Metabolism

On this page
🗺️ The Metabolic Mastery Map: Your Cellular Command Center
Metabolism is the body's economic system-every pathway, every enzyme, every cofactor exists to balance energy supply with cellular demand. Understanding how glucose oxidation, fat mobilization, amino acid catabolism, and ATP synthesis interconnect transforms isolated biochemical reactions into a unified clinical framework. Master these integration points, and you'll predict metabolic responses to feeding, fasting, exercise, stress, and disease states. This lesson builds your metabolic fluency through quantitative thresholds, regulatory nodes, and organ-specific adaptations that drive clinical decision-making.

The Metabolic Landscape Architecture
Metabolism organizes into three functional tiers that operate simultaneously across fed, fasting, and starved states:
-
Energy Currency Systems
- ATP production: 30-32 mol ATP per glucose via complete oxidation
- Phosphocreatine buffer: sustains muscle ATP for 8-10 seconds maximal effort
- NADH/FADH2 carriers: shuttle electrons to generate ≥90% of cellular ATP
- NADH yields 2.5 ATP per molecule through Complex I
- FADH2 yields 1.5 ATP per molecule through Complex II
- Total mitochondrial yield: 28 ATP from electron transport chain
-
Fuel Storage Hierarchies
- Glycogen reserves: 400-500 g total (300-400 g muscle, 100 g liver)
- Triglyceride stores: 10-15 kg in adipose tissue (135,000 kcal reserve)
- Protein mass: 6 kg structural protein (mobilized only in extreme starvation)
- Liver glycogen depletes in 12-18 hours fasting
- Muscle glycogen unavailable for blood glucose (lacks glucose-6-phosphatase)
- Fat oxidation provides ≥60% of resting energy after 12 hours fasting
-
Regulatory Control Points
- Insulin/glucagon ratio: primary fed-fast switch at <0.5 (fasting) vs >2.0 (fed)
- AMP-activated protein kinase (AMPK): cellular energy sensor activated when ATP/AMP ratio drops <5:1
- Allosteric modulators: acetyl-CoA, citrate, ATP inhibit glycolysis; AMP, ADP activate oxidation
- Phosphofructokinase-1 (PFK-1): rate-limiting glycolysis enzyme
- Fructose-2,6-bisphosphate: most potent PFK-1 activator (↑100-fold activity)
- Malonyl-CoA inhibits carnitine palmitoyltransferase I (CPT-I), blocking fat oxidation
📌 Remember: "GIFT" - Glucose, Insulin, Fat storage, Triglycerides characterize the fed state. When insulin >15 μU/mL, hepatic glucose output ceases and peripheral glucose uptake reaches 5-10 mg/kg/min, driving glycogen synthesis at 5-6 mg/kg/min and lipogenesis from excess carbohydrate.
| Metabolic State | Duration | Primary Fuel | Insulin:Glucagon | Hepatic Glucose Output | Ketone Bodies | Brain Fuel Source |
|---|---|---|---|---|---|---|
| Fed | 0-4 h post-meal | Glucose | >2.0 | 0 mg/kg/min | <0.1 mM | 100% glucose |
| Post-absorptive | 4-12 h | Glucose + Fat | 1.0-2.0 | 2 mg/kg/min | 0.1-0.5 mM | 100% glucose |
| Fasting | 12-24 h | Fat + Glucose | 0.5-1.0 | 2-3 mg/kg/min | 1-2 mM | 95% glucose, 5% ketones |
| Starvation | >24 h | Fat + Ketones | <0.5 | 1.5-2 mg/kg/min | 4-8 mM | 30% glucose, 70% ketones |
| Prolonged Starvation | >1 week | Ketones + Fat | <0.3 | 1 mg/kg/min | >8 mM | 20% glucose, 80% ketones |
⭐ Clinical Pearl: The brain consumes 120 g glucose daily (500 kcal) in the fed state but adapts to ketones during starvation, reducing glucose requirement to 40 g/day by day 7. This spares muscle protein from gluconeogenesis, extending survival from 60 days (without ketoadaptation) to >90 days with full ketone utilization.
Organ-Specific Metabolic Specialization
Each organ maintains unique metabolic machinery optimized for its physiological role:
-
Liver: The Metabolic Clearinghouse
- Glucose buffering: releases glucose at 2 mg/kg/min to maintain blood glucose 80-100 mg/dL
- Glycogen capacity: 100 g (400 kcal), depletes in 12-18 hours fasting
- Ketogenesis: produces 100-150 g ketones/day during starvation from fatty acid β-oxidation
- Acetyl-CoA accumulation when TCA cycle saturated
- HMG-CoA synthase: rate-limiting ketogenic enzyme
- Only liver and kidney cortex express ketogenic machinery
- Gluconeogenesis: synthesizes 180 g glucose/day from lactate, amino acids, glycerol
- Urea cycle: detoxifies ≥95% of ammonia to urea at 20-30 g/day
-
Muscle: The Metabolic Consumer
- Glycogen stores: 300-400 g (1,600 kcal), unavailable for blood glucose
- Fuel flexibility: switches from glucose (>50% at rest) to fat (>90% during prolonged exercise)
- Lactate production: 120 g/day exported to liver for Cori cycle gluconeogenesis
- Lactate dehydrogenase converts pyruvate to lactate, regenerating NAD+ for glycolysis
- During intense exercise, lactate production reaches 1 mmol/kg/min
- Amino acid catabolism: releases alanine and glutamine during fasting
- ATP consumption: ≥40% of resting metabolic rate
-
Adipose: The Energy Vault
- Triglyceride storage: 10-15 kg (135,000 kcal) in average adult
- Lipolysis rate: releases 50-100 g fatty acids/day during fasting
- Hormone-sensitive lipase: activated by glucagon, epinephrine, cortisol
- Insulin suppresses lipolysis at concentrations >10 μU/mL
- Complete lipolysis inhibition requires insulin >30 μU/mL
- Glycerol release: 10-20 g/day during fasting, substrate for hepatic gluconeogenesis
- Leptin secretion: signals energy stores to hypothalamus
💡 Master This: The Cori cycle and glucose-alanine cycle connect muscle and liver metabolism. Muscle produces 120 g lactate and 30 g alanine daily, which liver converts to 80 g glucose via gluconeogenesis. This costs 6 ATP per glucose synthesized but preserves blood glucose when glycogen depletes. Understanding these inter-organ cycles predicts metabolic responses in sepsis, trauma, and critical illness.
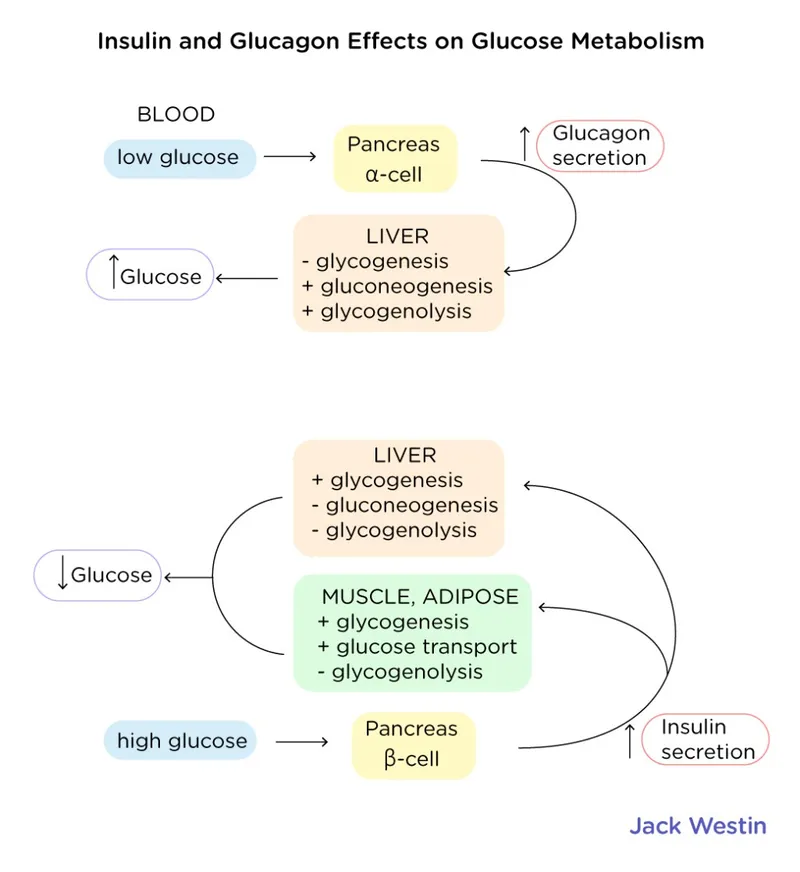
📌 Remember: "LAGS" - Liver, Adipose, Glucagon, Starvation. These four elements dominate fasting metabolism. When glucagon >150 pg/mL and insulin <5 μU/mL, hepatic glycogenolysis peaks at 3 mg/kg/min, adipose lipolysis reaches 100 g/day, and ketone production exceeds 2 mM within 24 hours.
Connect these foundational metabolic principles through the hormonal control systems that orchestrate fed-fast transitions in the next section.
🗺️ The Metabolic Mastery Map: Your Cellular Command Center
🎭 The Hormonal Command Network: Metabolic Maestros
Insulin and glucagon form the primary regulatory axis controlling substrate flux through metabolic pathways. These peptide hormones exert opposing effects through receptor-mediated signaling cascades that phosphorylate or dephosphorylate rate-limiting enzymes within seconds to minutes. Understanding their quantitative effects on glucose production, fat mobilization, and protein turnover enables precise interpretation of metabolic states from laboratory values.
Insulin: The Anabolic Orchestrator
Insulin secretion from pancreatic β-cells responds to plasma glucose with biphasic kinetics:
-
Secretion Dynamics
- Threshold: glucose >100 mg/dL triggers insulin release
- Peak secretion: glucose >140 mg/dL produces 50-100 μU/mL insulin
- First phase: 5-10 minute burst of pre-formed insulin
- Depletes within 15 minutes of sustained hyperglycemia
- Absent in type 2 diabetes mellitus
- Second phase: sustained synthesis and secretion for 2-3 hours
- Half-life: 4-6 minutes (rapid clearance requires continuous secretion)
-
Hepatic Effects (Insulin >15 μU/mL)
- Suppresses glucose output from 2 mg/kg/min to 0 mg/kg/min
- Activates glucokinase: increases glucose phosphorylation 3-fold
- Activates phosphofructokinase-2: increases fructose-2,6-bisphosphate 5-fold
- F-2,6-BP activates PFK-1 100-fold, driving glycolysis
- F-2,6-BP inhibits fructose-1,6-bisphosphatase, blocking gluconeogenesis
- Induces glycogen synthase: increases glycogen synthesis to 5-6 mg/kg/min
- Activates acetyl-CoA carboxylase: increases malonyl-CoA 10-fold
- Malonyl-CoA inhibits CPT-I, preventing fatty acid oxidation
- Malonyl-CoA provides substrate for fatty acid synthesis
- Induces lipogenic enzymes: ATP citrate lyase, fatty acid synthase increase 5-10 fold over 12-24 hours
-
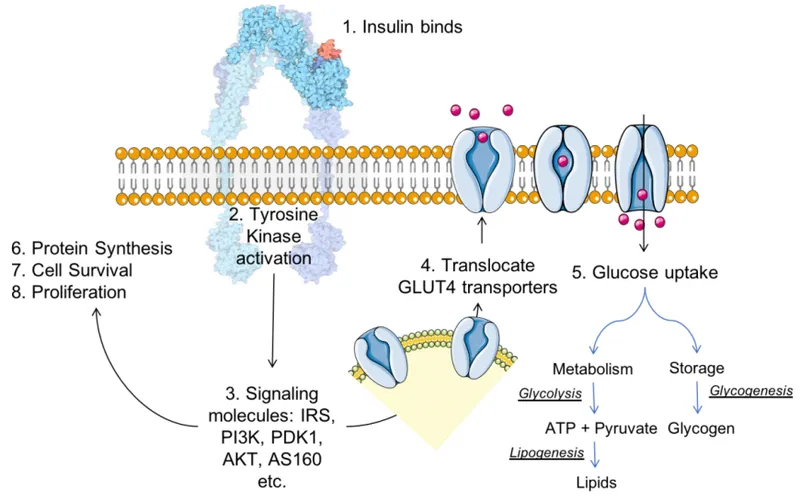
Muscle Effects (Insulin >10 μU/mL)
- Increases glucose uptake from 0.5 mg/kg/min to 5-10 mg/kg/min via GLUT4 translocation
- Activates glycogen synthase: muscle glycogen synthesis reaches 5 mg/kg/min
- Suppresses protein breakdown: reduces amino acid release 50%
- Activates protein synthesis: increases ribosomal translation 2-3 fold
- Activates pyruvate dehydrogenase: channels glucose to acetyl-CoA for oxidation
-
Adipose Effects (Insulin >5 μU/mL)
- Activates lipoprotein lipase: increases triglyceride uptake from chylomicrons and VLDL
- Suppresses hormone-sensitive lipase: reduces lipolysis >90% at insulin >30 μU/mL
- Increases glucose uptake: provides glycerol-3-phosphate for triglyceride synthesis
- Increases fatty acid esterification: triglyceride synthesis rate ↑5-fold
⭐ Clinical Pearl: Insulin resistance manifests when peripheral tissues require insulin >20 μU/mL to suppress hepatic glucose output or stimulate muscle glucose uptake to 50% of normal. Hepatic insulin resistance appears first, causing fasting hyperglycemia; muscle resistance follows, causing postprandial hyperglycemia. When β-cells cannot secrete sufficient insulin to overcome resistance, type 2 diabetes develops.
Glucagon: The Catabolic Commander
Glucagon secretion from pancreatic α-cells responds to falling glucose and rising amino acids:
-
Secretion Triggers
- Hypoglycemia: glucose <70 mg/dL increases glucagon 2-3 fold
- Amino acids: protein meal increases glucagon 50-100% (prevents hypoglycemia from insulin response)
- Exercise: glucagon rises 3-5 fold during prolonged activity
- Stress: epinephrine and cortisol amplify glucagon secretion
- Insulin suppression: high insulin (>20 μU/mL) suppresses glucagon release
-
Hepatic Effects (Glucagon >150 pg/mL)
- Activates glycogen phosphorylase: increases glycogenolysis to 3 mg/kg/min
- Inhibits glycogen synthase: prevents futile cycling
- Activates gluconeogenic enzymes: PEPCK and G6Pase increase 2-3 fold over 4-6 hours
- Provides 180 g glucose/day from lactate, alanine, glycerol during fasting
- Activates CPT-I: increases fatty acid oxidation 5-fold
- Activates ketogenesis: ketone production reaches 2-4 mM within 24 hours
- HMG-CoA synthase activity increases 10-fold
- Ketone bodies reach 8 mM by day 7 of starvation
-
Adipose Effects (Glucagon >100 pg/mL)
- Activates hormone-sensitive lipase: increases lipolysis to 100 g fatty acids/day
- Releases glycerol: provides 10-20 g/day substrate for gluconeogenesis
- No direct effect on muscle (lacks glucagon receptors)
📌 Remember: "GILA" - Glucagon Increases Lipolysis and Amino acid release. When insulin/glucagon ratio <0.5, the body shifts from glucose oxidation to fat oxidation, producing ketones at >2 mM and maintaining blood glucose through gluconeogenesis at 1.5-2 mg/kg/min.
| Hormone | Fed State Level | Fasting State Level | Primary Target | Glucose Effect | Fat Effect | Protein Effect |
|---|---|---|---|---|---|---|
| Insulin | 15-100 μU/mL | <5 μU/mL | Liver, Muscle, Adipose | Uptake ↑5-10× | Storage ↑5× | Synthesis ↑2-3× |
| Glucagon | 50-80 pg/mL | 150-300 pg/mL | Liver | Output ↑3× | Oxidation ↑5× | No direct effect |
| Epinephrine | <50 pg/mL | 50-200 pg/mL | Muscle, Adipose, Liver | Output ↑2× | Lipolysis ↑10× | Breakdown ↑ |
| Cortisol | 5-15 μg/dL | 15-25 μg/dL | Liver, Muscle | Gluconeogenesis ↑ | Lipolysis ↑2× | Breakdown ↑3× |
| Growth Hormone | <2 ng/mL | 5-20 ng/mL | Liver, Adipose | Output ↑ | Lipolysis ↑3× | Synthesis ↑ |
💡 Master This: The insulin/glucagon ratio determines metabolic state more accurately than either hormone alone. Ratio >2.0 drives anabolism; ratio <0.5 drives catabolism. In diabetic ketoacidosis, absolute insulin deficiency and relative glucagon excess (ratio <0.1) produce unrestrained lipolysis (>200 g/day), ketogenesis (>10 mM), and proteolysis, causing the classic triad of hyperglycemia, ketoacidosis, and muscle wasting.
Counter-Regulatory Hormone Integration
Epinephrine, cortisol, and growth hormone amplify glucagon's catabolic effects during stress:
-
Epinephrine (Stress Response)
- Secretion: increases 10-100 fold during hypoglycemia, exercise, stress
- Hepatic: activates glycogenolysis via β2-receptors (3 mg/kg/min output)
- Muscle: activates glycogenolysis for local ATP (lactate exported to liver)
- Adipose: activates hormone-sensitive lipase via β1-receptors (lipolysis ↑10-fold)
- Pancreas: suppresses insulin, stimulates glucagon secretion
- Peak effect: 5-10 minutes (immediate response)
-
Cortisol (Prolonged Stress)
- Secretion: increases 2-3 fold during fasting, illness, psychological stress
- Hepatic: induces PEPCK and G6Pase over 4-6 hours (gluconeogenesis ↑50%)
- Muscle: increases protein breakdown, releases amino acids (50 g/day in stress)
- Adipose: permissive effect for catecholamine-induced lipolysis
- Immune: suppresses inflammatory cytokines at >20 μg/dL
- Peak effect: 4-6 hours (genomic regulation)
-
Growth Hormone (Fasting Adaptation)
- Secretion: increases 5-10 fold during fasting, deep sleep
- Hepatic: stimulates IGF-1 production, increases gluconeogenesis
- Adipose: increases lipolysis (↑3-fold), spares glucose
- Muscle: promotes protein synthesis via IGF-1, opposes insulin
- Metabolic: shifts fuel utilization from glucose to fat
- Peak effect: 2-4 hours (mixed genomic and non-genomic)
⭐ Clinical Pearl: Hypoglycemia triggers counter-regulatory hormone surge in sequence: epinephrine at glucose <70 mg/dL (autonomic symptoms), glucagon at <60 mg/dL (hepatic response), cortisol and growth hormone at <55 mg/dL (sustained defense). Patients with hypoglycemia unawareness (autonomic neuropathy) lose epinephrine response, increasing severe hypoglycemia risk 6-fold.
Understanding these hormonal control mechanisms reveals how the body transitions between metabolic states through the pathway integration networks explored next.
🎭 The Hormonal Command Network: Metabolic Maestros
🔬 The Pathway Integration Nexus: Metabolic Crossroads
Metabolic pathways intersect at key intermediates that function as regulatory nodes, allowing substrate flux to shift between oxidation, storage, and biosynthesis based on cellular energy status. Acetyl-CoA, pyruvate, and glucose-6-phosphate serve as central hubs where glycolysis, gluconeogenesis, fatty acid metabolism, amino acid catabolism, and the TCA cycle converge. Mastering these integration points predicts how nutrients flow through competing pathways under different physiological conditions.
Acetyl-CoA: The Metabolic Crossroads
Acetyl-CoA stands at the intersection of carbohydrate, fat, and amino acid metabolism:
-
Sources of Acetyl-CoA
- Pyruvate oxidation: >50% of acetyl-CoA in fed state
- Pyruvate dehydrogenase complex produces acetyl-CoA in mitochondria
- Irreversible reaction commits glucose to oxidation
- Activated by insulin, inhibited by acetyl-CoA, NADH, ATP
- Fatty acid β-oxidation: >80% of acetyl-CoA during fasting
- Each 16-carbon palmitate yields 8 acetyl-CoA molecules
- Produces 106 ATP per palmitate (vs 30 ATP per glucose)
- Requires carnitine shuttle for mitochondrial entry
- Ketogenic amino acids: leucine and lysine catabolism
- Ketone body oxidation: brain consumes 100 g ketones/day during starvation
- Alcohol metabolism: ethanol → acetaldehyde → acetate → acetyl-CoA
- Pyruvate oxidation: >50% of acetyl-CoA in fed state
-
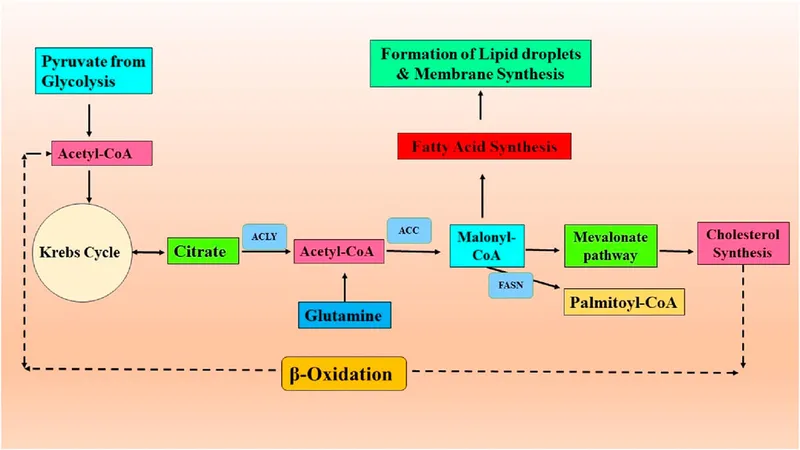
Fates of Acetyl-CoA
- TCA cycle oxidation: produces 10 ATP per acetyl-CoA
- Requires oxaloacetate availability (gluconeogenic precursor)
- When oxaloacetate depleted, acetyl-CoA accumulates
- Ketogenesis: 2 acetyl-CoA → acetoacetate → β-hydroxybutyrate
- Occurs only in hepatic mitochondria (HMG-CoA synthase)
- Produces 4-8 mM ketones during fasting
- Fatty acid synthesis: cytosolic acetyl-CoA → palmitate
- Requires citrate shuttle from mitochondria
- ATP citrate lyase cleaves citrate to acetyl-CoA + oxaloacetate
- Consumes 7 ATP and 14 NADPH per palmitate
- Cholesterol synthesis: 18 acetyl-CoA → cholesterol
- HMG-CoA reductase: rate-limiting enzyme (statin target)
- Produces 800-1,000 mg cholesterol/day
- TCA cycle oxidation: produces 10 ATP per acetyl-CoA
📌 Remember: "ACKOF" - Acetyl-CoA's Key Outcomes are Fatty acids, Ketones, Oxidation (TCA), and cholesterol Formation. In the fed state with high insulin, acetyl-CoA drives lipogenesis at 50-100 g/day. During fasting with high glucagon, the same acetyl-CoA produces ketones at 100-150 g/day instead.
Glucose-6-Phosphate: The Carbohydrate Dispatcher
G6P represents the first committed step of glucose metabolism and branches to multiple pathways:
-
G6P Formation
- Hexokinase (muscle, brain): Km 0.1 mM, always active
- Inhibited by product (G6P) for local regulation
- Traps glucose in cells (G6P cannot cross membranes)
- Glucokinase (liver, β-cells): Km 10 mM, glucose sensor
- Not inhibited by G6P (allows hepatic glucose buffering)
- Activity proportional to glucose concentration
- Mutations cause MODY-2 diabetes
- Glycogen phosphorylase: releases G6P from glycogen
- Produces 3 mg/kg/min during glycogenolysis
- Hexokinase (muscle, brain): Km 0.1 mM, always active
-
G6P Fates
- Glycolysis: >90% of G6P in fed state
- Produces 2 ATP (net) and 2 NADH per glucose
- Phosphofructokinase-1: rate-limiting step
- Glycogen synthesis: stores 5-6 mg/kg/min in fed state
- Glycogen synthase activated by insulin
- Requires UDP-glucose formation
- Pentose phosphate pathway: 5-10% of G6P flux
- Oxidative phase produces 2 NADPH per G6P
- Non-oxidative phase produces ribose-5-phosphate
- Active in liver, adipose, lactating breast, RBCs
- Gluconeogenesis: G6Pase hydrolyzes G6P → glucose
- Only in liver and kidney (release glucose to blood)
- Produces 180 g glucose/day during fasting
- Deficiency causes von Gierke disease (glycogen storage disease type I)
- Glycolysis: >90% of G6P in fed state
⭐ Clinical Pearl: The liver's unique expression of glucokinase and glucose-6-phosphatase enables bidirectional glucose flux. After a meal, glucokinase phosphorylates 50-100 g glucose for glycogen storage. During fasting, G6Pase releases 180 g glucose/day from gluconeogenesis and glycogenolysis. Muscle lacks G6Pase, so muscle glycogen cannot contribute to blood glucose-only liver glycogen maintains glycemia.
| G6P Pathway | Fed State Flux | Fasting State Flux | Rate-Limiting Enzyme | Insulin Effect | Glucagon Effect | Primary Tissues |
|---|---|---|---|---|---|---|
| Glycolysis | 90% | 10% | Phosphofructokinase-1 | Activate ↑5× | Inhibit ↓5× | All tissues |
| Glycogen Synthesis | 5-10% | 0% | Glycogen synthase | Activate ↑10× | Inhibit | Liver, Muscle |
| Pentose Phosphate | 5-10% | 5-10% | G6P dehydrogenase | Activate ↑2× | No effect | Liver, Adipose, RBCs |
| Gluconeogenesis | 0% | 80-90% | G6Pase | Inhibit | Activate ↑3× | Liver, Kidney |
Pyruvate: The Glycolytic Gateway
Pyruvate stands at the junction between cytosolic and mitochondrial metabolism:
-
Pyruvate Sources
- Glycolysis: produces 2 pyruvate per glucose
- Alanine transamination: ALT converts alanine → pyruvate
- Muscle releases 30 g alanine/day during fasting
- Glucose-alanine cycle: muscle exports nitrogen to liver
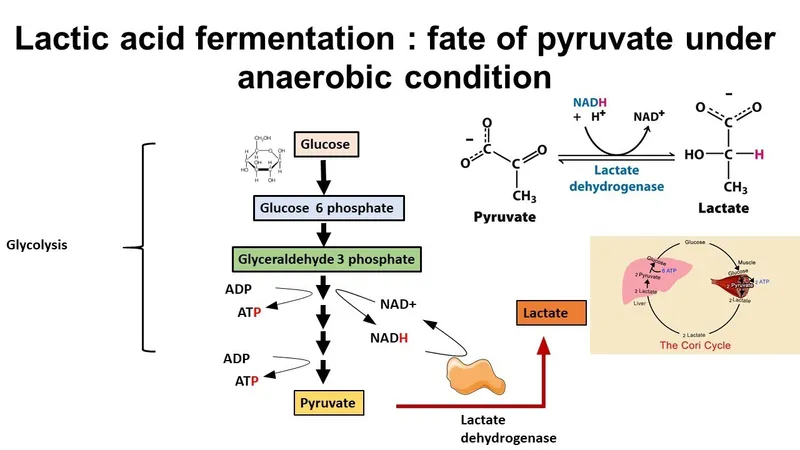
- Lactate oxidation: LDH converts lactate → pyruvate
- Cori cycle: muscle lactate → hepatic glucose
- 120 g lactate/day recycled to 80 g glucose
-
Pyruvate Fates
- Acetyl-CoA (oxidation): irreversible via pyruvate dehydrogenase
- Commits glucose to complete oxidation (30 ATP total)
- Activated by insulin, ADP, pyruvate
- Inhibited by acetyl-CoA, NADH, ATP
- Oxaloacetate (gluconeogenesis): pyruvate carboxylase
- Requires 1 ATP and biotin cofactor
- Activated by acetyl-CoA (ensures TCA cycle function)
- First committed step of gluconeogenesis
- Lactate (anaerobic glycolysis): lactate dehydrogenase
- Regenerates NAD+ for continued glycolysis
- Produces 1 mmol/kg/min during intense exercise
- Normal lactate 0.5-1.5 mM, rises to >10 mM in anaerobic conditions
- Alanine (amino acid synthesis): ALT
- Exports nitrogen from muscle to liver
- Prevents ammonia accumulation in muscle
- Acetyl-CoA (oxidation): irreversible via pyruvate dehydrogenase
💡 Master This: Pyruvate's fate depends on mitochondrial redox state (NADH/NAD+ ratio) and energy status (ATP/ADP ratio). High NADH and ATP inhibit pyruvate dehydrogenase, shunting pyruvate to lactate or oxaloacetate. Low NADH and ATP activate pyruvate dehydrogenase, driving complete oxidation. This explains why hypoxia (↑NADH) causes lactate accumulation even when oxygen delivery improves.
Citrate: The Mitochondrial-Cytosolic Messenger
Citrate links mitochondrial oxidation to cytosolic biosynthesis:
-
Citrate Synthesis and Export
- TCA cycle: citrate synthase condenses acetyl-CoA + oxaloacetate
- First step of TCA cycle, irreversible
- Inhibited by ATP, NADH, succinyl-CoA
- Citrate export: tricarboxylate transporter moves citrate to cytosol
- Occurs when mitochondrial ATP/ADP >10:1
- Signals energy excess, activates biosynthesis
- TCA cycle: citrate synthase condenses acetyl-CoA + oxaloacetate
-
Cytosolic Citrate Fates
- ATP citrate lyase: cleaves citrate → acetyl-CoA + oxaloacetate
- Provides acetyl-CoA for fatty acid and cholesterol synthesis
- Activated by insulin signaling
- Consumes 1 ATP per cleavage
- Allosteric regulation: citrate inhibits phosphofructokinase-1
- Reduces glycolytic flux when energy abundant
- Prevents futile cycling of glucose to pyruvate
- ATP citrate lyase: cleaves citrate → acetyl-CoA + oxaloacetate
📌 Remember: "CITRATE" - Citrate Indicates Tricarboxylic Richness, Activates Triacylglycerol Elongation. When citrate accumulates in mitochondria (ATP/ADP >10:1), it exits to cytosol, inhibits glycolysis, and provides acetyl-CoA for lipogenesis. This mechanism couples glucose oxidation to fat storage in the fed state.
These integration points coordinate substrate flux across the major energy-producing pathways examined in the next section.
🔬 The Pathway Integration Nexus: Metabolic Crossroads
🏥 The Energy Harvest Systems: ATP Production Hierarchies
The body employs three ATP-generating systems with different capacities, onset speeds, and durations. Understanding their quantitative contributions during rest, exercise, and metabolic stress predicts fuel utilization patterns and explains clinical presentations of mitochondrial disorders, glycogen storage diseases, and metabolic myopathies.
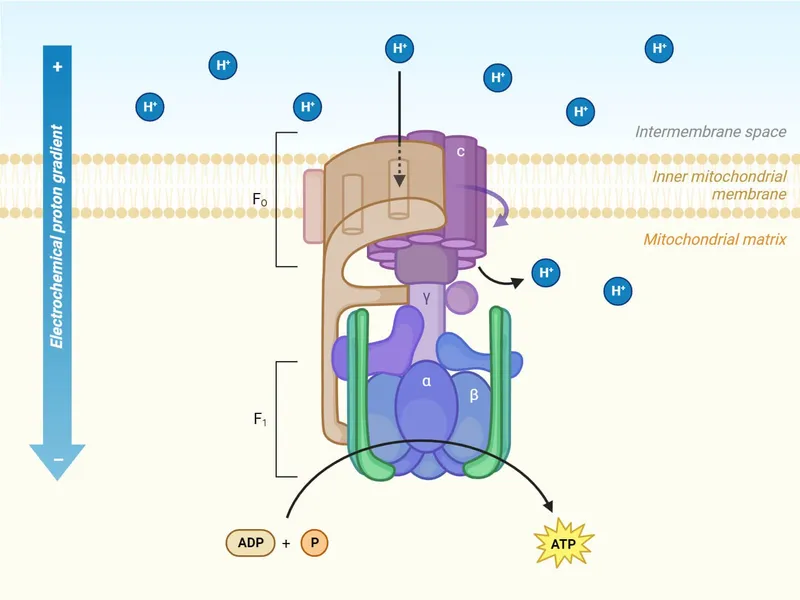
Substrate-Level Phosphorylation: Immediate Energy
Glycolysis and the TCA cycle generate ATP directly through high-energy phosphate transfer:
-
Glycolytic ATP Production
- Glucose → 2 pyruvate: net 2 ATP per glucose
- Phosphoglycerate kinase: 1,3-bisphosphoglycerate → 3-phosphoglycerate
- Pyruvate kinase: phosphoenolpyruvate → pyruvate
- Anaerobic capacity: 2 ATP per glucose (lactate endpoint)
- Aerobic capacity: 2 ATP per glucose (pyruvate → acetyl-CoA)
- Rate: 1 mmol ATP/kg/min maximal glycolytic flux
- Duration: limited by glycogen stores (400-500 g, 2,000 kcal)
- Glucose → 2 pyruvate: net 2 ATP per glucose
-
TCA Cycle ATP Production
- Succinyl-CoA → succinate: 1 GTP (equivalent to ATP) per cycle
- Complete glucose oxidation: 2 GTP per glucose (2 acetyl-CoA)
- Minimal direct ATP contribution (most energy via NADH/FADH2)
-
Clinical Significance
- Glycolysis sustains ATP 10-30 seconds during maximal effort
- Lactate accumulation when glycolytic rate exceeds oxidative capacity
- Normal lactate 0.5-1.5 mM, exercise 2-4 mM, pathologic >5 mM
- Lactate >10 mM indicates severe tissue hypoxia or mitochondrial dysfunction
⭐ Clinical Pearl: Type II glycogen storage diseases (Pompe disease, acid maltase deficiency) impair glycogenolysis, reducing glycolytic ATP production. Patients develop exercise intolerance, myoglobinuria after brief intense exercise, and normal lactate response (distinguishing from mitochondrial myopathies, which show elevated lactate at rest).
Oxidative Phosphorylation: Sustained Energy Production
The electron transport chain and ATP synthase generate >90% of cellular ATP:
-
Electron Transport Chain Components
- Complex I (NADH dehydrogenase): transfers electrons from NADH
- Pumps 4 H+ per NADH across inner membrane
- Yields 2.5 ATP per NADH oxidized
- Inhibited by rotenone, metformin
- Complex II (succinate dehydrogenase): transfers electrons from FADH2
- Pumps 0 H+ (does not contribute to gradient)
- Yields 1.5 ATP per FADH2 oxidized
- Part of TCA cycle (succinate → fumarate)
- Complex III (cytochrome bc1): transfers electrons to cytochrome c
- Pumps 4 H+ per electron pair
- Inhibited by antimycin A
- Complex IV (cytochrome oxidase): reduces O2 to H2O
- Pumps 2 H+ per electron pair
- Inhibited by cyanide, carbon monoxide, hydrogen sulfide
- Requires 0.5 O2 per NADH oxidized
- Complex I (NADH dehydrogenase): transfers electrons from NADH
-
ATP Synthase (Complex V)
- Proton gradient: 10-fold H+ concentration difference (pH 7.4 matrix vs 6.4 intermembrane space)
- Proton-motive force: -180 to -220 mV across inner membrane
- Coupling ratio: 3-4 H+ required per ATP synthesized
- Maximum efficiency: 40% of electron energy captured as ATP
- Heat production: 60% of energy dissipated as heat
-
Complete Glucose Oxidation Yield
- Glycolysis: 2 ATP + 2 NADH (cytosolic)
- Pyruvate → acetyl-CoA: 2 NADH (mitochondrial)
- TCA cycle (×2): 2 GTP + 6 NADH + 2 FADH2
- Total: 4 ATP (substrate-level) + 10 NADH + 2 FADH2
- Oxidative phosphorylation: (10 × 2.5) + (2 × 1.5) = 28 ATP
- Grand total: 30-32 ATP per glucose (depending on NADH shuttle)
📌 Remember: "COMPLEX" - Complexes One through Three Move Protons, Leading to Electron-driven X-phosphorylation. Complex I produces 2.5 ATP, Complex II produces 1.5 ATP, and together they generate 28 ATP from one glucose through oxidative phosphorylation->90% of total ATP yield.
| Metabolic Pathway | ATP Yield | NADH Yield | FADH2 Yield | Rate | Duration | Oxygen Required |
|---|---|---|---|---|---|---|
| Phosphocreatine | 1 ATP per PCr | 0 | 0 | Immediate | 8-10 sec | No |
| Glycolysis (anaerobic) | 2 ATP per glucose | 0 (→lactate) | 0 | 1 mmol/kg/min | 30-60 sec | No |
| Glycolysis (aerobic) | 2 ATP per glucose | 2 NADH | 0 | 0.5 mmol/kg/min | 2-3 min | Yes |
| Complete Oxidation | 30-32 ATP per glucose | 10 NADH | 2 FADH2 | 0.2 mmol/kg/min | Unlimited | Yes |
| Fatty Acid (Palmitate) | 106 ATP per C16 | 31 NADH | 15 FADH2 | 0.1 mmol/kg/min | Unlimited | Yes |
Metabolic Efficiency and Respiratory Quotient
The respiratory quotient (RQ) reveals substrate oxidation patterns:
-
RQ Calculation and Interpretation
- RQ = CO2 produced / O2 consumed
- Pure glucose oxidation: RQ = 1.0
- C6H12O6 + 6 O2 → 6 CO2 + 6 H2O
- 6 CO2 / 6 O2 = 1.0
- Pure fat oxidation: RQ = 0.7
- C16H32O2 + 23 O2 → 16 CO2 + 16 H2O
- 16 CO2 / 23 O2 = 0.7
- Pure protein oxidation: RQ = 0.8
- Mixed diet: RQ = 0.85 (typical resting state)
- Lipogenesis from glucose: RQ = >1.0 (excess CO2 production)
-
Clinical Applications
- Fed state: RQ **0.9-1.0
Practice Questions: Metabolism
Test your understanding with these related questions
Which of the following foods should be consumed to prevent thiamine deficiency?



