WNT signaling pathway US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for WNT signaling pathway. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
WNT signaling pathway US Medical PG Question 1: A group of researchers is studying molecules and DNA segments that are critical for important cellular processes in eukaryotic cells. They have identified a region that is located about 28 bases upstream of the 5’ coding region. This region promotes the initiation of transcription by binding with transcription factors. Which of the following regions have these researchers most likely identified?
- A. TATA Box (Correct Answer)
- B. RNA polymerase II
- C. Small nuclear ribonucleoprotein (SnRNPs)
- D. DNA methyltransferase
- E. CAAT Box
WNT signaling pathway Explanation: ***TATA Box***
- The **TATA box** is a core promoter element found in eukaryotic genes, typically located **25-35 base pairs upstream** of the transcription start site.
- It plays a crucial role in initiating transcription by serving as a binding site for **transcription factors**, which in turn recruit **RNA polymerase II**.
*RNA polymerase II*
- **RNA polymerase II** is the enzyme responsible for transcribing protein-coding genes into mRNA.
- While essential for transcription, it is an enzyme that binds to the promoter region (which includes the TATA box), rather than a regulatory DNA sequence itself.
*Small nuclear ribonucleoprotein (SnRNPs)*
- **SnRNPs** are components of the spliceosome, involved in the **splicing of pre-mRNA** to remove introns.
- They are involved in post-transcriptional modification, not in the initiation of transcription.
*DNA methyltransferase*
- **DNA methyltransferase** is an enzyme involved in **DNA methylation**, a process that typically represses gene expression.
- This enzyme modifies DNA, but it is not a DNA region that promotes transcription initiation.
*CAAT Box*
- The **CAAT box** is another common promoter element in eukaryotes, usually located further **upstream (70-80 base pairs)** from the transcription start site.
- While it also binds transcription factors and influences transcription initiation, its location is generally *more distant* than the 28 bases upstream described, making the TATA box a more accurate fit for the given distance.
WNT signaling pathway US Medical PG Question 2: A 38-year-old man presents to his primary care practitioner for 2 months of rectal bleeding. He also reports occasional diarrhea and abdominal pain. His family history is relevant for his father and uncle, who died from complications of colorectal cancer. Colonoscopy shows more than 10 colorectal adenomas. Which of the following genes is most likely affected in this patient?
- A. RAS
- B. TP53
- C. hMLH1
- D. PPAR
- E. APC (Correct Answer)
WNT signaling pathway Explanation: ***APC***
- This patient's presentation with **numerous colorectal adenomas** (over 10), early-onset symptoms (38 years old), and a strong **family history of colorectal cancer** (father and uncle) is highly characteristic of **Familial Adenomatous Polyposis (FAP)**.
- FAP is an **autosomal dominant** condition caused by a germline mutation in the **APC tumor suppressor gene**, leading to the development of hundreds to thousands of adenomatous polyps in the colon, which inevitably progress to colorectal cancer if untreated.
*RAS*
- **RAS mutations** are commonly found in sporadic colorectal cancers and play a role in tumor growth and progression, but they are not typically associated with the **hereditary syndrome of multiple adenomas** seen in this patient.
- RAS activation leads to an increase in **cell proliferation** and can contribute to the development of many cancers, but not as the primary genetic defect in a polyposis syndrome.
*TP53*
- **TP53** is a well-known tumor suppressor gene, and mutations are involved in various cancers, including colorectal cancer (often in its later stages). However, germline mutations in TP53 are associated with **Li-Fraumeni syndrome**, which involves a broad spectrum of early-onset cancers and is not primarily characterized by numerous colonic adenomas.
- TP53 mutations are generally hallmarks of **genomic instability** and are more often seen in the progression of sporadic cancers rather than initiating a polyposis syndrome.
*hMLH1*
- **hMLH1** is a gene involved in **DNA mismatch repair**. Germline mutations in this gene, along with other mismatch repair genes (e.g., MSH2, MSH6, PMS2), are responsible for **Lynch syndrome (hereditary non-polyposis colorectal cancer - HNPCC)**.
- While Lynch syndrome is an important cause of hereditary colorectal cancer, it is characterized by fewer polyps (typically <10) that progress rapidly to cancer, and an increased risk of other cancers (e.g., endometrial), which differs from the presentation of **hundreds of adenomas** seen in FAP.
*PPAR*
- **PPARs (Peroxisome Proliferator-Activated Receptors)** are a group of nuclear receptor proteins that play roles in metabolism, cell differentiation, and inflammation.
- While PPAR pathways have been investigated for their potential role in cancer development and as therapeutic targets, **mutations in PPAR genes are not directly linked** to a common hereditary colorectal cancer syndrome characterized by numerous adenomas like FAP.
WNT signaling pathway US Medical PG Question 3: A 40-year-old man presents with an episode of rectal bleeding. He is concerned because his mother died of colorectal cancer at 50 years of age. He has no further information about his family history. Physical examination and digital rectal examination are normal. He undergoes a colonoscopy and is found to have innumerable adenomas in the left side of the colon ranging in size from 4–15 mm. Which of the following is the most likely underlying mechanism of this patient illness?
- A. Mutation in DNA mismatch repair genes
- B. Inactivation of RB1 gene
- C. Alterations in STK11 gene
- D. Inactivation of BRCA1 and BRCA2 genes
- E. Mutations of the APC gene (Correct Answer)
WNT signaling pathway Explanation: ***Mutations of the APC gene***
- The description of **innumerable adenomas** in the colon, particularly at a relatively young age and with a family history of early-onset colorectal cancer, is highly suggestive of **familial adenomatous polyposis (FAP)**.
- FAP is an autosomal dominant condition caused by germline mutations in the **adenomatous polyposis coli (APC) gene**, a tumor suppressor gene, leading to the development of hundreds to thousands of adenomatous polyps and an almost 100% lifetime risk of colorectal cancer.
*Mutation in DNA mismatch repair genes*
- Mutations in **DNA mismatch repair genes** (e.g., MLH1, MSH2, MSH6, PMS2) are associated with **Lynch syndrome (hereditary nonpolyposis colorectal cancer)**.
- Lynch syndrome typically presents with fewer polyps (though an increased risk of colorectal cancer, especially right-sided) and other extracolonic cancers, which is less consistent with "innumerable adenomas."
*Inactivation of RB1 gene*
- The **RB1 gene** is a tumor suppressor gene primarily associated with **retinoblastoma** and has a role in other cancers like osteosarcoma.
- It is not the primary genetic mechanism for the development of multiple colonic adenomas described in this patient.
*Alterations in STK11 gene*
- Alterations in the **STK11 gene** are associated with **Peutz-Jeghers syndrome**, an autosomal dominant disorder characterized by the development of multiple **hamartomatous polyps** mainly in the gastrointestinal tract, especially the small intestine, and characteristic **mucocutaneous pigmentation**.
- These polyps are hamartomatous, not adenomatous, and while they carry a cancer risk, the presentation of innumerable adenomas points away from Peutz-Jeghers syndrome.
*Inactivation of BRCA1 and BRCA2 genes*
- **BRCA1 and BRCA2 genes** are critical tumor suppressor genes primarily associated with an increased risk of **breast cancer** and **ovarian cancer**, as well as some other cancers like prostate and pancreatic cancer.
- While these genes are important in cancer development, they are not directly implicated in the pathogenesis of familial adenomatous polyposis or the significant number of colonic adenomas described.
WNT signaling pathway US Medical PG Question 4: A 66-year-old man comes to the physician because of a 3-month history of constipation and streaks of blood in his stool. He has had a 10-kg (22-lb) weight loss during this period. Colonoscopy shows an exophytic tumor in the sigmoid colon. A CT scan of the abdomen shows liver metastases and enlarged mesenteric and para-aortic lymph nodes. A diagnosis of stage IV colorectal cancer is made, and palliative chemotherapy is initiated. The chemotherapy regimen includes a monoclonal antibody that inhibits tumor growth by preventing ligand binding to a protein directly responsible for epithelial cell proliferation and organogenesis. Which of the following proteins is most likely inhibited by this drug?
- A. VEGF
- B. TNF-α
- C. EGFR (Correct Answer)
- D. ALK
- E. CD52
WNT signaling pathway Explanation: ***EGFR***
- The description of a monoclonal antibody preventing ligand binding to a protein responsible for **epithelial cell proliferation** and organogenesis strongly points to the **epidermal growth factor receptor (EGFR)**.
- EGFR is highly expressed in many colorectal cancers and its activation by ligands like EGF promotes cell growth, survival, and metastasis. Inhibiting it reduces tumor progression.
*VEGF*
- **Vascular endothelial growth factor (VEGF)** is primarily involved in **angiogenesis**, the formation of new blood vessels.
- While anti-VEGF therapies (e.g., bevacizumab) are used in colorectal cancer, their mechanism is inhibiting blood supply to the tumor, not directly blocking a receptor responsible for epithelial cell proliferation as described.
*TNF-α*
- **Tumor necrosis factor-alpha (TNF-α)** is a **cytokine** primarily involved in inflammation and immune responses.
- Antibodies against TNF-α (e.g., infliximab) are used in inflammatory conditions like Crohn's disease, not typically as targeted therapy for colorectal cancer directly inhibiting epithelial proliferation.
*ALK*
- **Anaplastic lymphoma kinase (ALK)** is a **receptor tyrosine kinase** often implicated in lung cancer and lymphomas.
- ALK rearrangements lead to oncogenic fusion proteins, but it is not a primary target for widespread epithelial cell proliferation in colorectal cancer.
*CD52*
- **CD52** is a glycoprotein found on the surface of various immune cells, including lymphocytes.
- Antibodies targeting CD52 (e.g., alemtuzumab) are used in certain leukemias and lymphomas to deplete these cells, not for inhibiting epithelial cell proliferation in solid tumors.
WNT signaling pathway US Medical PG Question 5: A 62-year-old man comes to the physician because of progressive fatigue and dyspnea on exertion for 3 months. During this time, he has also had increased straining during defecation and a 10-kg (22-lb) weight loss. He has no personal or family history of serious medical illness. Physical examination shows conjunctival pallor. Laboratory studies show microcytic anemia. Test of the stool for occult blood is positive. Colonoscopy shows an exophytic mass in the ascending colon. Pathologic examination of the mass shows a well-differentiated adenocarcinoma. A gain-of-function mutation in which of the following genes is most likely involved in the pathogenesis of this patient's condition?
- A. APC
- B. TP53
- C. MLH1
- D. KRAS (Correct Answer)
- E. DCC
WNT signaling pathway Explanation: ***KRAS***
- A **gain-of-function mutation** in **KRAS** is a common early event in the development of colorectal adenocarcinoma, driving uncontrolled cell growth and proliferation.
- This mutation is frequently found in **sporadic colorectal cancers**, particularly in the advanced stages of adenoma to carcinoma progression.
*APC*
- **APC** is a **tumor suppressor gene**, and mutations in it are typically **loss-of-function**, not gain-of-function.
- While APC mutations are crucial early steps in the adenoma-carcinoma sequence, they lead to inactivation of the gene, not increased function.
*TP53*
- **TP53** is a **tumor suppressor gene** which, when mutated, usually involves **loss-of-function** or dominant-negative effects, impairing its ability to induce apoptosis or cell cycle arrest.
- Mutations in TP53 are typically associated with **later stages** of colorectal cancer progression and tend to be loss-of-function, not gain-of-function.
*MLH1*
- **MLH1** is involved in **DNA mismatch repair**, and mutations here lead to **microsatellite instability** and are characteristic of hereditary nonpolyposis colorectal cancer (Lynch syndrome).
- These are typically **loss-of-function mutations** that impair DNA repair, not gain-of-function mutations promoting oncogenesis directly through signaling pathways.
*DCC*
- **DCC** (**Deleted in Colorectal Carcinoma**) is a **tumor suppressor gene**, and its inactivation or loss is associated with colorectal cancer progression, particularly the transition from adenoma to carcinoma.
- Mutations or deletions in DCC result in a **loss-of-function**, not a gain-of-function, contributing to tumor growth by failing to regulate cell differentiation and apoptosis.
WNT signaling pathway US Medical PG Question 6: Antigen presentation of extracellular pathogens by antigen presenting cells requires endocytosis of the antigen, followed by the degradation in the acidic environment of the formed phagolysosome. Should the phagolysosome become unable to lower its pH, what is the most likely consequence?
- A. Deficient presentation of pathogens to CD4 T-cells (Correct Answer)
- B. Deficient cell extravasation
- C. Deficient presentation of pathogens to CD8 T-cells
- D. Deficient NK cell activation
- E. Deficient expression of B7
WNT signaling pathway Explanation: ***Deficient presentation of pathogens to CD4 T-cells***
- The acidic environment of the **phagolysosome** is crucial for optimal **antigen degradation** and processing into peptides that can bind to **MHC class II molecules**.
- Without proper acidification, peptide loading onto **MHC class II** is impaired, leading to deficient presentation of extracellular pathogens to **CD4 T-cells**.
*Deficient cell extravasation*
- **Cell extravasation** involves events like rolling, adhesion, and transendothelial migration, which are primarily regulated by **adhesion molecules** and **chemokines**, not phagolysosomal pH.
- A defect in phagolysosomal pH would not directly impede the ability of cells to exit the vasculature.
*Deficient presentation of pathogens to CD8 T-cells*
- **CD8 T-cell** activation primarily involves the presentation of **intracellular antigens** via **MHC class I molecules**, which typically occurs through degradation in the **cytosol** via proteasomes.
- While some cross-presentation pathways exist, the primary mechanism of CD8 T-cell antigen presentation is not dependent on the acidification of phagolysosomes for extracellular pathogens.
*Deficient NK cell activation*
- **Natural Killer (NK) cells** recognize and kill target cells based on the presence or absence of **MHC class I molecules** and activating ligands, not on the processing of extracellular antigens within phagolysosomes.
- Their activation depends on cytokine environments and surface receptor interactions, not directly on phagolysosomal pH.
*Deficient expression of B7*
- **B7 molecules (CD80/CD86)** are **co-stimulatory molecules** expressed by antigen-presenting cells that are crucial for full T-cell activation. While antigen processing can influence APC activation, a specific defect in phagolysosomal pH would primarily affect the *presentation* of peptides, not the *expression* of co-stimulatory molecules.
- The expression of B7 is more broadly regulated by inflammatory signals and toll-like receptor (TLR) engagement, rather than being solely dependent on proper phagolysosomal acidification.
WNT signaling pathway US Medical PG Question 7: A 24-year-old woman comes to the physician because of progressively worsening episodes of severe, crampy abdominal pain and nonbloody diarrhea for the past 3 years. Examination of the abdomen shows mild distension and generalized tenderness. There is a fistula draining stool in the perianal region. Immunohistochemistry shows dysfunction of the nucleotide oligomerization binding domain 2 (NOD2) protein. This dysfunction most likely causes overactivity of which of the following immunological proteins in this patient?
- A. Interferon-γ
- B. β-catenin
- C. IL-1β
- D. IL-10
- E. NF-κB (Correct Answer)
WNT signaling pathway Explanation: ***NF-κB***
- **NOD2** is a pattern recognition receptor that normally detects bacterial products and regulates inflammatory responses. In **Crohn's disease**, loss-of-function **NOD2 mutations** lead to impaired bacterial sensing and clearance.
- This defective NOD2 function results in **compensatory overactivation of NF-κB** through alternative inflammatory pathways (particularly TLR signaling), causing excessive **pro-inflammatory cytokine** production.
- This **NF-κB hyperactivation** is a key driver of chronic inflammation in **Crohn's disease**, contributing to symptoms like fistulas, strictures, and transmural inflammation.
*Interferon-γ*
- **Interferon-γ** is an important pro-inflammatory cytokine in Crohn's disease and is part of the Th1-mediated immune response.
- However, its production is downstream of **NF-κB** activation and other inflammatory cascades. **NOD2 dysfunction** does not directly cause **IFN-γ** overactivity through the primary molecular pathway.
*β-catenin*
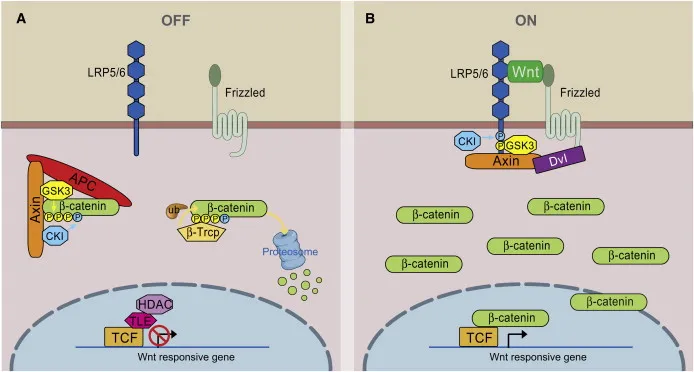
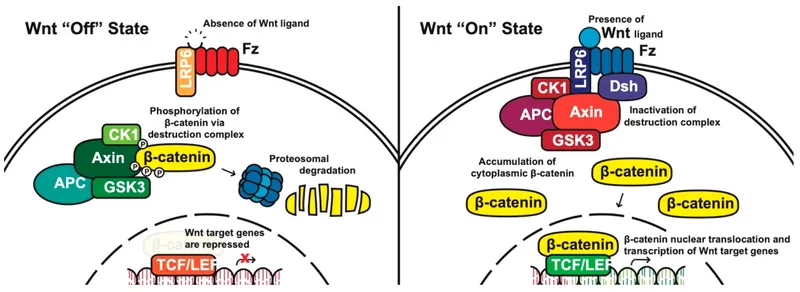
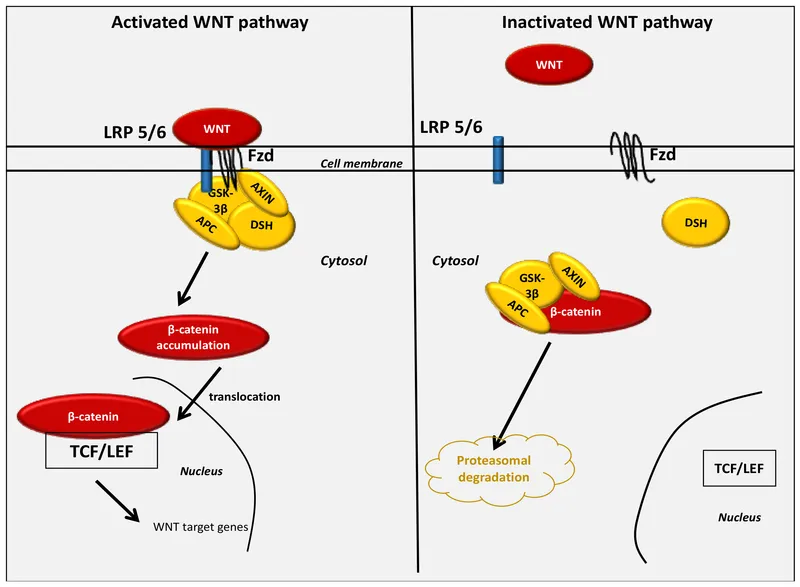
- **β-catenin** is a key component of the **Wnt signaling pathway** involved in cell adhesion, proliferation, and differentiation.
- It is not directly affected by **NOD2 dysfunction**. Dysregulation of **β-catenin** is more commonly associated with colorectal adenomas and cancer, not the inflammatory mechanisms of Crohn's disease.
*IL-1β*
- **IL-1β** is a potent pro-inflammatory cytokine that is indeed elevated in **Crohn's disease**.
- However, **IL-1β** is produced **downstream** of **NF-κB** activation. The primary molecular consequence of **NOD2 dysfunction** is the overactivity of **NF-κB**, which then drives production of various cytokines including **IL-1β**.
*IL-10*
- **IL-10** is an **anti-inflammatory cytokine** essential for maintaining intestinal immune homeostasis and suppressing excessive inflammatory responses.
- In Crohn's disease, **IL-10** signaling is often **impaired or deficient** rather than overactive. The question asks about overactivity, making this the opposite of what occurs in the disease.
WNT signaling pathway US Medical PG Question 8: A previously healthy 42-year-old man comes to the emergency room with constipation and diffuse, worsening abdominal pain for 2 days. He has no history of major medical illness. His father died in a car accident at the age of 32 years, and his mother has type 2 diabetes mellitus. A diagnosis of bowel obstruction is suspected and he is taken to the operating room for exploratory laparotomy. A partial resection of the colon is performed. The gross appearance of the patient's colonic tissue is shown. Microscopic examination shows tubular, tubulovillous, and villous adenomas. Assuming the patient's partner is not a carrier of the condition, which of the following is the likelihood that this patient’s children will develop this condition?
- A. 50% (Correct Answer)
- B. 25%
- C. 100%
- D. 0%
- E. 75%
WNT signaling pathway Explanation: ***50%***
- The image and clinical scenario are highly suggestive of **familial adenomatous polyposis (FAP)**, an autosomal dominant condition characterized by hundreds to thousands of colonic adenomas.
- Since FAP is an **autosomal dominant** disorder, an affected individual (heterozygous for the gene) has a **50% chance** of passing the mutated gene to each child, regardless of whether the partner is a carrier.
*25%*
- A 25% likelihood would be expected in **autosomal recessive inheritance** if both parents are carriers.
- FAP does not follow an autosomal recessive inheritance pattern; it is an **autosomal dominant** disease.
*100%*
- A 100% likelihood would occur only if the affected parent were **homozygous for the mutation** (extremely rare and typically lethal) or in certain non-Mendelian inheritance patterns.
- In typical FAP cases, the affected parent is heterozygous, resulting in a 50% chance of transmission to offspring, not 100%.
*0%*
- A 0% likelihood would imply that the condition is not hereditary or that the affected parent cannot transmit the disease, which is incorrect for FAP.
- FAP is a **hereditary condition** with a clear autosomal dominant inheritance pattern, so there is always a 50% risk of transmission per child.
*75%*
- A 75% likelihood does not correspond to any standard Mendelian inheritance pattern.
- This percentage does not fit the **autosomal dominant pattern** observed in FAP, which consistently shows 50% transmission risk per child.
WNT signaling pathway US Medical PG Question 9: A research team discovers a novel bacterial toxin that causes severe hypotension in infected patients. In vitro studies show the toxin ADP-ribosylates a specific amino acid on Gq alpha subunits, preventing their activation by GPCRs. Patients develop hypotension despite elevated levels of vasopressin, angiotensin II, and endothelin-1. Synthesize the pathophysiological mechanism explaining why multiple vasopressor hormones fail to maintain blood pressure in these patients.
- A. The toxin depletes intracellular ATP preventing myosin-actin interaction
- B. The toxin prevents receptor binding of vasopressor hormones through allosteric inhibition
- C. ADP-ribosylation of Gq prevents PLC activation, blocking IP3-mediated calcium release and vascular smooth muscle contraction (Correct Answer)
- D. ADP-ribosylation increases cAMP levels causing smooth muscle relaxation
- E. The toxin activates Gi proteins causing excessive vasodilation that overwhelms vasoconstrictor signals
WNT signaling pathway Explanation: ***ADP-ribosylation of Gq prevents PLC activation, blocking IP3-mediated calcium release and vascular smooth muscle contraction***
- Vasopressor hormones like **Vasopressin**, **Angiotensin II**, and **Endothelin-1** signal through **Gq-coupled receptors** to trigger **vascular smooth muscle contraction**.
- By ADP-ribosylating the **Gq alpha subunit**, the toxin inhibits **Phospholipase C (PLC)**, preventing the generation of **IP3 and DAG**, which are essential for releasing **intracellular calcium**.
*The toxin prevents receptor binding of vasopressor hormones through allosteric inhibition*
- The toxin targets the **G-protein (intracellular)** rather than the **extracellular binding site** of the G-protein coupled receptors (GPCRs).
- Since binding still occurs but **signal transduction** is blocked, this describes a post-receptor defect rather than **allosteric inhibition** of the receptor itself.
*The toxin activates Gi proteins causing excessive vasodilation that overwhelms vasoconstrictor signals*
- The prompt explicitly states the toxin modifies the **Gq alpha subunit**, not the **Gi subunit**.
- **Gi protein** activation primarily lowers **cAMP**, whereas the failure of pressors in this case is linked to the lack of **calcium mobilization** via Gq.
*ADP-ribosylation increases cAMP levels causing smooth muscle relaxation*
- Increased **cAMP** (via Gs activation or Gi inhibition) does cause relaxation, but this mechanism is associated with toxins like **Cholera** or **Pertussis**.
- The modification of **Gq** specifically disrupts the **phosphoinositol pathway**, not the **adenylyl cyclase** pathway that regulates cAMP.
*The toxin depletes intracellular ATP preventing myosin-actin interaction*
- ADP-ribosylation is a specific **post-translational modification** using **NAD+** as a substrate, which does not result in systemic **ATP depletion**.
- The failure of contraction is due to a lack of **calcium-calmodulin** activation of **Myosin Light Chain Kinase (MLCK)**, not a lack of energy supply for the motor proteins.
WNT signaling pathway US Medical PG Question 10: A 42-year-old woman with metastatic melanoma develops severe colitis while being treated with ipilimumab (anti-CTLA-4 antibody) and nivolumab (anti-PD-1 antibody). Her oncologist must decide whether to continue immunotherapy or treat the colitis with immunosuppression. Tumor analysis shows high PD-L1 expression and BRAF wild-type status. Previous conventional chemotherapy failed. Evaluate the optimal management strategy considering signal transduction implications.
- A. Stop both drugs permanently as immune-related adverse events indicate loss of self-tolerance mechanisms
- B. Continue both checkpoint inhibitors as tumor response depends on sustained T-cell receptor co-stimulation blockade
- C. Discontinue ipilimumab, continue nivolumab with corticosteroids, as PD-1 pathway blockade may be sufficient given high PD-L1 expression (Correct Answer)
- D. Continue both drugs but add TNF-alpha inhibitor to block inflammatory signaling without affecting anti-tumor immunity
- E. Stop all immunotherapy and switch to targeted therapy despite BRAF wild-type status
WNT signaling pathway Explanation: ***Discontinue ipilimumab, continue nivolumab with corticosteroids, as PD-1 pathway blockade may be sufficient given high PD-L1 expression***
- Severe colitis is a significant **immune-related adverse event (irAE)**; managing it requires **corticosteroids** to suppress the excessive T-cell response while balancing anti-tumor efficacy.
- In patients with **high PD-L1 expression**, the **PD-1 inhibitor (Nivolumab)** may provide sufficient anti-tumor signal transduction blockade alone, allowing for the discontinuation of the more toxic **anti-CTLA-4** agent.
*Continue both checkpoint inhibitors as tumor response depends on sustained T-cell receptor co-stimulation blockade*
- Sustaining treatment during severe colitis poses a high risk of **colon perforation** and death due to uncontrolled lymphocytic infiltration.
- **CTLA-4** blockade affects the priming phase in lymph nodes, and continuing it during high-grade toxicity is contraindicated by clinical safety guidelines.
*Stop all immunotherapy and switch to targeted therapy despite BRAF wild-type status*
- Targeted therapies like **BRAF and MEK inhibitors** require the **BRAF V600 mutation** to function; they are ineffective in **BRAF wild-type** status.
- Switching to these agents would leave the patient with no effective treatment for the underlying **metastatic melanoma**.
*Continue both drugs but add TNF-alpha inhibitor to block inflammatory signaling without affecting anti-tumor immunity*
- While **TNF-alpha inhibitors** like infliximab are used for refractory colitis, they are typically added only after **corticosteroids** fail to control symptoms.
- Clinical protocols mandate the suspension of the offending agents during **Grade 3/4 toxicity** to prevent further immune-mediated tissue damage.
*Stop both drugs permanently as immune-related adverse events indicate loss of self-tolerance mechanisms*
- Benefit-risk assessment often allows for the resumption of **PD-1 inhibitors** once toxicity resolves, especially if the tumor shows evidence of responding.
- **Permanent discontinuation** of all life-saving immunotherapy may not be necessary if the toxicity is managed and the clinical benefit of the **PD-1 pathway** blockade is high.
More WNT signaling pathway US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.