Genetic counseling for lysosomal diseases US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Genetic counseling for lysosomal diseases. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Genetic counseling for lysosomal diseases US Medical PG Question 1: A 28-year-old woman comes to the physician for genetic counseling prior to conception. For the past year, she has had intermittent episodes of headache, nausea, abdominal pain, and tingling of her fingers. She also complains of dark urine during the episodes. Her mother and maternal uncle have similar symptoms and her father is healthy. Her husband is healthy and there is no history of serious illness in his family. Serum studies show elevated concentrations of porphobilinogen and δ-aminolevulinic acid. What is the probability of this patient having a child with the same disease as her?
- A. 25%
- B. 67%
- C. 50% (Correct Answer)
- D. 100%
Genetic counseling for lysosomal diseases Explanation: ***50%***
- This patient's symptoms (headache, nausea, abdominal pain, tingling, dark urine) and elevated **porphobilinogen** and **δ-aminolevulinic acid** are highly suggestive of **Acute Intermittent Porphyria** (AIP).
- AIP is an **autosomal dominant** disorder. Therefore, there is a **50% chance** that any child of an affected parent will inherit the disease-causing allele.
- Since her husband is healthy with no family history, he does not carry the mutation, making this a straightforward autosomal dominant inheritance calculation.
*25%*
- This probability would be expected in an **autosomal recessive** inheritance pattern when two carrier parents have a child, which is not the case here.
- It would also be the probability for an X-linked recessive disorder if the mother is a carrier and the father is unaffected, and they are discussing a son's inheritance.
- The clinical presentation and family history (mother and maternal uncle affected, consistent with autosomal dominant pattern) rule out this probability.
*67%*
- This probability is seen in specific genetic scenarios, such as the chance of a phenotypically normal sibling of an individual with an autosomal recessive disease being a carrier.
- It's not a standard probability for direct offspring of an affected individual with an autosomal dominant condition.
- This does not apply to the straightforward inheritance question being asked here.
*100%*
- This probability would occur if the disease were inherited in an **autosomal dominant** manner and the affected parent was **homozygous dominant** for the mutation.
- However, this is extremely rare in AIP, as most affected individuals are **heterozygous**.
- The family history pattern (affected mother with unaffected father having an affected child) is consistent with heterozygosity, not homozygosity.
Genetic counseling for lysosomal diseases US Medical PG Question 2: A 7-month-old boy is brought by his parents to the pediatrician’s office. His mother says the child has been weakening progressively and is not as active as he used to be when he was born. His condition seems to be getting worse, especially over the last month. He was born at 41 weeks through normal vaginal delivery. There were no complications observed during the prenatal period. He was progressing well over the 1st few months and achieving the appropriate milestones. On examination, his abdomen appears soft with no liver enlargement. The patient appears to be dehydrated and lethargic. The results of a fundoscopic examination are shown in the picture. A blood test for which of the following enzymes is the next best assay to evaluate this patient's health?
- A. Acid alpha-glucosidase
- B. Hexosaminidase (Correct Answer)
- C. Sphingomyelinase
- D. Glucocerebrosidase
- E. Arylsulfatase A
Genetic counseling for lysosomal diseases Explanation: ***Hexosaminidase***
- The symptoms and history suggest **Tay-Sachs disease**, characterized by progressive weakness and developmental delay, often linked to **deficiency in hexosaminidase A**.
- A fundoscopic exam typically reveals a **cherry-red spot**, consistent with this condition, making hexosaminidase testing essential for diagnosis.
- Tay-Sachs results from accumulation of **GM2 ganglioside** in neurons due to hexosaminidase A deficiency.
*Glucocerebrosidase*
- This enzyme is primarily associated with **Gaucher's disease**, which does not match the clinical features presented here.
- Symptoms of Gaucher's disease include **hepatosplenomegaly** and bone pain, not primarily weakness or lethargy in a young infant.
*Acid alpha-glucosidase*
- Generally tested for **Pompe disease**, which typically presents with **muscle weakness and hypertrophic cardiomyopathy**, not solely lethargy and failure to thrive.
- The clinical presentation in this case does not indicate glycogen storage disorder symptoms.
*Arylsulfatase A*
- This enzyme deficiency relates to **metachromatic leukodystrophy**, which often features neurological decline rather than isolated lethargy in infants.
- The specific symptoms and age do not align with the typical findings of this condition.
*Sphingomyelinase*
- Linked to **Niemann-Pick disease**, characterized by **hepatosplenomegaly** and neurological deterioration, absent in this scenario.
- The presentation of weakness does not match the classic signs expected with sphingomyelinase deficiency.
Genetic counseling for lysosomal diseases US Medical PG Question 3: A 7-year-old boy is brought to the emergency department by his parents. He is complaining of left-sided knee pain which has progressively increased in severity over the past 2 days. It started when he was playing football with his brothers but he does not recall falling or getting any injury. Past medical history is significant for prolonged bleeding and easy bruising. His maternal uncle has similar problems. Physical exam reveals swollen and painful left knee. His laboratory investigations reveal:
Hemoglobin 11.8 g/dL
WBC count 7,000/mL
Platelets 250,000/mL
INR 0.9
aPTT 62 sec, fully corrected with a mixing study
Which of the following disorders have the same mode of inheritance as this patient’s disease?
- A. Hereditary spherocytosis
- B. Alkaptonuria
- C. Duchenne muscular dystrophy (Correct Answer)
- D. Sickle cell disease
- E. Huntington's disease
Genetic counseling for lysosomal diseases Explanation: ***Duchenne muscular dystrophy***
- The patient's presentation with **hemarthrosis** (knee pain and swelling without trauma), **prolonged bleeding**, and **easy bruising**, along with a **prolonged aPTT** that corrects with a mixing study, is highly suggestive of **hemophilia A or B**. Both conditions are **X-linked recessive disorders**, affecting males predominantly.
- **Duchenne muscular dystrophy** is also an **X-linked recessive disorder**, making its mode of inheritance identical to the suspected diagnosis of hemophilia in this patient.
*Hereditary spherocytosis*
- This condition is inherited in an **autosomal dominant** pattern, which is different from the mode of inheritance for hemophilia.
- It is characterized by **hemolytic anemia** due to a defect in red blood cell membrane proteins.
*Alkaptonuria*
- Alkaptonuria is an **autosomal recessive** disorder, caused by a deficiency of homogentisate 1,2-dioxygenase.
- It leads to the accumulation of **homogentisic acid**, causing **dark urine** when exposed to air, **ochronosis**, and **arthropathy**, distinct from the patient's bleeding disorder.
*Sickle cell disease*
- **Sickle cell disease** is an **autosomal recessive** disorder, characterized by abnormal hemoglobin leading to chronic hemolytic anemia and vaso-occlusive crises.
- While it can cause joint pain due to avascular necrosis or infarction, its inheritance pattern is different from the patient's condition.
*Huntington's disease*
- **Huntington's disease** is an **autosomal dominant** neurodegenerative disorder that manifests with progressive motor, cognitive, and psychiatric symptoms, typically in middle age.
- Its inheritance pattern and clinical presentation are distinct from the patient's bleeding disorder.
Genetic counseling for lysosomal diseases US Medical PG Question 4: A healthy 30-year-old woman comes to the physician with her husband for preconception counseling. Her husband is healthy but she is concerned because her brother was recently diagnosed with a genetic liver condition for which he takes penicillamine. Her father-in-law has liver cirrhosis and a tremor. The results of genetic testing show that both the patient and her husband are carriers of a mutation in the ATP7B gene. Which of the following is the chance that this patient’s offspring will eventually develop the hereditary condition?
- A. 0%
- B. 25% (Correct Answer)
- C. 100%
- D. 50%
- E. 75%
Genetic counseling for lysosomal diseases Explanation: ***25%***
- The familial history (brother with a genetic liver condition, father-in-law with cirrhosis and tremor) and the **ATP7B gene mutation** indicate **Wilson's disease**, which is typically inherited in an **autosomal recessive** pattern.
- If both parents are carriers (heterozygous for the mutation), the probability that their offspring will inherit two copies of the mutated gene (one from each parent) and, therefore, develop the condition is **25%** as per Mendelian inheritance.
*0%*
- This is incorrect because both parents are identified as carriers, meaning there is a definite risk of passing on the mutated genes to their offspring.
- For the risk to be 0%, at least one parent would need to be completely free of the mutation or the inheritance pattern would need to be dominant with no penetrance.
*100%*
- This would only be the case if both parents had the disease (were homozygous for the mutation) or if the condition were dominant and at least one parent had the disease and passed on the dominant allele.
- Since both are carriers, the chance of inheriting two mutated alleles is not 100%.
*50%*
- A 50% chance would apply if one parent had the disease (homozygous recessive) and the other was a carrier, or if it were an autosomal dominant condition with one affected heterozygous parent.
- This does not reflect the inheritance pattern for two carrier parents in an autosomal recessive condition.
*75%*
- A 75% chance is not typical for a single genetic outcome in standard Mendelian inheritance patterns from carrier parents.
- In the context of two carriers for an autosomal recessive trait, 75% represents the chance of the offspring either being a carrier (50%) or being completely unaffected (25%), but not the chance of developing the condition.
Genetic counseling for lysosomal diseases US Medical PG Question 5: A 34-year-old woman, gravida 1, para 0, at 18 weeks' gestation, comes to the physician for a prenatal visit. She recently read about a genetic disorder that manifests with gait ataxia, kyphoscoliosis, and arrhythmia and is concerned about the possibility of her child inheriting the disease. There is no personal or family history of this disorder. The frequency of unaffected carriers in the general population is 1/100. Assuming the population is in a steady state without selection, what is the probability that her child will develop this disease?
- A. 1/10,000
- B. 1/20,000
- C. 1/40,000 (Correct Answer)
- D. 1/200
- E. 1/400
Genetic counseling for lysosomal diseases Explanation: ***1/40,000***
- This disorder (Friedreich's ataxia) follows **autosomal recessive** inheritance, meaning both parents must be carriers for the child to be affected.
- Since there is no family history, we treat both parents as random individuals from the general population with carrier frequency 1/100.
- **Calculation**: Probability mother is carrier (1/100) × Probability father is carrier (1/100) × Probability child is affected given both parents are carriers (1/4) = **1/40,000**.
- This applies Hardy-Weinberg equilibrium principles for a steady-state population.
*1/10,000*
- This calculation (1/100 × 1/100 = 1/10,000) represents only the probability that both parents are carriers.
- It fails to account for the **1/4 chance** of an affected child when two carriers of an **autosomal recessive** condition conceive.
- This would be the answer if both parents being carriers automatically meant the child would be affected, which is incorrect.
*1/20,000*
- This result would occur if the probability of the child inheriting the disease from carrier parents was 1/2 instead of 1/4 (1/100 × 1/100 × 1/2 = 1/20,000).
- A 1/2 probability would apply to **autosomal dominant** conditions where one affected parent passes the disease, not for **autosomal recessive** inheritance.
- For autosomal recessive disorders, two carrier parents have a 1/4 (not 1/2) chance of an affected child.
*1/200*
- This probability (1/100 × 1/2 = 1/200) would suggest only one parent needed to be a carrier with a 1/2 transmission probability.
- This does not account for the requirement that **both parents must be carriers** for an **autosomal recessive** disorder.
- It represents a fundamental misunderstanding of recessive inheritance patterns.
*1/400*
- This calculation (1/100 × 1/4 = 1/400) incorrectly assumes only one parent needs to be a carrier.
- For **autosomal recessive** inheritance, **both parents must be carriers**, so both their carrier probabilities (1/100 each) must be included in the calculation.
- It omits the second parent's carrier probability entirely.
Genetic counseling for lysosomal diseases US Medical PG Question 6: A 3-year-old is brought to the pediatrician by his mother because she is concerned about recent changes to his behavior. She states that he has seemed to regress in his motor development and has been having occasional brief episodes of uncontrollable shaking. During the subsequent work up, a muscle biopsy is obtained which demonstrates red ragged fibers and a presumptive diagnosis of a genetic disease made. The mother asks if her other son will be affected. What should be the physician's response?
- A. There is a 50% chance he will be affected
- B. There is a 100% chance he will be affected, and the severity will be the same
- C. There is a 25% chance he will be affected
- D. There is a 100% chance he will be affected, but the severity may be different (Correct Answer)
- E. He will be unaffected
Genetic counseling for lysosomal diseases Explanation: ***There is a 100% chance he will be affected, but the severity may be different***
- The patient's symptoms (motor regression, seizures, red ragged fibers on muscle biopsy) are classic for a **mitochondrial disorder**, which are inherited via **maternal inheritance**.
- All children of an affected mother will inherit the affected mitochondria; however, the **heteroplasmy** (proportion of mutated mitochondria inherited) can vary, leading to different disease severities.
*There is a 50% chance he will be affected*
- This inheritance pattern is typical for **autosomal dominant** disorders, or occasionally X-linked disorders in males.
- Mitochondrial disorders do not follow autosomal dominant inheritance, as they are exclusively inherited from the mother.
*There is a 100% chance he will be affected, and the severity will be the same*
- While there is a 100% chance of inheriting the mutated mitochondria from an affected mother, the **phenotypic expression and severity can vary widely** due to heteroplasmy.
- The proportion of mutated mitochondria can differ in various tissues and between offspring, leading to variable clinical manifestations.
*There is a 25% chance he will be affected*
- This represents the risk of inheritance for an **autosomal recessive** disorder when both parents are carriers.
- Mitochondrial inheritance does not follow an autosomal recessive pattern.
*He will be unaffected*
- This would only be true if the mother's mitochondrial DNA were not affected or if the inheritance pattern allowed for some children to be completely spared, which is not the case for mitochondrial disorders.
- Since the mother is the carrier of the mitochondrial mutation, all her children will inherit the mutated mitochondria.
Genetic counseling for lysosomal diseases US Medical PG Question 7: A 28-year-old woman, gravida 1, para 0, at 20 weeks' gestation comes to the physician for genetic counseling. Her brother and maternal uncle both have anemia that worsens after taking certain medications. Based on the pedigree shown, what is the probability that her son will be affected by the disease?
- A. 12.5%
- B. 100%
- C. 50%
- D. 0%
- E. 25% (Correct Answer)
Genetic counseling for lysosomal diseases Explanation: ***25%***
- This is an **X-linked recessive** disorder, as evidenced by affected males born to unaffected parents, and the skipping of generations in females. The woman (III-2) is the daughter of a carrier mother (II-1) and an unaffected father (II-2), making her a **carrier** with a 50% probability (X<sup>A</sup>X<sup>a</sup>).
- Since her mother (II-1) is a carrier (as her brother IV-1 is affected and her parents (I-1 and I-2) are unaffected), and her father (II-2) is unaffected, the woman (III-2) has a 50% chance of being a **carrier**. If she is a carrier (X<sup>A</sup>X<sup>a</sup>) and her son inherits her affected X chromosome, he will be affected. The probability of her son being affected is (0.5 probability of her being a carrier) * (0.5 probability of passing on the affected X to her son) = **0.25 or 25%**.
*12.5%*
- This value would arise from an incorrect calculation of the woman's carrier status, or the inheritance pattern.
- It does not account for the direct inheritance from a carrier mother to a son in an X-linked recessive pattern.
*100%*
- This would only be possible if the mother was affected (which she is not, as she is female and not shaded) and passing on the affected X to all sons, or if the father was affected in a condition with male-to-male transmission.
- The woman (III-2) herself is not affected, indicating she is at most a carrier, not homozygous for the affected allele, nor is the disorder dominant.
*50%*
- This would be the probability of the son being affected *if* the woman (III-2) was a confirmed carrier, or if the disorder was autosomal dominant if she was affected.
- This option incorrectly assumes that the woman (III-2) is a *confirmed* carrier, ignoring the 50% probability that she might not have inherited the carrier status from her mother.
*0%*
- If the woman (III-2) were not a carrier, or if the disease was autosomal recessive and her partner was unaffected and not a carrier, then the probability would be 0%.
- Given the pattern of inheritance and her family history (affected brother and maternal uncle), there is a definite risk of her being a carrier and passing it on.
Genetic counseling for lysosomal diseases US Medical PG Question 8: A 7-month-old boy is brought to the pediatrician by his parents due to progressively worsening weakness for the last three months. The parents also describe the boy as having an exaggerated response when startled as well as diminishing response to visual stimuli. At birth, the boy was healthy and remained as such for the first few months of life. The mother says pregnancy was unremarkable, and the boy was born at 39 weeks with no complications during delivery. He is up to date on his vaccinations. The boy's grandparents immigrated from an eastern European country. Physical examination reveals hyperreflexia. Abdominal examination reveals no abnormalities. On fundoscopy, the following is seen. Which of the following is most likely deficient in this patient?
- A. Arylsulfatase A
- B. β-Glucosidase
- C. α-Galactosidase
- D. Hexosaminidase B
- E. Hexosaminidase A (Correct Answer)
Genetic counseling for lysosomal diseases Explanation: ***Hexosaminidase A***
- Deficiency of **Hexosaminidase A** leads to **Tay-Sachs disease**, characterized by deterioration in motor and cognitive functions, aligning with the symptoms of weakness and abnormal responses.
- The condition is associated with a **cherry-red spot** on the retina, often observed in patients, further confirming the diagnosis.
- **Ashkenazi Jewish ancestry** is a key risk factor, consistent with the patient's eastern European heritage.
*Arylsulfatase A*
- Deficiency causes **metachromatic leukodystrophy**, which typically presents with **ataxia** and loss of previously attained skills, but not the exaggerated startle response noted in this case.
- The condition is not commonly associated with the specific visual and neurologic symptoms observed in the patient.
*β-Glucosidase*
- Deficiency leads to **Gaucher's disease**, which presents with splenomegaly, bone pain, and anemia, rather than the neurological symptoms seen here.
- The symptoms do not match the **progressive weakness** and startle reflex changes described.
*α-Galactosidase*
- Lack of this enzyme results in **Fabry disease**, which mainly causes pain episodes, skin lesions, and organ dysfunction, especially renal involvement.
- Neurological symptoms described here do not fit the typical presentation seen in Fabry disease.
*Hexosaminidase B*
- Deficiency causes **Sandhoff disease**, which presents similarly to Tay-Sachs with developmental regression and cherry-red spot.
- However, Sandhoff disease typically includes **hepatosplenomegaly**, which is notably absent in this patient on abdominal examination.
- The normal abdominal findings help distinguish this from Hexosaminidase A deficiency.
Genetic counseling for lysosomal diseases US Medical PG Question 9: An 18-month-old girl is brought to the pediatrician’s office for failure to thrive and developmental delay. The patient’s mother says she has not started speaking and is just now starting to pull herself up to standing position. Furthermore, her movement appears to be restricted. Physical examination reveals coarse facial features and restricted joint mobility. Laboratory studies show increased plasma levels of several enzymes. Which of the following is the underlying biochemical defect in this patient?
- A. Congenital lack of lysosomal formation
- B. Inappropriate protein targeting to endoplasmic reticulum
- C. Failure of mannose phosphorylation (Correct Answer)
- D. Inappropriate degradation of lysosomal enzymes
- E. Misfolding of nuclear proteins
Genetic counseling for lysosomal diseases Explanation: ***Failure of mannose phosphorylation***
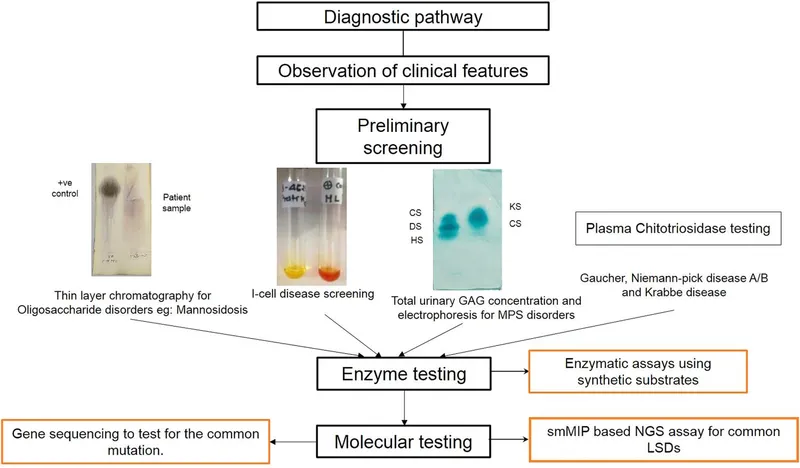
- The constellation of **failure to thrive**, **developmental delay**, **coarse facial features**, restricted joint mobility, and elevated plasma enzymes in an 18-month-old girl is highly suggestive of **I-cell disease** (mucolipidosis type II).
- **I-cell disease** is caused by the deficiency of **N-acetylglucosaminyl-1-phosphotransferase**, an enzyme responsible for phosphorylating mannose residues on lysosomal enzymes, which is crucial for proper targeting to the lysosome.
*Congenital lack of lysosomal formation*
- **Lysosomes** are present in this condition, but their enzymes are misdirected.
- A congenital lack of lysosomal formation would present with even more severe and widespread cellular dysfunction, possibly incompatible with life beyond early embryonic stages.
*Inappropriate protein targeting to endoplasmic reticulum*
- Proteins destined for the endoplasmic reticulum (ER) are typically targeted by an N-terminal signal peptide and then processed within the ER.
- While ER dysfunction can cause various disorders, the specific symptoms and enzyme elevations point away from a primary ER targeting defect related to lysosomal enzymes.
*Inappropriate degradation of lysosomal enzymes*
- In I-cell disease, lysosomal enzymes are synthesized but are **not properly targeted to the lysosomes**; instead, they are secreted into the bloodstream, leading to their elevated plasma levels.
- While some degradation might occur, the primary issue is mis-packaging and secretion, not increased degradation within the cell.
*Misfolding of nuclear proteins*
- Misfolding of nuclear proteins can lead to a variety of genetic disorders and cellular stress responses, but the clinical presentation, particularly the accumulation of undegraded material and elevated plasma lysosomal enzymes, is not characteristic of primary nuclear protein misfolding.
- The pathology in I-cell disease centers on lysosomal dysfunction rather than nuclear protein abnormalities.
Genetic counseling for lysosomal diseases US Medical PG Question 10: An investigator is studying the outcomes of a malaria outbreak in an endemic region of Africa. 500 men and 500 women with known malaria exposure are selected to participate in the study. Participants with G6PD deficiency are excluded from the study. The clinical records of the study subjects are reviewed and their peripheral blood smears are evaluated for the presence of Plasmodium trophozoites. Results show that 9% of the exposed population does not have clinical or laboratory evidence of malaria infection. Which of the following best explains the absence of infection seen in this subset of participants?
- A. Translocation of c-myc gene
- B. Glutamic acid substitution in the β-globin chain (Correct Answer)
- C. Inherited mutation affecting ribosome synthesis
- D. Inherited defect in erythrocyte membrane ankyrin protein
- E. Defective X-linked ALA synthase gene
Genetic counseling for lysosomal diseases Explanation: ***Glutamic acid substitution in the β-globin chain***
- This describes **sickle cell trait (heterozygous HbS)**, which confers significant protection against severe malaria, explaining the absence of infection despite exposure.
- Individuals with sickle cell trait have **abnormally shaped red blood cells** under low oxygen conditions, which are less hospitable for **Plasmodium falciparum** growth and are more rapidly cleared by the spleen.
*Translocation of c-myc gene*
- A **t(8;14) translocation of the c-myc gene** is characteristic of **Burkitt lymphoma**, a B-cell malignancy, and has no protective effect against malaria.
- This genetic alteration leads to overexpression of **c-myc**, a proto-oncogene, contributing to uncontrolled cell growth.
*Inherited mutation affecting ribosome synthesis*
- Defects in **ribosome synthesis** can lead to various **ribosomopathies**, affecting cell proliferation and function, but they are not known to provide protection against malaria.
- Such mutations often result in syndromes with **developmental abnormalities** or **bone marrow failure**.
*Inherited defect in erythrocyte membrane ankyrin protein*
- Defects in **ankyrin protein** are associated with **hereditary spherocytosis**, causing fragile, spherical red blood cells that are prematurely destroyed.
- While hereditary spherocytosis can reduce malaria severity, its role in preventing initial infection is less pronounced, and the question refers to absence of infection.
*Defective X-linked ALA synthase gene*
- A defective **X-linked ALA synthase gene** (ALAS2) is associated with **X-linked sideroblastic anemia**, causing impaired heme synthesis.
- This condition is characterized by **microcytic, hypochromic anemia** and iron overload in erythroid precursors, with no known protective effect against malaria.
More Genetic counseling for lysosomal diseases US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.