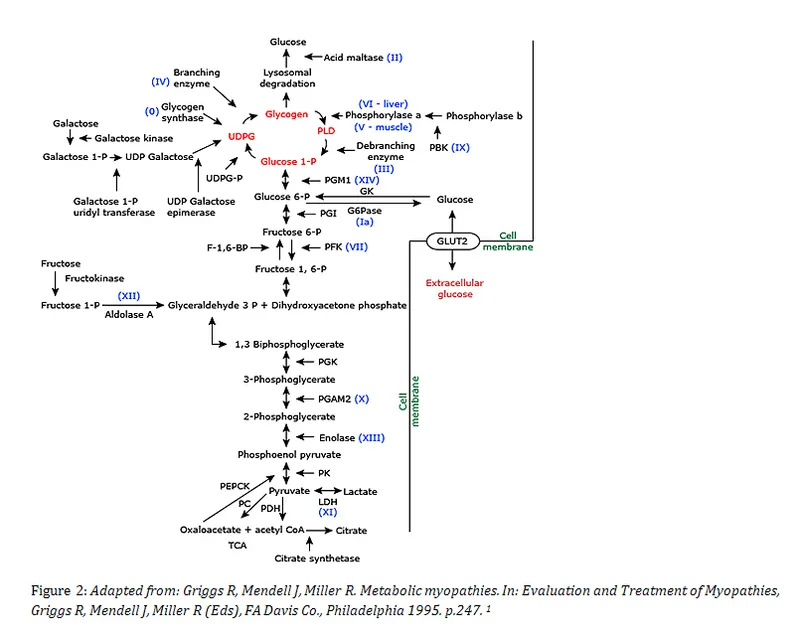
Glycogen metabolism US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Glycogen metabolism. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Glycogen metabolism US Medical PG Question 1: A 45-year-old man is brought to the emergency department by ambulance after vomiting blood. The patient reports that he only ate a small snack the morning before and had not eaten anything for over 24 hours. At the hospital, the patient is stabilized. He is admitted to a surgical floor and placed on NPO with a nasogastric tube set to intermittent suction. He has been previously diagnosed with liver cirrhosis. An esophagogastroduodenoscopy (EGD) has been planned for the next afternoon. At the time of endoscopy, some pathways were generating glucose to maintain serum glucose levels. Which of the following enzymes catalyzes the irreversible biochemical reaction of this process?
- A. Glucose-6-phosphate dehydrogenase
- B. Glycogen phosphorylase
- C. Enolase
- D. Glyceraldehyde-3-phosphate dehydrogenase
- E. Fructose-1,6-bisphosphatase (Correct Answer)
Glycogen metabolism Explanation: ***Fructose-1,6-bisphosphatase***
- The scenario describes a patient in a fasting state for over 24 hours, during which **gluconeogenesis** is crucial for maintaining blood glucose levels.
- **Fructose-1,6-bisphosphatase** is one of the key regulatory enzymes in gluconeogenesis, catalyzing an **irreversible reaction** that bypasses the phosphofructokinase-1 step of glycolysis.
*Glucose-6-phosphate dehydrogenase*
- This enzyme is involved in the **pentose phosphate pathway**, which generates NADPH and precursors for nucleotide synthesis.
- It does not directly participate in gluconeogenesis to produce glucose from non-carbohydrate sources.
*Glycogen phosphorylase*
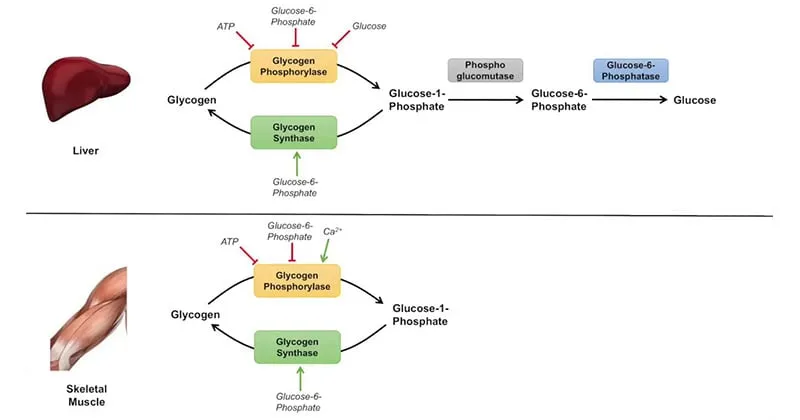
- This enzyme is involved in **glycogenolysis**, the breakdown of glycogen into glucose-1-phosphate.
- While it releases glucose, the body's glycogen stores would likely be depleted after over 24 hours of fasting, making gluconeogenesis the primary pathway for glucose production.
*Enolase*
- Enolase is an enzyme in the glycolytic pathway, catalyzing the reversible conversion of 2-phosphoglycerate to phosphoenolpyruvate.
- It is not an enzyme of gluconeogenesis, nor does it catalyze an irreversible step in the glucose production process during fasting.
*Glyceraldehyde-3-phosphate dehydrogenase*
- This enzyme is also part of glycolysis, catalyzing the reversible oxidation and phosphorylation of glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate.
- Like enolase, it is not an irreversible enzyme in gluconeogenesis that would be generating glucose under fasting conditions.
Glycogen metabolism US Medical PG Question 2: A 24-year-old man presents for an annual check-up. He is a bodybuilder and tells you he is on a protein-rich diet that only allows for minimal carbohydrate intake. His friend suggests he try exogenous glucagon to help him lose some excess weight before an upcoming competition. Which of the following effects of glucagon is he attempting to exploit?
- A. Increased glucose utilization by tissues
- B. Decreased blood cholesterol level
- C. Increased hepatic gluconeogenesis
- D. Increased lipolysis in adipose tissues (Correct Answer)
- E. Increased hepatic glycogenolysis
Glycogen metabolism Explanation: ***Increased lipolysis in adipose tissues***
- While **glucagon's primary target is the liver**, it can have **modest lipolytic effects** on adipose tissue by opposing insulin's anti-lipolytic actions.
- Glucagon stimulates cAMP production, which can activate **hormone-sensitive lipase** to break down triglycerides into **fatty acids** and **glycerol**.
- However, **catecholamines (epinephrine/norepinephrine)** are far more potent direct stimulators of adipose tissue lipolysis than glucagon.
- The friend is attempting to exploit this lipolytic effect for fat loss, though **exogenous glucagon is not an evidence-based or safe weight-loss strategy**.
*Increased glucose utilization by tissues*
- This is **opposite** to glucagon's actual effect. **Glucagon raises blood glucose** levels; it does not promote glucose uptake by peripheral tissues.
- **Insulin** is the hormone responsible for promoting glucose uptake and utilization by muscle, adipose, and other tissues.
*Decreased blood cholesterol level*
- Glucagon does not have a direct, clinically significant effect on reducing blood cholesterol levels.
- While glucagon affects overall lipid metabolism through its catabolic actions, it is not used therapeutically for hypercholesterolemia.
*Increased hepatic gluconeogenesis*
- **Glucagon strongly stimulates hepatic gluconeogenesis**, which is the synthesis of glucose from non-carbohydrate precursors (amino acids, lactate, glycerol) in the liver.
- This action **raises blood glucose** levels and would not directly contribute to fat loss or weight reduction.
- In the context of a low-carbohydrate diet, increased gluconeogenesis would maintain blood glucose but not promote the fat loss the bodybuilder seeks.
*Increased hepatic glycogenolysis*
- **Glucagon is a potent stimulator of hepatic glycogenolysis**, the breakdown of stored liver glycogen into glucose.
- This rapidly increases blood glucose levels during fasting or hypoglycemia.
- However, this does not directly target adipose tissue for fat loss; it mobilizes glucose stores rather than fat stores, so it's not the mechanism relevant to weight loss goals.
Glycogen metabolism US Medical PG Question 3: A 65-year-old male prisoner goes on a hunger strike to protest the conditions of his detainment. After 5 days without food, he suffers a seizure for which he is taken into a medical facility. On physical examination, he looks pale and diaphoretic. His blood glucose level is 50 mg/dL. In order to keep a constant supply of energy to his brain, which of the following molecules is his liver releasing into the bloodstream?
- A. Glycogen
- B. Glucose-6-phosphate
- C. ß-hydroxybutyric acid (Correct Answer)
- D. Fatty acids
- E. Glucose-1-phosphate
Glycogen metabolism Explanation: ***ß-hydroxybutyric acid***
- After 5 days of a hunger strike, **glycogen stores** are depleted, forcing the body to rely on **fatty acid oxidation** and **ketone body production** in the liver as an alternative fuel source for the brain.
- **ß-hydroxybutyrate** is one of the primary ketone bodies released by the liver into the bloodstream to provide energy, especially for the brain, during prolonged fasting.
*Glycogen*
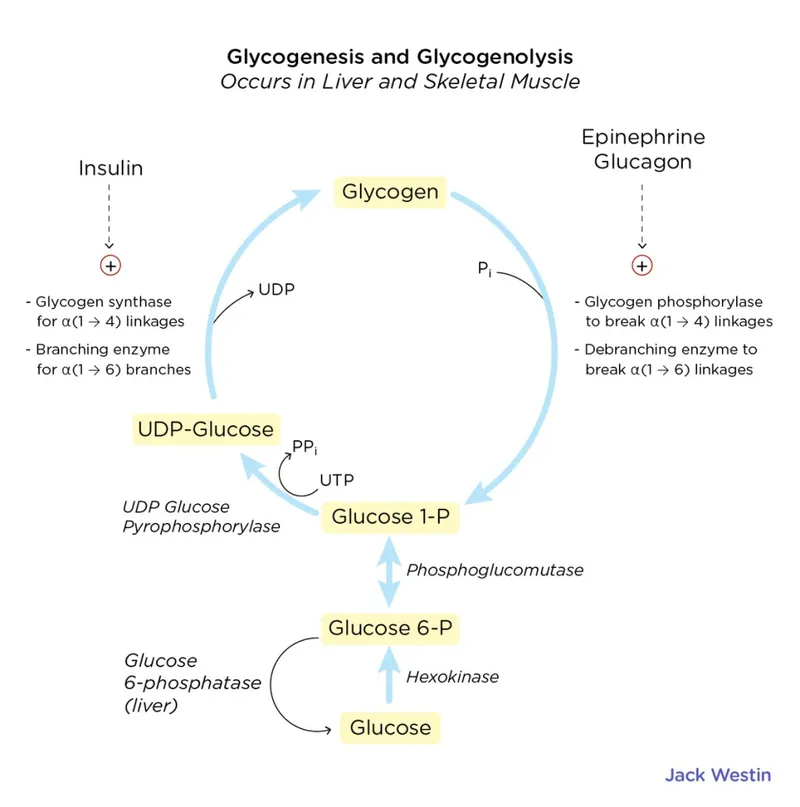
- **Glycogenolysis** (breakdown of glycogen) is a short-term response to low blood glucose and supplies glucose for only about 24-36 hours of fasting. After 5 days, **hepatic glycogen stores** would be largely depleted.
- The liver releases **free glucose** into the bloodstream, not intact glycogen, from glycogen breakdown.
*Glucose-6-phosphate*
- **Glucose-6-phosphate** is an intermediate in glycolysis and gluconeogenesis, but it is not directly released into the bloodstream by the liver.
- It must be converted to **free glucose** by glucose-6-phosphatase before it can exit the hepatocyte and enter circulation.
*Fatty acids*
- The liver takes up **fatty acids** from adipose tissue breakdown during prolonged fasting to convert them into **ketone bodies**.
- While fatty acids are a major energy source for other tissues, the **brain cannot directly utilize fatty acids** for energy due to the inability of long-chain fatty acids to cross the blood-brain barrier.
*Glucose-1-phosphate*
- **Glucose-1-phosphate** is an intermediate formed during the breakdown of glycogen (glycogenolysis).
- Like glucose-6-phosphate, it is not directly released into the bloodstream but is further metabolized within the hepatocyte, eventually leading to the release of **free glucose**.
Glycogen metabolism US Medical PG Question 4: A 6-month-old boy is referred to a geneticist after he is found to have persistent hypotonia and failure to thrive. He has also had episodes of what appears to be respiratory distress and has an enlarged heart on physical exam. There is a family history of childhood onset hypertrophic cardiomyopathy, so a biopsy is performed showing electron dense granules within the lysosomes. Genetic testing is performed showing a defect in glycogen processing. A deficiency in which of the following enzymes is most likely to be responsible for this patient's symptoms?
- A. Lysosomal alpha 1,4-glucosidase (Correct Answer)
- B. Branching enzyme
- C. Muscle phosphorylase
- D. Debranching enzyme
- E. Glucose-6-phosphatase
Glycogen metabolism Explanation: ***Lysosomal alpha 1,4-glucosidase***
- The constellation of **hypotonia**, **failure to thrive**, **respiratory distress**, and **cardiomegaly** in an infant, along with **electron-dense granules in lysosomes** and a defect in **glycogen processing**, is characteristic of **Pompe disease (Type II glycogen storage disease)**.
- **Pompe disease** is caused by a deficiency of **lysosomal alpha 1,4-glucosidase** (also known as acid maltase), which is responsible for breaking down glycogen in lysosomes.
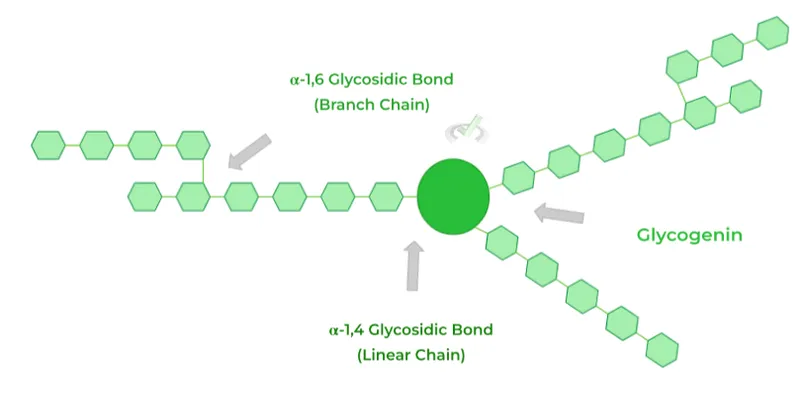
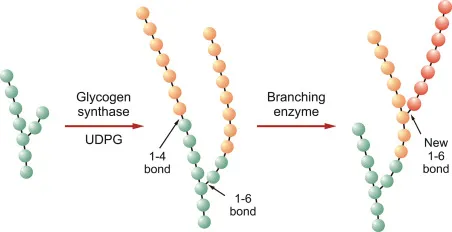
*Branching enzyme*
- A deficiency in **branching enzyme (amylo-alpha-1,4-to-alpha-1,6-transglucosidase)** causes **Andersen disease (Type IV glycogen storage disease)**, which typically presents with **hepatosplenomegaly**, **cirrhosis**, and **failure to thrive**.
- While it involves glycogenopathy, the specific features of **cardiomyopathy** and **lysosomal accumulation** are not primary to this disorder.
*Muscle phosphorylase*
- A deficiency in **muscle phosphorylase** causes **McArdle disease (Type V glycogen storage disease)**, which primarily affects **skeletal muscle**.
- Symptoms include **exercise intolerance**, **muscle cramps**, and **myoglobinuria**, typically presenting later in childhood or adolescence, and does not involve cardiomyopathy or lysosomal storage.
*Debranching enzyme*
- A deficiency in **debranching enzyme (alpha-1,6-glucosidase)** causes **Cori disease (Type III glycogen storage disease)**, which presents with **hepatomegaly**, **hypoglycemia**, and **muscle weakness**.
- While it can sometimes involve a milder form of cardiomyopathy, the significant **lysosomal involvement** and severe infantile onset with respiratory distress and profound hypotonia point away from Cori disease.
*Glucose-6-phosphatase*
- A deficiency in **glucose-6-phosphatase** causes **Von Gierke disease (Type I glycogen storage disease)**, characterized by **severe fasting hypoglycemia**, **lactic acidosis**, **hepatomegaly**, and **hyperlipidemia**.
- This condition primarily affects the liver and kidneys, and typically does not present with primary cardiomyopathy, hypotonia, or lysosomal glycogen accumulation.
Glycogen metabolism US Medical PG Question 5: The balance between glycolysis and gluconeogenesis is regulated at several steps, and accumulation of one or more products/chemicals can either promote or inhibit one or more enzymes in either pathway. Which of the following molecules if increased in concentration can promote gluconeogenesis?
- A. ADP
- B. Acetyl-CoA (Correct Answer)
- C. AMP
- D. Fructose-2,6-bisphosphate
- E. Insulin
Glycogen metabolism Explanation: ***Acetyl-CoA***
- **Acetyl-CoA** promotes gluconeogenesis by activating **pyruvate carboxylase**, the enzyme that converts pyruvate to oxaloacetate, effectively pushing the pathway forward.
- High levels of **Acetyl-CoA** generally signal a state of abundant energy from fatty acid oxidation, indicating that glucose is not immediately needed for energy and can be synthesized for storage or use elsewhere.
*ADP*
- **ADP** is a key indicator of low cellular energy and **stimulates** glycolysis while **inhibiting** gluconeogenesis to produce ATP.
- Its presence signals a need for energy synthesis rather than glucose production.
*AMP*
- **AMP** also signals low energy status and is a powerful **allosteric activator** of **phosphofructokinase-1 (PFK-1)**, the rate-limiting enzyme in glycolysis.
- Activates **AMP-activated protein kinase (AMPK)**, which promotes catabolic processes like glycolysis and inhibits anabolic processes like gluconeogenesis.
*Fructose-2,6-bisphosphate*
- **Fructose-2,6-bisphosphate** is a potent **allosteric activator** of **PFK-1** in glycolysis and a strong **inhibitor** of **fructose-1,6-bisphosphatase** in gluconeogenesis.
- Its levels increase in response to insulin, promoting glucose utilization and inhibiting glucose production.
*Insulin*
- **Insulin** is a hormone that **promotes glucose uptake** and utilization by tissues and **inhibits gluconeogenesis**.
- It achieves this by activating enzymes involved in glycolysis and glycogen synthesis while inhibiting key enzymes in gluconeogenesis, such as **fructose-1,6-bisphosphatase**.
Glycogen metabolism US Medical PG Question 6: A 12-year-old girl comes to the clinic with a grossly enlarged abdomen. She has a history of frequent episodes of weakness, sweating, and pallor that are eliminated by eating. Her development has been slow. She started to walk unassisted at 2 years and was not performing well at school. Physical examination reveals a blood pressure of 100/60 mm Hg, heart rate of 80/min, and temperature of 36.9°C (98.4℉). On physical examination, the liver is enlarged, firm, and palpable up to the pelvis. The spleen and kidney are not palpable. Laboratory investigation reveals low blood glucose and pH with high lactate, triglycerides, ketones, and free fatty acids. The liver biopsy revealed high glycogen content. Hepatic glycogen structure was normal. The enzyme assay performed on the biopsy tissue revealed very low glucose-6-phosphatase levels. What is the most likely diagnosis?
- A. Pompe's disease
- B. Cori's disease
- C. Hereditary hemochromatosis
- D. Von-Gierke's disease (Correct Answer)
- E. McArdle disease
Glycogen metabolism Explanation: ***Von-Gierke's disease***
- The combination of **hepatomegaly**, **hypoglycemia** (causing weakness, sweating, pallor), **lactic acidosis**, **hyperlipidemia**, and elevated ketones points to a severe defect in glucose metabolism.
- **Very low glucose-6-phosphatase levels** on liver biopsy and normal hepatic glycogen structure are pathognomonic for Von-Gierke's disease (Glycogen Storage Disease Type I).
*Pompe's disease*
- This is a **lysosomal storage disease** affecting **alpha-1,4-glucosidase**, leading to glycogen accumulation in lysosomes.
- It primarily affects the **heart** and skeletal muscles and would not present with severe lactic acidosis and hyperlipidemia.
*Cori's disease*
- This is **Glycogen Storage Disease Type III**, caused by a deficiency in the **debranching enzyme** (amylo-alpha-1,6-glucosidase).
- While it can cause hepatomegaly and hypoglycemia, the hepatic glycogen structure would be abnormal due to incompletely debranched glycogen, and glucose-6-phosphatase levels would be normal.
*Hereditary hemochromatosis*
- This is an **iron overload disorder** leading to iron deposition in organs like the liver, heart, and pancreas.
- It would present with symptoms related to organ damage from iron accumulation, such as liver cirrhosis and diabetes, not the metabolic derangements seen here.
*McArdle disease*
- This is **Glycogen Storage Disease Type V**, due to a deficiency in **muscle glycogen phosphorylase**.
- It primarily causes exercise-induced muscle pain, cramping, and fatigue due to an inability to break down muscle glycogen for energy, not systemic metabolic disturbances or hepatomegaly.
Glycogen metabolism US Medical PG Question 7: A 5-month-old boy presents with increasing weakness for the past 3 months. The patient’s mother says that the weakness is accompanied by dizziness, sweating, and vertigo early in the morning. Physical examination shows hepatomegaly. Laboratory findings show an increased amount of lactate, uric acid, and elevated triglyceride levels. Which of the following enzymes is most likely deficient in this patient?
- A. Hepatic glycogen phosphorylase
- B. Debranching enzyme
- C. Glucose-6-phosphatase (Correct Answer)
- D. Muscle glycogen phosphorylase
- E. Lysosomal α-1,4-glucosidase
Glycogen metabolism Explanation: ***Glucose-6-phosphatase***
- The constellation of **hypoglycemia** (weakness, dizziness, sweating, vertigo, especially early morning), **hepatomegaly**, **lactic acidosis**, **hyperuricemia**, and **hypertriglyceridemia** are classic features of **Type I glycogen storage disease (von Gierke disease)**, which is caused by a deficiency of **glucose-6-phosphatase**.
- This enzyme is crucial for the final step of both **glycogenolysis** and **gluconeogenesis**, releasing free glucose into the bloodstream; its deficiency leads to an inability to maintain normal blood glucose levels during fasting and accumulation of glucose-6-phosphate, which shunts into other metabolic pathways.
*Hepatic glycogen phosphorylase*
- Deficiency in **hepatic glycogen phosphorylase** (Type VI glycogen storage disease, Hers disease) would cause **hepatomegaly** and **hypoglycemia**, but typically does not present with severe **lactic acidosis**, **hyperuricemia**, or **hypertriglyceridemia** to the same degree as von Gierke disease.
- The primary defect is in breaking down glycogen, leading to its accumulation in the liver, but the products of glycolysis can still exit the liver via gluconeogenesis.
*Debranching enzyme*
- Deficiency in **debranching enzyme** (Type III glycogen storage disease, Cori or Forbes disease) causes **hepatomegaly** and **hypoglycemia**, but usually presents with milder symptoms and less severe **lactic acidosis**, **hyperuricemia**, and **hypertriglyceridemia**.
- Patients often present with symptoms similar to Type I, but muscle involvement is also common, and **glycogen structures with short outer branches** are characteristic.
*Muscle glycogen phosphorylase*
- Deficiency in **muscle glycogen phosphorylase** (Type V glycogen storage disease, McArdle disease) primarily affects **skeletal muscle**, leading to exercise intolerance, muscle pain, and myoglobinuria.
- It does not typically cause **hypoglycemia** or **hepatomegaly**, as the liver enzyme is functional, and the symptoms described are systemic rather than muscle-specific.
*Lysosomal α-1,4-glucosidase*
- Deficiency in **lysosomal α-1,4-glucosidase** (Type II glycogen storage disease, Pompe disease) primarily affects the **heart, muscle, and liver**, causing severe **cardiomyopathy**, hypotonia, and **hepatomegaly**.
- While it involves glycogen accumulation, it typically does not present with **hypoglycemia** (as cytoplasmic glycogen metabolism is intact), **lactic acidosis**, or the specific metabolic derangements seen in this patient.
Glycogen metabolism US Medical PG Question 8: A 2-month-old boy is brought to the emergency department 25 minutes after having a seizure. He has had multiple seizures during the past week. His mother has noticed that he has become lethargic and has had a weak cry for the past month. He was born at 37 weeks' gestation. He is at the 20th percentile for height and 15th percentile for weight. His temperature is 36.7°C (98°F), respirations are 50/min, and pulse is 140/min. Examination shows a soft and nontender abdomen. The liver is palpated 4 cm below the right costal margin; there is no splenomegaly. Serum studies show:
Na+ 137 mEq/L
Cl- 103 mEq/L
K+ 3.9 mEq/L
Glucose 32 mg/dL
Calcium 9.6 mg/dL
Total cholesterol 202 mg/dL
Triglycerides 260 mg/dL
Lactate 4.2 mEq/L (N = 0.5 - 2.2 mEq/L)
A deficiency of which of the following enzymes is the most likely cause of this infant's symptoms?
- A. Galactose 1-phosphate uridyltransferase
- B. Glycogen branching enzyme
- C. Glucose 6-phosphatase (Correct Answer)
- D. Fructokinase
- E. Acid maltase
Glycogen metabolism Explanation: ***Glucose 6-phosphatase***
- The constellation of **hypoglycemia**, **lactic acidosis**, **hepatomegaly**, and **hyperlipidemia** in an infant is characteristic of **Type I glycogen storage disease (von Gierke's disease)**, which is caused by a deficiency of glucose 6-phosphatase.
- Seizures and lethargy are common manifestations of severe hypoglycemia in infants.
*Galactose 1-phosphate uridyltransferase*
- Deficiency of this enzyme causes **classic galactosemia**, which typically presents with **jaundice**, **cataracts**, **vomiting**, and **failure to thrive**, usually after initiation of milk feeds.
- While patients can develop hepatomegaly and hypoglycemia, the prominent lactic acidosis and hyperlipidemia seen here are less typical.
*Glycogen branching enzyme*
- Deficiency causes **Type IV glycogen storage disease (Andersen's disease)**, characterized by **hepatosplenomegaly**, **failure to thrive**, and progressive cirrhosis.
- Hypoglycemia is generally less severe, and lactic acidosis and hyperlipidemia are not primary features in the same way as Type I GSD.
*Fructokinase*
- Deficiency causes **essential fructosuria**, a benign condition where fructose accumulates in the urine.
- It is typically asymptomatic and does not lead to hypoglycemia, lactic acidosis, or hepatomegaly.
*Acid maltase*
- Deficiency (alpha-1,4-glucosidase) causes **Type II glycogen storage disease (Pompe's disease)**, which primarily affects skeletal and cardiac muscle.
- The infantile form presents with **severe hypotonia** ("floppy baby"), **cardiomyopathy**, and **macroglossia**. Hepatomegaly, hypoglycemia, and lactic acidosis are not prominent features.
Glycogen metabolism US Medical PG Question 9: A 9-month-old girl is brought to the physician because of a 1-month history of poor feeding and irritability. She is at the 15th percentile for height and 5th percentile for weight. Examination shows hypotonia and wasting of skeletal muscles. Cardiopulmonary examination shows no abnormalities. There is hepatomegaly. Her serum glucose is 61 mg/dL, creatinine kinase is 100 U/L, and lactic acid is within the reference range. Urine ketone bodies are elevated. Which of the following enzymes is most likely deficient in this patient?
- A. Glucose-6-phosphatase
- B. Muscle phosphorylase
- C. Acid alpha-glucosidase
- D. Glycogen debrancher (Correct Answer)
- E. Glucocerebrosidase
Glycogen metabolism Explanation: ***Glycogen debrancher***
- The patient's symptoms of **hepatomegaly**, **hypoglycemia**, **poor feeding**, **growth failure**, and **elevated urine ketones** in the presence of normal lactic acid suggest Type III glycogen storage disease (Cori disease), caused by a deficiency in **glycogen debrancher enzyme**.
- **Muscle wasting** and **hypotonia** are also consistent with Type III GSD, as the debranching enzyme is present in both liver and muscle.
*Glucose-6-phosphatase*
- Deficiency in **glucose-6-phosphatase** (Type I GSD, Von Gierke disease) also presents with **hepatomegaly** and **hypoglycemia**.
- However, Type I GSD is characterized by **lactic acidosis**, which is explicitly stated as normal in this patient, and **hyperlipidemia**, which is not mentioned.
*Muscle phosphorylase*
- Deficiency in **muscle phosphorylase** (Type V GSD, McArdle disease) primarily affects skeletal muscle, causing **exercise intolerance** and **muscle pain**.
- It does not typically present with **hypoglycemia**, **hepatomegaly**, or **growth failure** in infancy.
*Acid alpha-glucosidase*
- Deficiency in **acid alpha-glucosidase** (Type II GSD, Pompe disease) causes accumulation of glycogen in lysosomes, leading to severe **cardiomyopathy**, **hypotonia**, and **muscle weakness**.
- While hypotonia is present, the absence of **cardiomegaly** and significant **liver involvement** makes this diagnosis less likely.
*Glucocerebrosidase*
- Deficiency in **glucocerebrosidase** causes Gaucher disease, a lysosomal storage disorder, not a glycogen storage disorder.
- Symptoms include **hepatosplenomegaly**, **bone crises**, and neurological symptoms, but not **hypoglycemia** or isolated muscle wasting directly related to glycogen metabolism.
Glycogen metabolism US Medical PG Question 10: A 38-year-old man presents to his physician with double vision persisting for a week. When he enters the exam room, the physician notes that the patient has a broad-based gait. The man’s wife informs the doctor that he has been an alcoholic for the last 5 years and his consumption of alcohol has increased significantly over the past few months. She also reports that he has become indifferent to his family members over time and is frequently agitated. She also says that his memory has been affected significantly, and when asked about a particular detail, he often recollects it incorrectly, though he insists that his version is the true one. On physical examination, his vital signs are stable, but when the doctor asks him where he is, he seems to be confused. His neurological examination also shows nystagmus. Which of the following options describes the earliest change in the pathophysiology of the central nervous system in this man?
- A. Increased astrocyte lactate
- B. Increased extracellular concentration of glutamate
- C. Increased fragmentation of deoxyribonucleic acid within the neurons
- D. Breakdown of the blood-brain barrier
- E. Decreased α-ketoglutarate dehydrogenase activity in astrocytes (Correct Answer)
Glycogen metabolism Explanation: ***Decreased α-ketoglutarate dehydrogenase activity in astrocytes***
- Chronic **alcoholism** leads to **thiamine deficiency**, which impairs the activity of **α-ketoglutarate dehydrogenase** in the **Krebs cycle**.
- This enzyme is crucial for **neuronal energy metabolism** and its deficiency contributes to the earliest **neuropathological changes** observed in Wernicke-Korsakoff syndrome.
*Increased astrocyte lactate*
- While chronic alcoholism and thiamine deficiency can lead to metabolic dysfunction, increased **astrocyte lactate** is typically a response to **hypoxia** or **ischemia**, not the primary initiating event of thiamine deficiency.
- Lactate accumulation can be a downstream effect of impaired glucose metabolism, but not the earliest or most direct consequence of **α-ketoglutarate dehydrogenase** inhibition.
*Increased extracellular concentration of glutamate*
- **Glutamate excitotoxicity** can occur in chronic alcoholism, particularly during withdrawal, leading to neuronal damage.
- However, the primary insult in Wernicke-Korsakoff syndrome, which this patient's symptoms suggest, is **thiamine deficiency**, impacting the **Krebs cycle** before widespread glutamate release.
*Increased fragmentation of deoxyribonucleic acid within the neurons*
- **DNA fragmentation** indicates significant **neuronal damage** and **apoptosis**, which are later consequences of prolonged metabolic stress and oxidative injury.
- It is not the earliest pathophysiological change but rather a result of the progression of metabolic dysfunction caused by **thiamine deficiency**.
*Breakdown of the blood-brain barrier*
- **Blood-brain barrier (BBB) disruption** can occur in various neurological conditions, including chronic alcoholism, leading to inflammation and edema.
- While BBB dysfunction might contribute to the pathology, the initial and most direct effect of thiamine deficiency is on **cellular energy metabolism** within neurons and astrocytes, preceding widespread BBB breakdown.
More Glycogen metabolism US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.