Tyrosine kinase receptors US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Tyrosine kinase receptors. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Tyrosine kinase receptors US Medical PG Question 1: An investigator is studying a drug that acts on a G protein-coupled receptor in the pituitary gland. Binding of the drug to this receptor leads to increased production of inositol triphosphate (IP3) in the basophilic cells of the anterior pituitary. Administration of this drug every 90 minutes is most likely to be beneficial in the treatment of which of the following conditions?
- A. Prostate cancer
- B. Variceal bleeding
- C. Central diabetes insipidus
- D. Anovulatory infertility (Correct Answer)
- E. Hyperkalemia
Tyrosine kinase receptors Explanation: ***Anovulatory infertility***
- The drug's action on a G protein-coupled receptor leading to increased **IP3 production** in pituitary basophils suggests activation of the **gonadotropin-releasing hormone (GnRH) receptor**.
- **Pulsatile administration** (e.g., every 90 minutes) of GnRH or its agonists is crucial for stimulating the release of **FSH and LH**, which can induce ovulation in women with anovulatory infertility due to hypothalamic-pituitary dysfunction.
*Prostate cancer*
- While GnRH agonists are used in prostate cancer, they are typically administered **continuously or in depot forms** to desensitize the GnRH receptor, thereby suppressing testosterone production.
- **Pulsatile administration** would rather stimulate testosterone release, which is detrimental in prostate cancer.
*Variceal bleeding*
- **Variceal bleeding** is primarily managed with vasoconstrictors like **octreotide** (a somatostatin analog) or **vasopressin**, which are unrelated to GnRH receptor activation.
- The mechanism of action described (increased IP3 in pituitary basophils) does not align with treatments for variceal bleeding.
*Central diabetes insipidus*
- **Central diabetes insipidus** is caused by a deficiency in **vasopressin (ADH)**, which regulates water balance in the kidneys.
- Treatment involves synthetic ADH (**desmopressin**), not drugs acting on GnRH receptors and affecting pituitary basophils.
*Hyperkalemia*
- **Hyperkalemia** is an electrolyte imbalance characterized by high potassium levels and is managed with medications that shift potassium intracellularly (e.g., insulin, beta-agonists) or promote its excretion (e.g., diuretics, potassium binders).
- The described drug action on pituitary GnRH receptors is unrelated to potassium homeostasis.
Tyrosine kinase receptors US Medical PG Question 2: An investigator is studying the effect of a high-lipid diet on glucose metabolism in Wistar rats. The experimental rat group is fed a high-lipid diet while the control group is fed a low-lipid diet. Two months after initiation of the experiment, the rats in both groups are injected with insulin and serum glucose measurements are obtained. Compared to the control group, the high-lipid diet group has a significantly higher average serum glucose after receiving insulin. Which of the following intracellular changes is most likely involved in the pathogenesis of this finding?
- A. Decreased activation of caspase 8
- B. Decreased production of protein kinase A
- C. Decreased expression of TP53
- D. Increased exposure of nuclear localization signal
- E. Increased activity of serine kinases (Correct Answer)
Tyrosine kinase receptors Explanation: ***Increased activity of serine kinases***
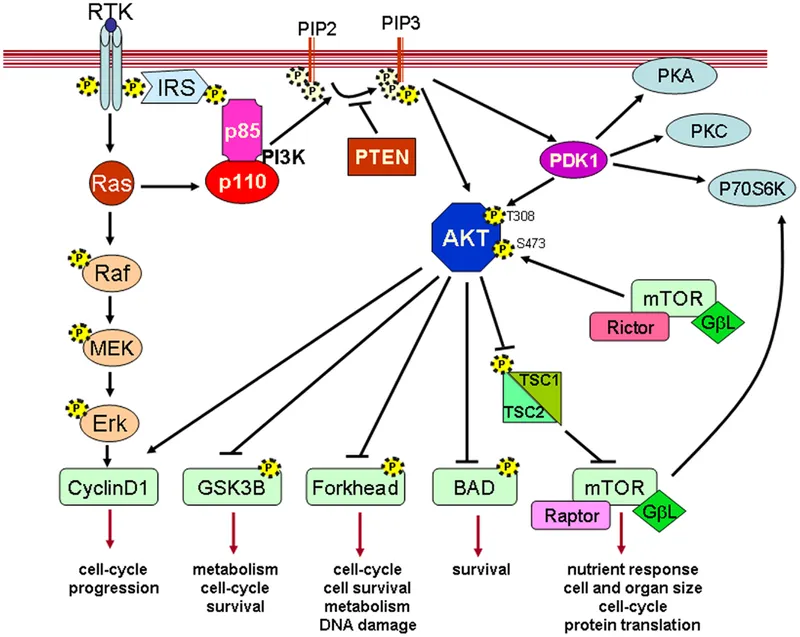
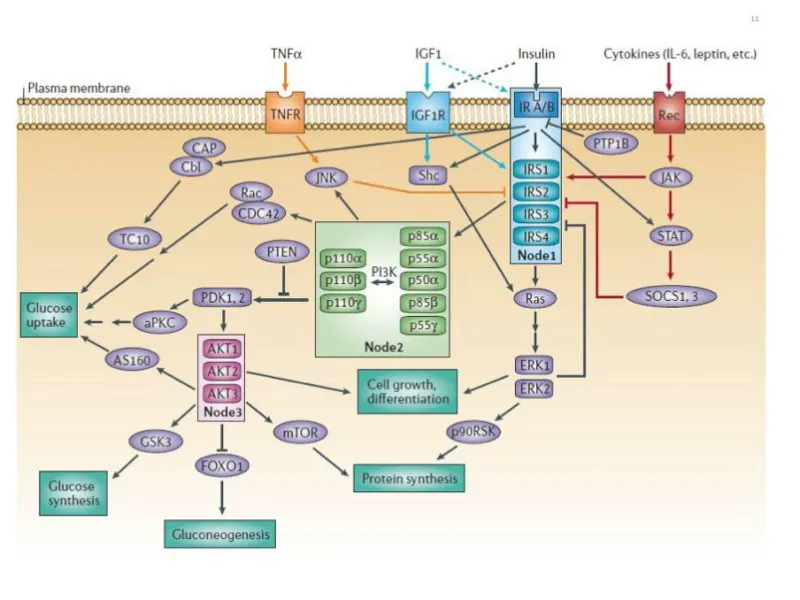
- High-fat diets lead to **increased levels of intracellular fatty acids** and their metabolites, which activate **serine kinases** (e.g., JNK, IKK).
- These serine kinases phosphorylate the **insulin receptor substrate (IRS) proteins** at serine residues, rather than tyrosine residues, thereby **inhibiting insulin signal transduction** and leading to insulin resistance.
*Decreased activation of caspase 8*
- **Caspase 8** is primarily involved in the **extrinsic apoptotic pathway** and is not directly implicated in a decrease in glucose uptake in the context of insulin resistance.
- While prolonged insulin resistance and lipotoxicity can eventually lead to β-cell apoptosis, decreased caspase 8 activation would generally *reduce* apoptosis, not directly cause impaired glucose metabolism in this acute scenario.
*Decreased production of protein kinase A*
- **Protein kinase A (PKA)** is activated by cAMP and plays a role in various metabolic processes, including glucose homeostasis, but its *decreased* production is not the primary mechanism by which a high-lipid diet causes insulin resistance.
- In some contexts, PKA activity can even be involved in counter-regulatory responses to insulin or may play complex roles, but the direct inhibition of insulin signaling via IRS serine phosphorylation is more central to lipid-induced resistance.
*Decreased expression of TP53*
- **TP53** (p53) is a tumor suppressor gene involved in cell cycle arrest, DNA repair, and apoptosis.
- While p53 can indirectly influence metabolism in various cancer-related contexts, its decreased expression is not a direct or primary mechanism for high-lipid diet-induced **insulin resistance** in this setting.
*Increased exposure of nuclear localization signal*
- An **increased exposure of a nuclear localization signal (NLS)** would facilitate the transport of proteins into the nucleus, which is relevant for transcription factors or nuclear processes.
- This is not a direct or well-established mechanism for the development of **insulin resistance** caused by a high-lipid diet, which primarily affects early insulin signaling pathways at the cell membrane and in the cytoplasm.
Tyrosine kinase receptors US Medical PG Question 3: A 30-year-old woman presents to her physician for her annual checkup. She has diabetes mellitus, type 1 and takes insulin regularly. She reports no incidents of elevated or low blood sugar and that she is feeling energetic and ready to face the morning every day. Her vital signs and physical are normal. On the way home from her checkup she stops by the pharmacy and picks up her prescription of insulin. Later that night she takes a dose. What is the signaling mechanism associated with this medication?
- A. Increased concentration intracellular cAMP
- B. Increased permeability of the cell membrane to negatively charged molecules
- C. Activation of tyrosine kinase (Correct Answer)
- D. Rapid and direct upregulation of enzyme transcription
- E. Increased permeability of the cell membrane to positively charged molecules
Tyrosine kinase receptors Explanation: ***Activation of tyrosine kinase***
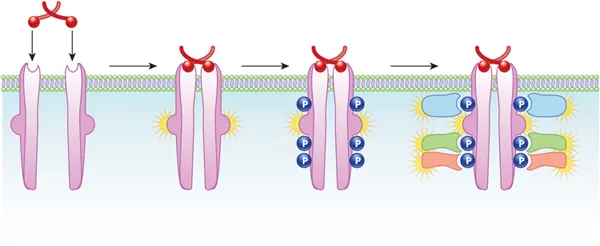
- **Insulin** primarily binds to the **insulin receptor**, which is a **receptor tyrosine kinase**.
- Upon insulin binding, the intrinsic tyrosine kinase activity of the receptor is activated, leading to **autophosphorylation** and phosphorylation of downstream signaling proteins like **IRS-1**.
*Increased concentration intracellular cAMP*
- This mechanism is characteristic of signaling pathways involving **G protein-coupled receptors** that activate adenylyl cyclase, such as those for **glucagon** or **catecholamines**.
- Insulin does not primarily signal through **cAMP** as a second messenger.
*Increased permeability of the cell membrane to negatively charged molecules*
- Changes in membrane permeability to negatively charged molecules are usually associated with **GABAergic** or **glycinergic neurotransmission**, leading to inhibitory postsynaptic potentials.
- This is not a primary mechanism for **insulin signaling**.
*Rapid and direct upregulation of enzyme transcription*
- While insulin does influence gene expression over longer periods, its immediate effects involve **protein phosphorylation** and translocation, not direct, rapid transcriptional changes at the moment of receptor binding.
- Steroid hormones typically mediate more direct transcriptional regulation.
*Increased permeability of the cell membrane to positively charged molecules*
- This mechanism is characteristic of **ligand-gated ion channels** that allow influx of ions like **Na+** or **Ca2+**, important in neuronal excitation or muscle contraction.
- Insulin signaling primarily involves **kinase cascades** and not direct changes in membrane permeability to positive ions.
Tyrosine kinase receptors US Medical PG Question 4: A 58-year-old male undergoes a surveillance colonoscopy in which a 2 cm adenoma is identified and removed. Had this adenoma not been excised, the patient would have been at risk of progression to carcinoma. Which of the following is the final mutational step in the progression from adenoma to carcinoma?
- A. p53 inactivation (Correct Answer)
- B. APC mutation
- C. COX-2 overexpression
- D. SMAD 2/4 loss
- E. K-ras mutation
Tyrosine kinase receptors Explanation: ***p53 inactivation***
- **p53 loss of function** is typically the final genetic event in the **adenoma-to-carcinoma sequence**, facilitating unrestricted cell growth and preventing apoptosis in dysplastic cells.
- The **p53 tumor suppressor gene** normally checkpoints cell division and induces programmed cell death, making its inactivation critical for malignant transformation.
*APC mutation*
- **APC (adenomatous polyposis coli) mutation** is often the **initiating event** in colorectal adenoma formation, leading to aberrant crypt foci and polyp formation.
- While critical for early tumor genesis, it does not represent the final step in progression to invasive carcinoma.
*COX-2 overexpression*
- **Cyclooxygenase-2 (COX-2) overexpression** leads to increased prostaglandin production, which can promote cell proliferation, angiogenesis, and inhibit apoptosis.
- It is an important factor in tumor growth and progression but occurs earlier in the sequence and is not the terminal mutational step for carcinoma.
*SMAD 2/4 loss*
- **SMAD 2/4 loss of function** disrupts the **TGF-β signaling pathway**, which normally inhibits cell growth and promotes differentiation.
- This event typically occurs in the late adenoma stage, contributing to dysplasia, but **p53 inactivation** is considered the final critical step for full malignant transformation.
*K-ras mutation*
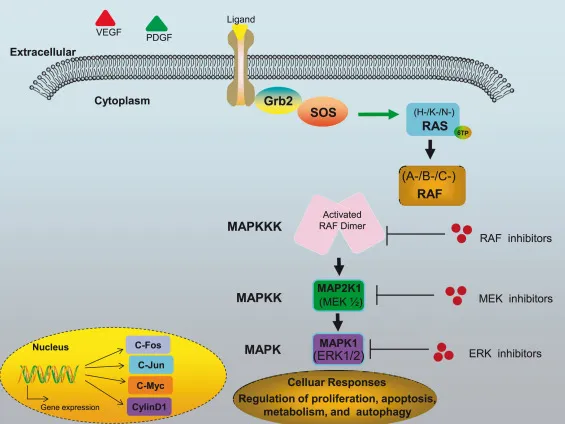
- **K-ras mutation** is a well-known event in the **adenoma-to-carcinoma sequence**, occurring earlier than p53 inactivation, usually in intermediate-sized adenomas.
- It leads to constitutive activation of the RAS/MAPK pathway, promoting cell growth and survival, but generally before full malignant transformation.
Tyrosine kinase receptors US Medical PG Question 5: A 66-year-old man comes to the physician because of a 3-month history of constipation and streaks of blood in his stool. He has had a 10-kg (22-lb) weight loss during this period. Colonoscopy shows an exophytic tumor in the sigmoid colon. A CT scan of the abdomen shows liver metastases and enlarged mesenteric and para-aortic lymph nodes. A diagnosis of stage IV colorectal cancer is made, and palliative chemotherapy is initiated. The chemotherapy regimen includes a monoclonal antibody that inhibits tumor growth by preventing ligand binding to a protein directly responsible for epithelial cell proliferation and organogenesis. Which of the following proteins is most likely inhibited by this drug?
- A. VEGF
- B. TNF-α
- C. EGFR (Correct Answer)
- D. ALK
- E. CD52
Tyrosine kinase receptors Explanation: ***EGFR***
- The description of a monoclonal antibody preventing ligand binding to a protein responsible for **epithelial cell proliferation** and organogenesis strongly points to the **epidermal growth factor receptor (EGFR)**.
- EGFR is highly expressed in many colorectal cancers and its activation by ligands like EGF promotes cell growth, survival, and metastasis. Inhibiting it reduces tumor progression.
*VEGF*
- **Vascular endothelial growth factor (VEGF)** is primarily involved in **angiogenesis**, the formation of new blood vessels.
- While anti-VEGF therapies (e.g., bevacizumab) are used in colorectal cancer, their mechanism is inhibiting blood supply to the tumor, not directly blocking a receptor responsible for epithelial cell proliferation as described.
*TNF-α*
- **Tumor necrosis factor-alpha (TNF-α)** is a **cytokine** primarily involved in inflammation and immune responses.
- Antibodies against TNF-α (e.g., infliximab) are used in inflammatory conditions like Crohn's disease, not typically as targeted therapy for colorectal cancer directly inhibiting epithelial proliferation.
*ALK*
- **Anaplastic lymphoma kinase (ALK)** is a **receptor tyrosine kinase** often implicated in lung cancer and lymphomas.
- ALK rearrangements lead to oncogenic fusion proteins, but it is not a primary target for widespread epithelial cell proliferation in colorectal cancer.
*CD52*
- **CD52** is a glycoprotein found on the surface of various immune cells, including lymphocytes.
- Antibodies targeting CD52 (e.g., alemtuzumab) are used in certain leukemias and lymphomas to deplete these cells, not for inhibiting epithelial cell proliferation in solid tumors.
Tyrosine kinase receptors US Medical PG Question 6: A 16-year-old male presents to the emergency department complaining of episodes of pounding headache, chest fluttering, and excessive sweating. He has a past history of kidney stones that are composed of calcium oxalate. He does not smoke or drink alcohol. Family history reveals that his mother died of thyroid cancer. Vital signs reveal a temperature of 37.1°C (98.7°F), blood pressure of 200/110 mm Hg and pulse of 120/min. His 24-hour urine calcium, serum metanephrines, and serum normetanephrines levels are all elevated. Mutation of which of the following genes is responsible for this patient's condition?
- A. RET proto-oncogene (Correct Answer)
- B. BCL2
- C. BRAF
- D. BCR-ABL
- E. HER-2/neu (C-erbB2)
Tyrosine kinase receptors Explanation: ***RET proto-oncogene***
- The patient's symptoms (pounding headache, chest fluttering, sweating, hypertension, tachycardia), elevated metanephrines, and a history of kidney stones (suggesting **hyperparathyroidism**) combined with a family history of **thyroid cancer** are classic for **Multiple Endocrine Neoplasia type 2A (MEN2A)**.
- **MEN2A** is caused by a germline mutation in the **RET proto-oncogene** and typically involves **medullary thyroid carcinoma**, **pheochromocytoma** (explaining the adrenal symptoms), and **primary hyperparathyroidism**.
*BCL2*
- The **BCL2 gene** is an **anti-apoptotic gene** primarily associated with lymphomas, particularly **follicular lymphoma**, where its overexpression promotes cell survival.
- Mutations or translocations involving BCL2 are not linked to endocrine disorders like MEN2A or the specific combination of symptoms seen in this patient.
*BRAF*
- The **BRAF gene** encodes a protein involved in cell growth signaling and is commonly mutated in various cancers, most notably **melanoma** and **papillary thyroid carcinoma**.
- While associated with thyroid cancer, a BRAF mutation does not explain the pheochromocytoma, hyperparathyroidism, or the specific family history indicative of MEN2A.
*BCR-ABL*
- The **BCR-ABL fusion gene** results from the **Philadelphia chromosome translocation (t(9;22))** and is the hallmark of **chronic myeloid leukemia (CML)** and some cases of acute lymphoblastic leukemia.
- This gene is a potent oncogene in hematopoietic malignancies and has no association with the endocrine tumors or symptoms described in the patient.
*HER-2/neu (C-erbB2)*
- **HER-2/neu (C-erbB2)** is an oncogene that encodes a receptor tyrosine kinase and is primarily associated with **breast cancer** and some **gastric cancers**, where its overexpression indicates a more aggressive tumor and guides targeted therapy.
- This gene is not implicated in the pathogenesis of MEN2A or the constellation of symptoms observed in this patient.
Tyrosine kinase receptors US Medical PG Question 7: A 37-year-old woman, gravida 3, para 2, at 28 weeks' gestation comes to the physician for a follow-up examination. One week ago, an oral glucose tolerance screening test showed elevated serum glucose levels. She has complied with the recommended diet and lifestyle modifications. Over the past week, home blood glucose monitoring showed elevated fasting and post-prandial blood glucose levels. Which of the following describes the mechanism of action of the most appropriate pharmacotherapy for this patient?
- A. Inhibition of alpha-glucosidase
- B. Closure of ATP-dependent K+-channels
- C. Inhibition of dipeptidyl peptidase 4
- D. Activation of peroxisome proliferator-activated receptor-gamma
- E. Binding to receptors with tyrosine kinase activity (Correct Answer)
Tyrosine kinase receptors Explanation: ***Binding to receptors with tyrosine kinase activity***
- This mechanism describes **insulin**, which is the **first-line pharmacotherapy for gestational diabetes mellitus (GDM)** that is not controlled by diet and lifestyle modifications. Insulin binds to its receptor, activating **tyrosine kinase activity** and initiating a cascade of intracellular signaling that promotes glucose uptake and utilization.
- Given the **elevated fasting and post-prandial blood glucose levels** despite dietary changes, insulin therapy is indicated to manage GDM, which relies on this mechanism of action.
*Inhibition of alpha-glucosidase*
- **Alpha-glucosidase inhibitors** (e.g., acarbose, miglitol) delay carbohydrate absorption in the gut, primarily targeting post-prandial hyperglycemia.
- While these can be used in some forms of diabetes, they are generally **not preferred for GDM due to potential gastrointestinal side effects** and less effective control over fasting hyperglycemia compared to insulin.
*Closure of ATP-dependent K+-channels*
- Drugs like **sulfonylureas** (e.g., glyburide) act by **closing ATP-dependent K+-channels** on pancreatic beta cells, leading to depolarization and insulin release.
- **Glyburide** can be used in GDM but is a controversial choice due to its potential to **cross the placenta** and cause fetal hypoglycemia.
*Inhibition of dipeptidyl peptidase 4*
- **Dipeptidyl peptidase-4 (DPP-4) inhibitors** (e.g., sitagliptin, saxagliptin) enhance the action of incretin hormones, stimulating glucose-dependent insulin release and suppressing glucagon secretion.
- There is **limited data on the safety and efficacy of DPP-4 inhibitors in pregnancy**, and they are not typically recommended for GDM.
*Activation of peroxisome proliferator-activated receptor-gamma*
- This mechanism describes **thiazolidinediones (TZDs)**, such as pioglitazone and rosiglitazone, which improve insulin sensitivity.
- **TZDs are contraindicated in pregnancy** due to potential adverse effects on the fetus, including increased risk of cardiovascular defects and fluid retention.
Tyrosine kinase receptors US Medical PG Question 8: A 55-year-old male presents with complaints of intermittent facial flushing. He also reports feeling itchy after showering. On review of systems, the patient says he has been having new onset headaches recently. On physical exam, his vital signs, including O2 saturation, are normal. He has an abnormal abdominal mass palpable in the left upper quadrant. A complete blood count reveals: WBCs 6500/microliter; Hgb 18.2 g/dL; Platelets 385,000/microliter. Which of the following is most likely responsible for his presentation?
- A. BCR-ABL fusion
- B. Chronic hypoxemia
- C. Tyrosine kinase mutation (Correct Answer)
- D. Fibrosis of bone marrow
- E. Elevated serum erythropoietin levels
Tyrosine kinase receptors Explanation: ***Tyrosine kinase mutation***
- The patient's symptoms (facial flushing, post-showering pruritus, headaches) along with **elevated hemoglobin** (18.2 g/dL) and **splenomegaly** (abdominal mass in LUQ) strongly suggest **Polycythemia Vera (PV)**.
- PV is a **myeloproliferative neoplasm** typically caused by a **JAK2 V617F mutation**, which is a type of **tyrosine kinase mutation**, leading to constitutive activation of the JAK-STAT pathway and uncontrolled erythropoiesis.
*BCR-ABL fusion*
- A **BCR-ABL fusion gene** is characteristic of **Chronic Myeloid Leukemia (CML)**, which typically presents with markedly elevated WBCs and splenomegaly.
- While splenomegaly is present here, the patient's symptoms and hematologic findings (elevated Hgb, normal WBCs) are not consistent with CML.
*Chronic hypoxemia*
- **Chronic hypoxemia** can cause **secondary erythrocytosis** due to increased erythropoietin production in response to low oxygen levels.
- However, the patient's **O2 saturation is normal**, ruling out chronic hypoxemia as the cause of his elevated hemoglobin.
*Fibrosis of bone marrow*
- **Bone marrow fibrosis** is a hallmark of **Primary Myelofibrosis**, another myeloproliferative neoplasm, which typically presents with anemia, marked splenomegaly, and teardrop cells on peripheral smear.
- While it can manifest with constitutional symptoms, the patient's **high hemoglobin** and absent anemia make primary myelofibrosis less likely.
*Elevated serum erythropoietin levels*
- **Elevated serum erythropoietin (EPO) levels** are characteristic of **secondary erythrocytosis**, where EPO production increases due to conditions like hypoxemia, renal tumors, or exogenous EPO use.
- In **Polycythemia Vera**, EPO levels are typically **low or undetectable** due to feedback inhibition from the high red blood cell mass.
Tyrosine kinase receptors US Medical PG Question 9: A 34-year-old man comes to the physician because of blurry vision and fatigue for 2 months. During this period, he has also had occasional bleeding from his gums after brushing his teeth. One month ago, he was diagnosed with deep vein thrombosis after returning from an overseas business meeting. His pulse is 118/min, respirations are 19/min, and blood pressure is 149/91 mm Hg. Pulse oximetry on room air shows an oxygen saturation of 97%. Examination shows bluish discoloration of the lips. The tip of the spleen is palpable 1 cm below the left costal margin. Sensory examination of the hands shows paresthesia. Hemoglobin concentration is 18 g/dL, hematocrit is 65%, leukocytes are 15,000/μL, and platelets are 470,000/μL. His serum erythropoietin concentration is decreased. Activation of which of the following is the most likely underlying cause of this patient's condition?
- A. Serine/threonine kinase
- B. Antiapoptotic molecule
- C. Nonreceptor tyrosine kinase (Correct Answer)
- D. Transcription factor
- E. Cytokine receptor
Tyrosine kinase receptors Explanation: ***Nonreceptor tyrosine kinase***
- This patient's symptoms (blurry vision, fatigue, gum bleeding, deep vein thrombosis, splenomegaly, **elevated hemoglobin, hematocrit, leukocytes, and platelets**, and **decreased erythropoietin**) are highly suggestive of **polycythemia vera**.
- Polycythemia vera is a myeloproliferative neoplasm characterized by a mutation in the **JAK2 gene**, which encodes a **nonreceptor tyrosine kinase**. This mutation leads to constitutive activation of the JAK-STAT pathway, resulting in uncontrolled proliferation of myeloid cells independent of growth factors.
*Serine/threonine kinase*
- While serine/threonine kinases are involved in various cellular signaling pathways, their constitutive activation is not the primary underlying cause of polycythemia vera.
- Mutations in serine/threonine kinases are more commonly associated with other conditions, such as certain cancers, but not specifically with the **JAK2 V617F mutation** characteristic of PV.
*Antiapoptotic molecule*
- Activation of antiapoptotic molecules plays a role in the survival of cancer cells, but it is a downstream effect rather than the primary initiating event in polycythemia vera.
- The **JAK2 mutation** leads to increased cell proliferation and reduced apoptosis indirectly by enhancing survival signals.
*Transcription factor*
- Transcription factors regulate gene expression, and their dysregulation can contribute to various diseases, including cancers. However, the direct activation of a transcription factor is not the root cause of polycythemia vera.
- The **JAK-STAT pathway** ultimately affects transcription factors, but the initial genetic defect is in the JAK2 kinase.
*Cytokine receptor*
- Cytokine receptors bind cytokines and initiate signaling cascades, often involving JAK kinases. While cytokine receptor signaling is hyperactive in polycythemia vera, the primary defect is not in the receptor itself but in the downstream **JAK2 kinase**.
- The **JAK2 V617F mutation** causes **cytokine-independent activation** of the signaling pathway, meaning the cells don't need external cytokines to proliferate.
Tyrosine kinase receptors US Medical PG Question 10: An investigator is conducting an experiment to study different pathways of glucose metabolism. He obtains cells cultured from various tissues to study the effect of increased extracellular glucose concentration. Following the incubation of these cells in 5% dextrose, he measures the intracellular fructose concentration. The concentration of fructose is expected to be highest in cells obtained from which of the following tissues?
- A. Ovary
- B. Retina
- C. Myelin sheath
- D. Kidney
- E. Lens (Correct Answer)
Tyrosine kinase receptors Explanation: ***Lens***
- The **lens** is rich in the enzyme **aldose reductase**, which converts glucose to sorbitol, and then **sorbitol dehydrogenase** converts sorbitol to fructose via the **polyol pathway**.
- In a high-glucose environment, this pathway becomes highly active in the lens, leading to an increased production and accumulation of **fructose**, which can contribute to osmotic stress and cataract formation.
*Ovary*
- While other reproductive tissues can metabolize glucose, the **ovary** is not a primary site for significant fructose accumulation through the **polyol pathway** in response to elevated glucose.
- Its metabolic activity is more geared towards steroidogenesis and oocyte development rather than high fructose production from glucose.
*Retina*
- The **retina** contains some aldose reductase activity, and increased glucose can activate the **polyol pathway**, leading to sorbitol and fructose accumulation.
- However, the lens typically shows a more pronounced increase in fructose concentration due to its higher metabolic flux through this pathway and its susceptibility to osmotic damage.
*Myelin sheath*
- The **myelin sheath**, primarily composed of lipids, is part of the nervous system and is not known for significant **fructose production** via the **polyol pathway** in response to high glucose.
- Damage to myelin in diabetic conditions is often linked to other mechanisms like glycation and oxidative stress rather than direct fructose accumulation.
*Kidney*
- The **kidney** can utilize the **polyol pathway**, and conditions like **diabetic nephropathy** involve increased sorbitol and fructose production.
- However, the magnitude of fructose accumulation and its direct pathogenic role differ from that in the lens, where osmotic effects of polyols are particularly critical.
More Tyrosine kinase receptors US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.