Post-translational modifications US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Post-translational modifications. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Post-translational modifications US Medical PG Question 1: An 18-month-old girl is brought to the pediatrician’s office for failure to thrive and developmental delay. The patient’s mother says she has not started speaking and is just now starting to pull herself up to standing position. Furthermore, her movement appears to be restricted. Physical examination reveals coarse facial features and restricted joint mobility. Laboratory studies show increased plasma levels of several enzymes. Which of the following is the underlying biochemical defect in this patient?
- A. Congenital lack of lysosomal formation
- B. Inappropriate protein targeting to endoplasmic reticulum
- C. Failure of mannose phosphorylation (Correct Answer)
- D. Inappropriate degradation of lysosomal enzymes
- E. Misfolding of nuclear proteins
Post-translational modifications Explanation: ***Failure of mannose phosphorylation***
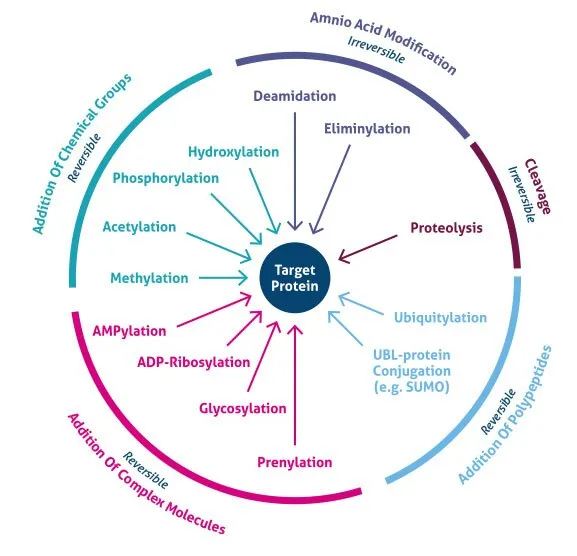
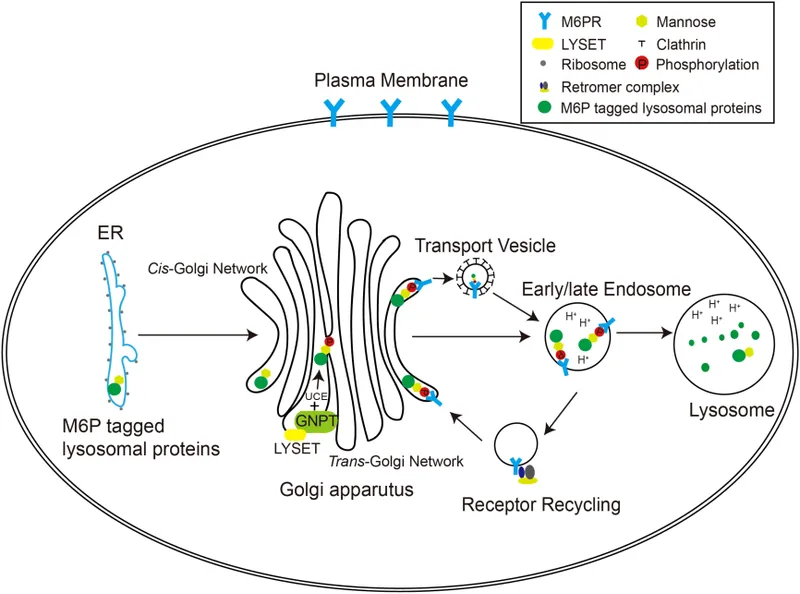
- The constellation of **failure to thrive**, **developmental delay**, **coarse facial features**, restricted joint mobility, and elevated plasma enzymes in an 18-month-old girl is highly suggestive of **I-cell disease** (mucolipidosis type II).
- **I-cell disease** is caused by the deficiency of **N-acetylglucosaminyl-1-phosphotransferase**, an enzyme responsible for phosphorylating mannose residues on lysosomal enzymes, which is crucial for proper targeting to the lysosome.
*Congenital lack of lysosomal formation*
- **Lysosomes** are present in this condition, but their enzymes are misdirected.
- A congenital lack of lysosomal formation would present with even more severe and widespread cellular dysfunction, possibly incompatible with life beyond early embryonic stages.
*Inappropriate protein targeting to endoplasmic reticulum*
- Proteins destined for the endoplasmic reticulum (ER) are typically targeted by an N-terminal signal peptide and then processed within the ER.
- While ER dysfunction can cause various disorders, the specific symptoms and enzyme elevations point away from a primary ER targeting defect related to lysosomal enzymes.
*Inappropriate degradation of lysosomal enzymes*
- In I-cell disease, lysosomal enzymes are synthesized but are **not properly targeted to the lysosomes**; instead, they are secreted into the bloodstream, leading to their elevated plasma levels.
- While some degradation might occur, the primary issue is mis-packaging and secretion, not increased degradation within the cell.
*Misfolding of nuclear proteins*
- Misfolding of nuclear proteins can lead to a variety of genetic disorders and cellular stress responses, but the clinical presentation, particularly the accumulation of undegraded material and elevated plasma lysosomal enzymes, is not characteristic of primary nuclear protein misfolding.
- The pathology in I-cell disease centers on lysosomal dysfunction rather than nuclear protein abnormalities.
Post-translational modifications US Medical PG Question 2: An investigator is studying the function of the endoplasmic reticulum in genetically modified lymphocytes. A gene is removed that facilitates the binding of ribosomes to the endoplasmic reticulum. Which of the following processes is most likely to be impaired as a result of this genetic modification?
- A. Production of secretory proteins (Correct Answer)
- B. Neutralization of toxins
- C. Ubiquitination of proteins
- D. α-Oxidation of fatty acids
- E. Synthesis of ketone bodies
Post-translational modifications Explanation: ***Production of secretory proteins***
- Ribosomes bound to the **rough endoplasmic reticulum (RER)** are responsible for synthesizing proteins destined for secretion, insertion into membranes, or delivery to organelles like lysosomes.
- If ribosomes cannot bind to the ER, these proteins will be synthesized in the **cytosol** and lack the proper signals and processing for their intended destination and function.
*Neutralization of toxins*
- The **smooth endoplasmic reticulum (SER)**, not the RER, is primarily involved in **detoxification** processes, particularly drug metabolism and neutralization of toxins.
- This function relies on enzymes embedded within the SER membrane and is largely independent of ribosome binding.
*Ubiquitination of proteins*
- **Ubiquitination** is a post-translational modification that tags proteins for degradation by the **proteasome** or for trafficking to specific cellular compartments.
- This process occurs primarily in the **cytosol** and does not directly rely on ribosome binding to the ER for protein synthesis.
*α-Oxidation of fatty acids*
- **α-oxidation of fatty acids** is a metabolic pathway that occurs primarily in the **peroxisomes**.
- It is distinct from protein synthesis on the ER and would not be directly impacted by the inability of ribosomes to bind to the ER.
*Synthesis of ketone bodies*
- The **synthesis of ketone bodies** (ketogenesis) primarily occurs in the **mitochondria** of liver cells.
- This metabolic pathway is not directly dependent on ribosome binding to the endoplasmic reticulum for its function.
Post-translational modifications US Medical PG Question 3: A genetic population study is being conducted to find the penetrance of a certain disease. This disease is associated with impaired iron metabolism and primarily affects the liver. Patients often present with diabetes and bronze skin pigmentation. After a genetic screening of 120 inhabitants with a family history of this disease, 40 were found to have the disease-producing genotype, but only 10 presented with symptoms. What are the chances of the screened patients with said genotype developing the disease phenotype?
- A. 0.4%
- B. 40%
- C. 3%
- D. 4%
- E. 25% (Correct Answer)
Post-translational modifications Explanation: ***25%***
- **Penetrance** is calculated as the proportion of individuals with a specific genotype who express the associated phenotype.
- In this case, 10 individuals out of 40 with the disease-producing genotype developed symptoms, so (10 / 40) * 100% = **25%**.
*0.4%*
- This value is significantly lower than the actual penetrance and likely results from an incorrect calculation or misinterpretation of the given data.
- It does not accurately reflect the proportion of genotypically affected individuals who express the phenotype.
*40%*
- This percentage represents the proportion of screened individuals with the disease-producing genotype (40 out of 120 are ~33%), not the penetrance itself.
- It incorrectly equates the presence of the genotype in the population with the expression of the phenotype.
*3%*
- This value is likely obtained by an erroneous calculation, possibly by dividing the symptomatic individuals by the total screened population (10/120 ≈ 8.3%), which does not represent penetrance.
- It does not account for the specific individuals who possess the genotype.
*4%*
- This percentage might arise from an incorrect division or a misunderstanding of what constitutes penetrance.
- It is an inaccurate representation of the ratio between phenotype expression and genotype presence.
Post-translational modifications US Medical PG Question 4: An experimental compound added to a protein disrupts both alpha helices as well as beta-pleated sheets. Which of the following has the experimental compound affected?
- A. Ionic bonds between amino acids
- B. Hydrogen bonds between amino acids (Correct Answer)
- C. Covalent peptide bonds between amino acids
- D. Disulfide bonds between amino acids
- E. The primary structure of the protein
Post-translational modifications Explanation: ***Hydrogen bonds between amino acids***
- Both **alpha helices** and **beta-pleated sheets** are formed and stabilized by **hydrogen bonds** between the backbone atoms (carbonyl oxygen and amide hydrogen) of different amino acids.
- Disrupting these specific bonds would destabilize and unravel these **secondary protein structures**.
*Ionic bonds between amino acids*
- **Ionic bonds** are electrostatic interactions between charged R-groups of amino acids and primarily contribute to **tertiary** and **quaternary protein structures**.
- While important for overall protein folding, they are not the primary stabilizing force for **alpha helices** and **beta-pleated sheets**.
*Covalent peptide bonds between amino acids*
- **Peptide bonds** are the **covalent links** that form the **primary structure** (amino acid sequence) of a protein.
- Disrupting these would lead to the protein breaking down into smaller peptides or individual amino acids, which is a much more severe disruption than just affecting secondary structures.
*Disulfide bonds between amino acids*
- **Disulfide bonds** are **covalent bonds** formed between the sulfur atoms of two **cysteine residues**.
- These bonds contribute significantly to the stability of **tertiary protein structure** and sometimes **quaternary structure**, but not directly to the formation of alpha helices or beta-pleated sheets.
*The primary structure of the protein*
- The **primary structure** is the unique linear sequence of amino acids linked by **peptide bonds**.
- Disruption of **alpha helices** and **beta-pleated sheets** indicates an effect on the **secondary structure**, not the primary sequence itself.
Post-translational modifications US Medical PG Question 5: A 3-year-old is brought to the pediatrician by his mother because she is concerned about recent changes to his behavior. She states that he has seemed to regress in his motor development and has been having occasional brief episodes of uncontrollable shaking. During the subsequent work up, a muscle biopsy is obtained which demonstrates red ragged fibers and a presumptive diagnosis of a genetic disease made. The mother asks if her other son will be affected. What should be the physician's response?
- A. There is a 50% chance he will be affected
- B. There is a 100% chance he will be affected, and the severity will be the same
- C. There is a 25% chance he will be affected
- D. There is a 100% chance he will be affected, but the severity may be different (Correct Answer)
- E. He will be unaffected
Post-translational modifications Explanation: ***There is a 100% chance he will be affected, but the severity may be different***
- The patient's symptoms (motor regression, seizures, red ragged fibers on muscle biopsy) are classic for a **mitochondrial disorder**, which are inherited via **maternal inheritance**.
- All children of an affected mother will inherit the affected mitochondria; however, the **heteroplasmy** (proportion of mutated mitochondria inherited) can vary, leading to different disease severities.
*There is a 50% chance he will be affected*
- This inheritance pattern is typical for **autosomal dominant** disorders, or occasionally X-linked disorders in males.
- Mitochondrial disorders do not follow autosomal dominant inheritance, as they are exclusively inherited from the mother.
*There is a 100% chance he will be affected, and the severity will be the same*
- While there is a 100% chance of inheriting the mutated mitochondria from an affected mother, the **phenotypic expression and severity can vary widely** due to heteroplasmy.
- The proportion of mutated mitochondria can differ in various tissues and between offspring, leading to variable clinical manifestations.
*There is a 25% chance he will be affected*
- This represents the risk of inheritance for an **autosomal recessive** disorder when both parents are carriers.
- Mitochondrial inheritance does not follow an autosomal recessive pattern.
*He will be unaffected*
- This would only be true if the mother's mitochondrial DNA were not affected or if the inheritance pattern allowed for some children to be completely spared, which is not the case for mitochondrial disorders.
- Since the mother is the carrier of the mitochondrial mutation, all her children will inherit the mutated mitochondria.
Post-translational modifications US Medical PG Question 6: A 9-year-old boy is brought to his primary care physician after his mom noticed that he was limping. He says that he has been experiencing significant hip and knee pain over the last 2 months but thought he may have just strained a muscle. Radiographs show a collapse of the femoral head, and he is diagnosed with Legg-Calve-Perthes disease. He undergoes surgery and is placed in a Petrie cast from his hips to his toes bilaterally so that he is unable to move his knees or ankles. Eight weeks later, the cast is removed, and he is found to have significantly smaller calves than before the cast was placed. Which process in myocytes is most likely responsible for this finding?
- A. Decreased formation of double membrane bound vesicles
- B. Increased formation of double membrane bound vesicles
- C. Monoubiquitination of proteins
- D. Inhibition of gene transcription
- E. Polyubiquitination of proteins (Correct Answer)
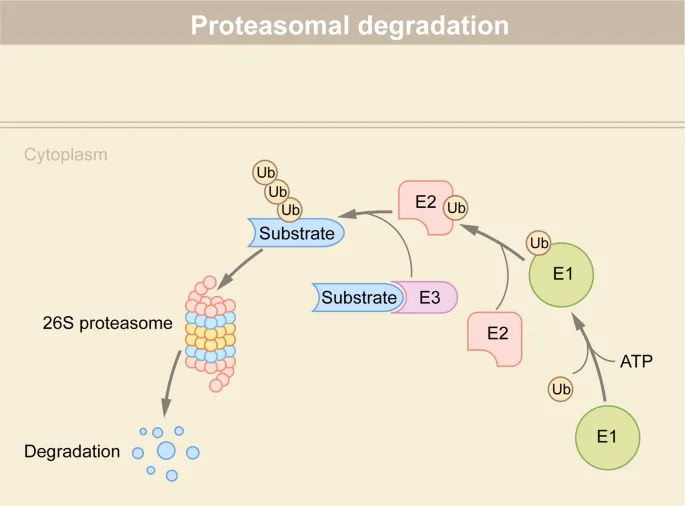
Post-translational modifications Explanation: ***Polyubiquitination of proteins***
- **Polyubiquitination** targets proteins for degradation by the **proteasome**, a key mechanism in skeletal muscle atrophy due to disuse.
- The cast-induced disuse leads to muscle fiber shrinkage, as the cellular machinery for protein breakdown becomes more active than protein synthesis.
*Decreased formation of double membrane bound vesicles*
- This option refers to a decrease in **autophagy**, a process where cells degrade and recycle cellular components. While autophagy is involved in muscle atrophy, a *decrease* in this process would generally lead to accumulation of cellular components rather than a reduction in muscle mass.
- Autophagy would typically be *increased* during disuse atrophy to break down unnecessary components, not decreased.
*Increased formation of double membrane bound vesicles*
- This describes **increased autophagy**, a process that contributes to muscle atrophy by degrading cell components, including organelles and proteins. However, the primary and most direct mechanism for the rapid degradation of muscle proteins in disuse atrophy is the ubiquitin-proteasome pathway, rather than autophagy in this specific context.
- While autophagy does play a role in muscle wasting, **ubiquitin-proteasome system** is considered the dominant pathway for targeted protein degradation in disuse atrophy.
*Monoubiquitination of proteins*
- **Monoubiquitination** is typically involved in regulatory processes like **endomembrane trafficking**, **DNA repair**, and changing protein activity or localization, not directly in targeting proteins for proteasomal degradation.
- Unlike polyubiquitination, which marks proteins for destruction, monoubiquitination typically serves as a regulatory signal for various cellular functions without leading to rapid protein breakdown.
*Inhibition of gene transcription*
- While prolonged disuse can lead to changes in gene expression, including the downregulation of genes involved in muscle growth, the immediate and direct cause of muscle mass reduction in the context of acute disuse is the **accelerated degradation of existing proteins**.
- **Reduced gene transcription** would reduce the *synthesis* of new proteins, but the significant and rapid atrophy observed also requires the active breakdown of existing muscle proteins.
Post-translational modifications US Medical PG Question 7: A 67-year-old man comes to the physician for a follow-up examination after he was diagnosed with mantle cell lymphoma. The physician recommends a chemotherapeutic regimen containing bortezomib. Which of the following best describes the effect of this drug?
- A. Crosslinking of purine bases
- B. Preventing the relaxation of DNA supercoils
- C. Inhibition of tyrosine kinase receptors
- D. Accumulation of ubiquitinated proteins (Correct Answer)
- E. Stabilization of tubulin polymers
Post-translational modifications Explanation: ***Accumulation of ubiquitinated proteins***
- **Bortezomib** is a **proteasome inhibitor**, specifically targeting the 26S proteasome, which is responsible for degrading ubiquitinated proteins.
- Its inhibition leads to the accumulation of various **ubiquitinated proteins**, including pro-apoptotic factors, ultimately inducing **apoptosis** in cancer cells.
*Crosslinking of purine bases*
- This mechanism is characteristic of **alkylating agents** such as cyclophosphamide or cisplatin, which form covalent bonds with DNA, preventing replication and transcription.
- **Bortezomib** does not directly crosslink DNA bases; its primary action is on protein degradation pathways.
*Preventing the relaxation of DNA supercoils*
- This describes the mechanism of **topoisomerase inhibitors**, such as etoposide (topoisomerase II) or irinotecan (topoisomerase I), which block DNA replication and repair.
- Bortezomib has a distinct mechanism involving proteasome inhibition, not direct interaction with DNA or topoisomerases.
*Inhibition of tyrosine kinase receptors*
- This is the action of **tyrosine kinase inhibitors**, a class of drugs like imatinib or gefitinib, that target specific signaling pathways involved in cell growth and proliferation.
- Bortezomib's anti-cancer effects are mediated through protein degradation pathways, not by inhibiting receptor tyrosine kinases.
*Stabilization of tubulin polymers*
- This mechanism is characteristic of **taxanes** (e.g., paclitaxel), which hyperstabilize microtubules, interfering with cell division.
- **Bortezomib** does not affect microtubule dynamics; its action is focused on the proteasomal degradation system.
Post-translational modifications US Medical PG Question 8: A 12-year-old male presents to the emergency department following several days of facial edema. A urinalysis confirms proteinuria and hematuria. Once admitted, a kidney biopsy is viewed under an electron microscope to confirm the diagnosis of minimal change disease. In the following electron micrograph, what process occurs in the structure marked with an arrow?
- A. Podocyte foot process effacement (Correct Answer)
- B. Normal podocyte foot process interdigitation
- C. Mesangial cell proliferation
- D. Endothelial cell fenestration
- E. Glomerular basement membrane thickening
Post-translational modifications Explanation: **Podocyte foot process effacement**
- Minimal change disease is characterized by the **effacement (flattening and fusion)** of podocyte foot processes, which are the structures indicated by the arrow in the image.
- This effacement leads to the loss of the **slit diaphragm barrier**, causing massive proteinuria.
*Normal podocyte foot process interdigitation*
- Normal podocyte foot processes exhibit a distinct, highly organized **interdigitating pattern**, which is clearly not observed in the image due to the flattening.
- This normal interdigitation is crucial for maintaining the **glomerular filtration barrier** and preventing protein leakage.
*Mesangial cell proliferation*
- **Mesangial cell proliferation** is characteristic of conditions like IgA nephropathy or mesangioproliferative glomerulonephritis, and is not the primary feature of minimal change disease.
- The image illustrates changes in the podocytes, not the mesangial cells (labeled 'M').
*Endothelial cell fenestration*
- **Endothelial cell fenestrations** are normal pores in the glomerular endothelial cells that allow for filtration, and are not directly affected or effaced in minimal change disease.
- The arrow in the image points to the podocyte layer, not the endothelial cells.
*Glomerular basement membrane thickening*
- **Glomerular basement membrane (GBM) thickening** is seen in conditions like diabetic nephropathy or membranous nephropathy, but not typically in minimal change disease.
- In minimal change disease, the GBM typically appears normal under electron microscopy.
Post-translational modifications US Medical PG Question 9: Given the mRNA sequence shown below, if translation were to start at the first base, what would the tRNA anticodon be for the last amino acid translated in the chain?
5'----GCACCGGCCUGACUAUAA---3'
- A. 3' GCG 5'
- B. 3' CGC 5'
- C. 5' CGG 3'
- D. 3' CGG 5' (Correct Answer)
- E. 3' GAU 5'
Post-translational modifications Explanation: ***3' CGG 5'***
- The mRNA sequence is 5'-GCACCGGCCUGACUAUAA-3'. We need to identify the **open reading frame** starting from the first base and translate codons until a stop codon is reached.
- The codons are **GCA** (Ala), **CCG** (Pro), **GCC** (Ala), **UGA** (Stop). The **last amino acid** translated is Alanine, corresponding to the mRNA codon **GCC**. The tRNA anticodon for GCC is **3'-CGG-5'** because base pairing rules dictate C pairs with G, and G pairs with C, in an antiparallel orientation.
*3' GCG 5'*
- This anticodon would pair with an mRNA codon of 5'-CGC-3', which codes for Arginine, not the alanine derived from the last amino acid in the given sequence.
- It does not correctly reflect the antiparallel binding and base pairing required for the mRNA codon GCC.
*5' CGG 3'*
- While it contains the correct bases for pairing with GCC, the **orientation is incorrect**. tRNA anticodons are written 3' to 5'.
- A 5'-CGG-3' anticodon would pair with an mRNA codon of 3'-GCC-5', which is not consistent with the standard 5' to 3' mRNA codon reading.
*3' GAU 5'*
- This anticodon would pair with an mRNA codon of 5'-CUA-3', which codes for Leucine.
- Leucine is not the last amino acid translated from the given mRNA sequence before a stop codon.
*3' CGC 5'*
- This anticodon would pair with an mRNA codon of 5'-GCG-3', which codes for Alanine.
- However, the last amino acid translated is encoded by 5'-GCC-3', not 5'-GCG-3'.
Post-translational modifications US Medical PG Question 10: An 8-month-old female infant from a first-degree consanguineous couple was brought to the physician because the mother noticed abnormalities in the growth of her child as well as the different lengths of her child's legs. The infant had gingival hyperplasia, restricted movement in both shoulders, a prominent, pointed forehead, and enophthalmos with a slight opacity in both corneas. A blood test revealed 10 fold higher than normal levels of the following enzymes: N-acetyl-ß-glucosaminidase, ß-glucuronidase, ß-hexosaminidase A, and alkaline phosphatase. Which of the following is most likely deficient in this patient?
- A. Lysosomal alpha-1,4-glucosidase
- B. Glucose-6-phosphate dehydrogenase
- C. N-acetyl-glucosamine-1-phosphotransferase (Correct Answer)
- D. Glucocerebrosidase
- E. Alpha-galactosidase A
Post-translational modifications Explanation: ***N-acetyl-glucosamine-1-phosphotransferase***
- The clinical presentation with **gingival hyperplasia**, **restricted joint movement**, **skeletal abnormalities** (growth abnormalities, leg length discrepancy, prominent forehead), and **corneal opacity** with elevated lysosomal enzymes (N-acetyl-ß-glucosaminidase, ß-glucuronidase, ß-hexosaminidase A) is highly characteristic of **I-cell disease** (mucolipidosis II).
- I-cell disease is caused by a deficiency in **N-acetyl-glucosamine-1-phosphotransferase**, an enzyme crucial for phosphorylating mannose residues on lysosomal enzymes, tagging them for delivery to lysosomes. Without this tag, lysosomal enzymes are secreted extracellularly, leading to their accumulation in the blood and their deficiency within lysosomes, causing the clinical features.
*Lysosomal alpha-1,4-glucosidase*
- Deficiency of **lysosomal alpha-1,4-glucosidase** causes **Pompe disease (glycogen storage disease type II)**, which is characterized by **cardiomegaly**, hypotonia, and liver involvement, but typically does not present with the skeletal dysplasias, gingival hyperplasia, or corneal clouding seen in this patient.
- While it is a lysosomal storage disorder, the specific clinical features and panel of elevated enzymes differ significantly from this case.
*Glucose-6-phosphate dehydrogenase*
- Deficiency of **glucose-6-phosphate dehydrogenase (G6PD)** causes **G6PD deficiency**, an X-linked disorder leading to **hemolytic anemia** in response to oxidative stress (e.g., fava beans, certain drugs, infections).
- It does not present with the systemic skeletal, connective tissue, and corneal abnormalities described, nor does it involve elevated lysosomal enzyme levels.
*Glucocerebrosidase*
- Deficiency of **glucocerebrosidase** causes **Gaucher disease**, which presents with **hepatosplenomegaly**, bone crises, pancytopenia, and sometimes neurological involvement.
- While it is a lysosomal storage disorder, the clinical features (e.g., absence of gingival hyperplasia, corneal opacity, or specific skeletal dysplasias like restricted joint movement) and the pattern of elevated enzymes do not match the patient's presentation.
*Alpha-galactosidase A*
- Deficiency of **alpha-galactosidase A** causes **Fabry disease**, an X-linked lysosomal storage disorder characterized by **neuropathic pain**, **angiokeratomas**, renal failure, and cardiac involvement.
- The clinical picture of Fabry disease does not include gingival hyperplasia, prominent skeletal abnormalities, or the specific pattern of elevated lysosomal enzymes observed in this patient.
More Post-translational modifications US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.