline characteristics.
*Statistical power*
- **Statistical power** refers to the probability of correctly rejecting a false null hypothesis and detecting a true effect.
- It is determined by factors like sample size and effect size, not by the **balance of baseline characteristics** between groups.
*Effect modification*
- **Effect modification** occurs when the effect of an exposure on an outcome varies across different levels of a third variable.
- This is an **analytical consideration** explored in later stages of data analysis, not a concern addressed by comparing baseline characteristics.
*Confounding*
- **Confounding** occurs when an extraneous variable is associated with both the exposure and the outcome, distorting the true relationship.
- While the baseline table helps verify that potential confounders are evenly distributed, the primary purpose is to evaluate whether **randomization was successful**, not to directly assess confounding as an analysis concern.
Historical cohorts US Medical PG Question 5: You are currently employed as a clinical researcher working on clinical trials of a new drug to be used for the treatment of Parkinson's disease. Currently, you have already determined the safe clinical dose of the drug in a healthy patient. You are in the phase of drug development where the drug is studied in patients with the target disease to determine its efficacy. Which of the following phases is this new drug currently in?
- A. Phase 4
- B. Phase 1
- C. Phase 2 (Correct Answer)
- D. Phase 0
- E. Phase 3
Historical cohorts Explanation: ***Phase 2***
- **Phase 2 trials** involve studying the drug in patients with the target disease to assess its **efficacy** and further evaluate safety, typically involving a few hundred patients.
- The question describes a stage after safe dosing in healthy patients (Phase 1) and before large-scale efficacy confirmation (Phase 3), focusing on efficacy in the target population.
*Phase 4*
- **Phase 4 trials** occur **after a drug has been approved** and marketed, monitoring long-term effects, optimal use, and rare side effects in a diverse patient population.
- This phase is conducted post-market approval, whereas the question describes a drug still in development prior to approval.
*Phase 1*
- **Phase 1 trials** primarily focus on determining the **safety and dosage** of a new drug in a **small group of healthy volunteers** (or sometimes patients with advanced disease if the drug is highly toxic).
- The question states that the safe clinical dose in a healthy patient has already been determined, indicating that Phase 1 has been completed.
*Phase 0*
- **Phase 0 trials** are exploratory, very early-stage studies designed to confirm that the drug reaches the target and acts as intended, typically involving a very small number of doses and participants.
- These trials are conducted much earlier in the development process, preceding the determination of safe clinical doses and large-scale efficacy studies.
*Phase 3*
- **Phase 3 trials** are large-scale studies involving hundreds to thousands of patients to confirm **efficacy**, monitor side effects, compare it to commonly used treatments, and collect information that will allow the drug to be used safely.
- While Phase 3 does assess efficacy, it follows Phase 2 and is typically conducted on a much larger scale before submitting for regulatory approval.
Historical cohorts US Medical PG Question 6: You are interested in studying the etiology of heart failure reduced ejection fraction (HFrEF) and attempt to construct an appropriate design study. Specifically, you wish to look for potential causality between dietary glucose consumption and HFrEF. Which of the following study designs would allow you to assess for and determine this causality?
- A. Cross-sectional study
- B. Case series
- C. Cohort study (Correct Answer)
- D. Case-control study
- E. Randomized controlled trial
Historical cohorts Explanation: ***Cohort study***
- A **cohort study** observes a group of individuals over time to identify risk factors and outcomes, allowing for the assessment of **temporal relationships** between exposure (dietary glucose) and outcome (HFrEF).
- This design is suitable for establishing a potential **causal link** as it tracks participants from exposure to outcome, enabling the calculation of incidence rates and relative risks.
*Cross-sectional study*
- A **cross-sectional study** measures exposure and outcome simultaneously at a single point in time, making it impossible to determine the **temporal sequence** of events.
- This design can only identify **associations** or correlations, not causation, as it cannot establish whether high glucose consumption preceded HFrEF.
*Case series*
- A **case series** describes characteristics of a group of patients with a particular disease or exposure, often to highlight unusual clinical features, but it lacks a **comparison group**.
- It cannot assess causality because it does not provide information on the frequency of exposure in healthy individuals or the incidence of the disease in unexposed individuals.
*Case-control study*
- A **case-control study** compares individuals with the outcome (cases) to those without the outcome (controls) to determine past exposures, which makes it prone to **recall bias**.
- While it can suggest associations, it cannot definitively establish a temporal relationship or causation as the outcome is already known when exposure is assessed.
*Randomized controlled trial*
- A **randomized controlled trial (RCT)** is the gold standard for establishing causation by randomly assigning participants to an intervention or control group, but it may not be ethical or feasible for studying long-term dietary exposures and chronic diseases like HFrEF due to the long follow-up period and complexity of diet.
- While ideal for causality, directly controlling and randomizing dietary glucose intake over decades to observe HFrEF development might be practically challenging or unethical.
Historical cohorts US Medical PG Question 7: A researcher wants to determine whether there is an association between CRP values and the risk of MI or cancer. Four relative risk (RR) values were plotted $(0.5,1.5,1.7,1.8)$ with respect to CRP levels. What conclusion can be drawn?
- A. CRP has no relationship
- B. CRP decreases & disease decreases
- C. CRP increases disease/cancer risk (Correct Answer)
- D. No association in first interval
- E. CRP shows protective effect in first interval
Historical cohorts Explanation: ***CRP increases disease/cancer risk***
- A **relative risk (RR)** greater than 1 indicates an increased risk of the outcome (MI or cancer) in the exposed group (higher CRP levels) compared to the unexposed group.
- The plots show RRs of 1.5, 1.7, and 1.8, all of which are greater than 1, consistently indicating that higher CRP levels are associated with an elevated risk for MI or cancer.
- The overall trend across the four intervals demonstrates a positive association between CRP and disease risk.
*CRP has no relationship*
- This conclusion is incorrect because three of the four plotted RR values (1.5, 1.7, 1.8) are above 1, indicating a positive association or increased risk.
- An RR of 1 signifies no relationship, but the majority of values clearly deviate from 1, showing a definite association.
*CRP decreases & disease decreases*
- While one RR value (0.5) suggests a decreased risk, the majority of the given RRs (1.5, 1.7, 1.8) are greater than 1, indicating an increased risk.
- This option would only be true if all or most RR values were less than 1, implying a protective effect, which is not the overall trend here.
*No association in first interval*
- The first interval shows an RR of 0.5. An RR of 1 indicates no association, while an RR of 0.5 actually indicates a **decreased risk or protective effect**, rather than no association.
- Therefore, stating "no association" for the first interval is inaccurate given the definition of relative risk.
*CRP shows protective effect in first interval*
- While the first interval RR of 0.5 does suggest a protective effect in isolation, this option fails to capture the **overall conclusion** from all four data points.
- When interpreting multiple RR values together, the predominant pattern (three values >1) indicates an overall increased risk, making this a misleading conclusion for the study as a whole.
Historical cohorts US Medical PG Question 8: A researcher wants to study the carcinogenic effects of a food additive. From the literature, he finds that 7 different types of cancers have been linked to the consumption of this food additive. He wants to study all 7 possible outcomes. He conducts interviews with people who consume food containing these additives and people who do not. He then follows both groups for several years to see if they develop any of these 7 cancers or any other health outcomes. Which of the following study models best represents this study?
- A. Cohort study (Correct Answer)
- B. Case-control study
- C. Cross-sectional study
- D. Randomized clinical trial
- E. Crossover study
Historical cohorts Explanation: ***Cohort study***
- This study design involves selecting a group based on their **exposure status** (consumers vs. non-consumers of the food additive) and **following them forward in time** to observe the incidence of outcomes (cancers).
- It is ideal for studying **multiple potential outcomes** from a single exposure and for establishing the **temporal relationship** between exposure and disease.
*Case-control study*
- This design starts by identifying individuals with a particular **outcome (cases)** and comparing them to individuals without the outcome (controls) to look back for **past exposures**.
- It is efficient for studying **rare diseases** or when multiple exposures are suspected for a single outcome, which is inverse to the scenario described.
*Cross-sectional study*
- This study measures both **exposure and outcome simultaneously** at a single point in time, providing a snapshot of prevalence.
- It cannot establish a **temporal relationship** between exposure and outcome and is less suitable for studying incident diseases that develop over time.
*Randomized clinical trial*
- This design involves **randomly assigning participants** to an intervention group or a control group and following them for outcomes.
- It is primarily used to evaluate the **efficacy of interventions** or treatments, not to study the carcinogenic effects of naturally occurring exposures.
*Crossover study*
- In a crossover design, participants **receive all interventions** in a specific sequence, making each subject serve as their own control.
- This design is generally used for evaluating **short-term effects of treatments** in chronic, stable conditions and is unsuitable for observing the development of diseases like cancer over extended periods.
Historical cohorts US Medical PG Question 9: A 52-year-old man comes to the physician for a follow-up examination 1 year after an uncomplicated liver transplantation. He feels well but wants to know how long he can expect his donor graft to function. The physician informs him that the odds of graft survival are 90% at 1 year, 78% at 5 years, and 64% at 10 years. At this time, given that the graft has already survived 1 year, the probability of the patient's graft surviving to 10 years after transplantation is closest to which of the following?
- A. 82%
- B. 58%
- C. 71% (Correct Answer)
- D. 64%
- E. 45%
Historical cohorts Explanation: ***71%***
- This question tests understanding of **conditional probability** in survival analysis.
- The patient is currently at 1 year post-transplant with a functioning graft. We need to calculate the probability of surviving to 10 years **given survival to 1 year**.
- Using the conditional probability formula: P(survive to 10 years | survived to 1 year) = P(S10) / P(S1) = 64% / 90% = 0.711 ≈ **71%**
- This represents the probability that a graft that has already "made it" through the first year will continue functioning until year 10.
- In **Kaplan-Meier survival analysis**, conditional probabilities are crucial for counseling patients at different timepoints post-procedure.
*64%*
- This represents the **absolute probability** of 10-year graft survival measured from the time of transplantation (time zero).
- However, the question asks "at this time" (1 year post-transplant) for the conditional probability, not the absolute probability from transplantation.
- This would be correct if asking a patient at time zero what their 10-year survival odds are.
*82%*
- This does not represent any valid calculation from the given survival data.
- It may result from incorrect manipulation of the probabilities (e.g., incorrectly adding or averaging values).
*58%*
- This is not derived from proper statistical calculation of the given survival probabilities.
- It does not represent either absolute or conditional probability for any relevant timepoint.
*45%*
- This is incorrect and does not correspond to any valid calculation.
- It might arise from incorrectly multiplying probabilities (e.g., 0.90 × 0.50) but has no basis in survival analysis.
Historical cohorts US Medical PG Question 10: The study is performed to examine the association between type 2 diabetes mellitus (DM2) and Alzheimer's disease (AD). Group of 250 subjects diagnosed with DM2 and a matched group of 250 subjects without DM2 are enrolled. Each subject is monitored regularly over their lifetime for the development of symptoms of dementia or mild cognitive impairment. If symptoms are present, an autopsy is performed after the patient's death to confirm the diagnosis of AD. Which of the following is most correct regarding this study?
- A. It is a retrospective observational study.
- B. Relative risk cannot be determined from this study.
- C. It is a prospective observational study. (Correct Answer)
- D. It can provide proof of causation between DM2 and AD.
- E. It is a case-control study.
Historical cohorts Explanation: ***It is a prospective observational study.***
- The study enrolls subjects and then follows them forward in time ("**monitored regularly over their lifetime**") to observe the development of an outcome (AD), which defines a **prospective study**.
- Since the researchers are observing and not actively intervening (e.g., administering a treatment), it is an **observational study**.
*It is a retrospective observational study.*
- A **retrospective study** looks back in time to examine outcomes that have already occurred, which is contrary to the description of following subjects over their lifetime.
- In a retrospective study, data on exposures and outcomes are collected from past records or participant recall.
*Relative risk cannot be determined from this study.*
- This study design, a **prospective cohort study**, allows for the calculation of **relative risk** because it follows groups defined by their exposure (DM2 vs. no DM2) to determine the incidence of the outcome (AD) in each group.
- Relative risk compares the incidence rate of an outcome in an exposed group to the incidence rate in an unexposed group.
*It can provide proof of causation between DM2 and AD.*
- **Observational studies** like this can identify **associations** and suggest potential causal links, but they generally cannot **prove causation** due to the possibility of confounding variables.
- While it can strengthen the hypothesis of a causal link, randomized controlled trials are often considered the gold standard for establishing causation.
*It is a case-control study.*
- A **case-control study** begins by identifying individuals with an outcome (cases, e.g., AD patients) and comparing them to individuals without the outcome (controls) to determine past exposures, which is different from following exposed and unexposed groups forward.
- This study design defines groups based on their exposure (DM2 status) at the beginning, not based on the presence or absence of the outcome.
More Historical cohorts US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.