Lysosomal storage diseases

On this page
🧬 Lysosomal Storage Diseases: The Cellular Waste Crisis
Lysosomes are the cell's recycling centers, but when their enzymes fail, undegraded substrates accumulate and trigger a cascade of devastating multisystem disease. You'll master how specific enzyme deficiencies produce distinct clinical phenotypes across sphingolipidoses and mucopolysaccharidoses, learn to recognize their diagnostic patterns through physical and laboratory findings, and understand how enzyme replacement, substrate reduction, and emerging gene therapies are transforming once-fatal conditions into manageable diseases.
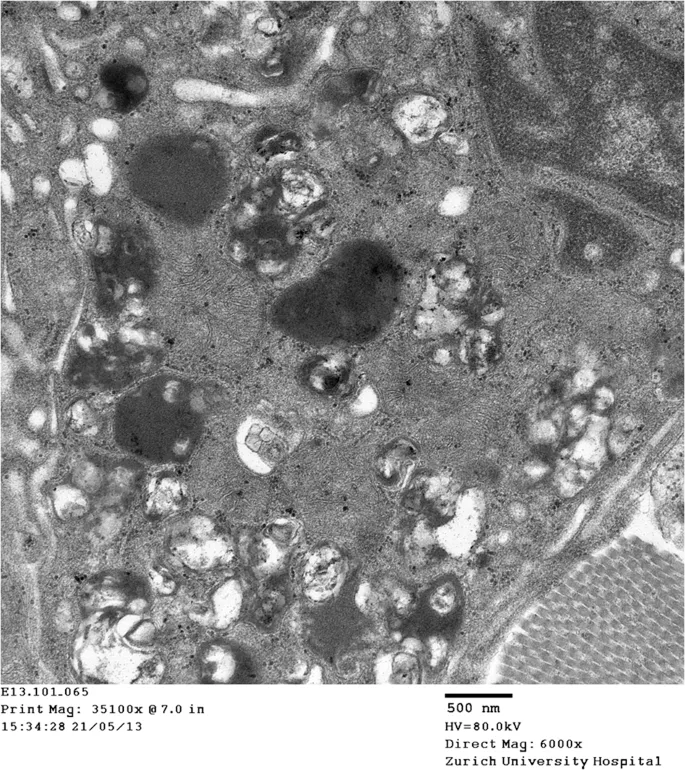
The Lysosomal Architecture: Precision Engineering
Lysosomes function as cellular digestive powerhouses, containing 60+ hydrolytic enzymes operating at pH 4.5-5.0. These membrane-bound organelles process 30% of cellular protein turnover and degrade complex substrates including:
- Sphingolipids - membrane components requiring 8 sequential enzymes
- GM2 ganglioside degradation involves β-hexosaminidase A with Km = 0.1 mM
- Glucocerebroside processing by β-glucocerebrosidase at optimal pH 5.2
- Sphingomyelin hydrolysis requiring acid sphingomyelinase activity
- Glycosaminoglycans (GAGs) - structural polysaccharides needing 12+ enzymes
- Heparan sulfate degradation through 4 distinct sulfatases
- Dermatan sulfate processing requiring α-L-iduronidase activity
- Keratan sulfate breakdown involving β-galactosidase and neuraminidase
- Glycoproteins - complex molecules requiring 15+ processing steps
- N-linked oligosaccharide removal by α-mannosidase (pH optimum 4.0)
- Sialic acid cleavage by neuraminidase with Km = 0.05 mM
📌 Remember: LAMP - Lysosomal Associated Membrane Proteins maintain the pH gradient essential for enzyme function. Without proper acidification, even normal enzymes fail.
Enzyme Targeting: The Mannose-6-Phosphate Postal System
Lysosomal enzymes reach their destination through the mannose-6-phosphate (M6P) pathway, a sophisticated cellular addressing system with 99.5% targeting accuracy:
- Step 1: N-acetylglucosamine phosphotransferase adds GlcNAc-1-phosphate to mannose residues
- Step 2: Phosphodiesterase removes GlcNAc, exposing M6P recognition signal
- Step 3: M6P receptors bind enzymes with Kd = 10-8 M affinity
- Step 4: Clathrin-coated vesicles transport enzyme-receptor complexes
- Step 5: Acidic pH (4.5) releases enzymes from receptors
⭐ Clinical Pearl: I-cell disease results from phosphotransferase deficiency, causing 10-20x elevated plasma lysosomal enzymes as they're secreted instead of targeted to lysosomes.
| Targeting Component | Normal Function | Deficiency Disease | Plasma Enzyme Levels |
|---|---|---|---|
| GlcNAc phosphotransferase | M6P signal creation | I-cell disease | ↑ 10-20x |
| M6P receptor | Enzyme recognition | Pseudo-Hurler | ↑ 3-5x |
| Clathrin machinery | Vesicle transport | Rare variants | ↑ 2-3x |
| V-ATPase pump | pH maintenance | Multiple LSDs | Variable |
| LAMP proteins | Membrane integrity | Danon disease | ↑ 5-10x |
When lysosomal enzymes fail, specific substrates accumulate with exponential kinetics, creating cellular toxicity through multiple mechanisms:
- Primary storage - direct substrate accumulation at mg/g tissue levels
- GM2 ganglioside in Tay-Sachs: 5-10x normal in neurons
- Glucocerebroside in Gaucher: 20-50x normal in macrophages
- Heparan sulfate in Sanfilippo: 10-15x normal in brain
- Secondary storage - downstream metabolic disruption
- Cholesterol accumulation due to membrane dysfunction
- Autophagy impairment with LC3-II/LC3-I ratio ↑ 3-5x
- Mitochondrial dysfunction with ATP production ↓ 40-60%
💡 Master This: Storage material accumulation follows zero-order kinetics when enzyme activity falls below 10% of normal, explaining why mild enzyme deficiencies may remain asymptomatic while severe deficiencies cause rapid progression.
The cellular consequences extend beyond simple storage, triggering inflammatory cascades with TNF-α levels ↑ 5-10x and IL-1β elevation ↑ 3-8x, creating a self-perpetuating cycle of tissue damage that explains the progressive nature of these devastating disorders.
Connect this foundational understanding of lysosomal precision through the major disease categories to understand how specific enzyme defects create distinct clinical patterns.
🧬 Lysosomal Storage Diseases: The Cellular Waste Crisis
🎭 The Sphingolipidosis Spectrum: Lipid Metabolism Mayhem
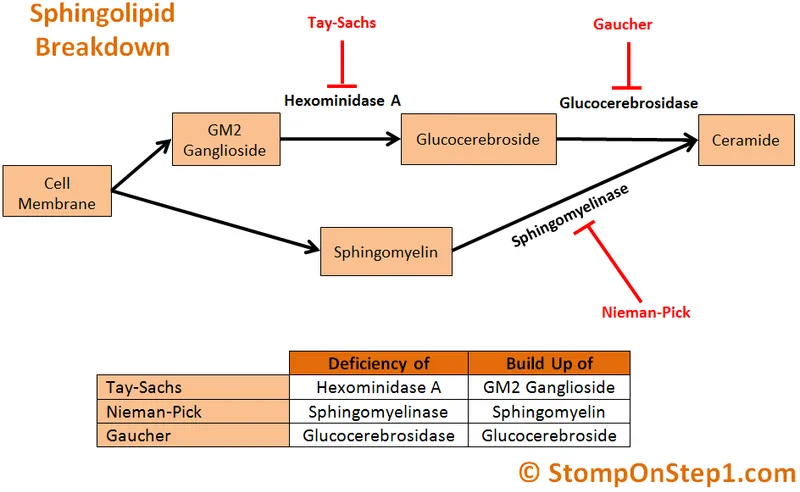
Tay-Sachs Disease: The GM2 Ganglioside Catastrophe
Tay-Sachs disease results from β-hexosaminidase A deficiency, blocking GM2 ganglioside degradation and causing massive neuronal storage with clinical onset by 6 months:
- Enzyme defect: HEXA gene mutations causing <5% residual activity
- Classic infantile: 0% enzyme activity, onset 3-6 months
- Juvenile form: 2-4% activity, onset 2-5 years
- Adult-onset: 5-10% activity, onset 20-30 years
- Pathological accumulation: GM2 ganglioside 10-100x normal in neurons
- Gray matter: 5-15 mg/g tissue (normal <0.5 mg/g)
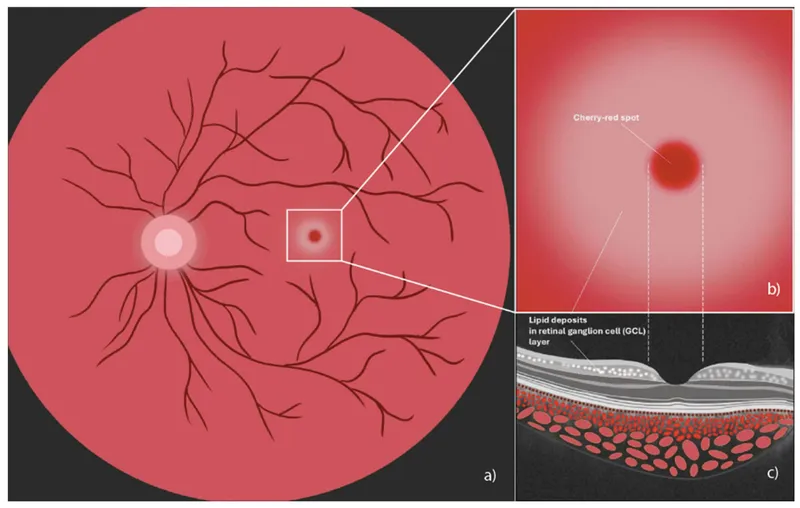
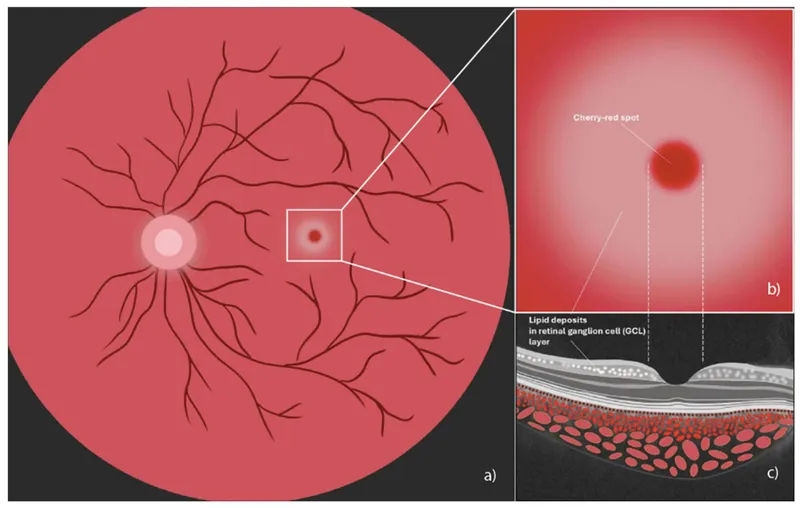
- Retinal ganglion cells: cherry-red spot in 90% of patients
- Cerebral cortex: neuronal ballooning with PAS-positive storage
📌 Remember: HEXA deficiency = Hexosaminidase A = Tay-Sachs. The cherry-red spot represents normal foveal color contrasting with pale, lipid-laden perifoveal retina.
- Clinical progression: Relentless neurodegeneration with death by 4 years
- 6 months: Loss of motor skills, hyperacusis to sound
- 12 months: Seizures in 80%, macrocephaly (head circumference >97th percentile)
- 18 months: Spasticity, blindness, feeding difficulties
- 24+ months: Vegetative state, recurrent infections
Gaucher Disease: The Glucocerebroside Accumulation Syndrome
Gaucher disease, the most common LSD (1 in 40,000 births, 1 in 800 Ashkenazi Jews), results from β-glucocerebrosidase deficiency with three distinct phenotypes based on neurological involvement:
- Type 1 (Non-neuronopathic) - 95% of cases
- Enzyme activity: 10-25% of normal
- Hepatosplenomegaly: Spleen 5-20x normal size
- Bone disease: Erlenmeyer flask deformity, avascular necrosis
- Hematologic: Thrombocytopenia (<100,000/μL), anemia (Hgb <10 g/dL)
- Type 2 (Acute neuronopathic) - <1% of cases
- Enzyme activity: <5% of normal
- Neurological onset: 3-6 months with death by 2 years
- Brainstem involvement: Stridor, swallowing dysfunction
- Type 3 (Chronic neuronopathic) - 5% of cases
- Enzyme activity: 5-10% of normal
- Horizontal gaze palsy: Pathognomonic sign in 90%
- Survival: Into adulthood with variable progression
⭐ Clinical Pearl: Gaucher cells (lipid-laden macrophages with wrinkled tissue paper appearance) contain glucocerebroside levels 20-100x normal and stain positive with PAS and acid phosphatase.
| Gaucher Type | Enzyme Activity | Neurological | Organomegaly | Bone Disease | Survival |
|---|---|---|---|---|---|
| Type 1 | 10-25% | None | Severe | Moderate-Severe | Normal |
| Type 2 | <5% | Severe acute | Moderate | Mild | <2 years |
| Type 3 | 5-10% | Chronic progressive | Severe | Severe | Adulthood |
| Perinatal | <1% | Severe | Massive | Severe | <6 months |
| Cardiovascular | <5% | Variable | Moderate | Mild | Variable |
Niemann-Pick Disease: The Sphingomyelin Storage Crisis
Niemann-Pick diseases encompass distinct disorders with different enzyme defects but similar clinical presentations involving hepatosplenomegaly and foam cell accumulation:
- Type A (Acute neuronopathic): Acid sphingomyelinase deficiency
- Enzyme activity: <5% of normal
- Clinical onset: First 6 months with death by 3 years
- Neurodegeneration: Cherry-red spot (50% of patients), seizures
- Hepatosplenomegaly: Massive with foam cells in bone marrow
- Type B (Non-neuronopathic): Partial sphingomyelinase deficiency
- Enzyme activity: 5-10% of normal
- Pulmonary involvement: Interstitial lung disease in 90%
- Survival: Into adulthood with respiratory complications
- Type C (Cholesterol transport defect): NPC1/NPC2 gene mutations
- Cholesterol accumulation: 5-10x normal in late endosomes
- Vertical gaze palsy: Pathognomonic in 90% by age 10
- Cataplexy: Gelastic seizures triggered by laughter/excitement
💡 Master This: Filipin staining shows intense perinuclear fluorescence in Niemann-Pick C fibroblasts due to unesterified cholesterol accumulation, providing a 95% sensitive diagnostic test.
The sphingolipidoses demonstrate how substrate specificity and tissue distribution determine clinical phenotype, with neurotropic substrates causing devastating CNS disease while systemic storage creates organomegaly and hematological complications.
Connect these lipid storage mechanisms through mucopolysaccharide accumulation patterns to understand how different substrate classes create distinct clinical syndromes.
🎭 The Sphingolipidosis Spectrum: Lipid Metabolism Mayhem
🏗️ Mucopolysaccharidosis Architecture: The GAG Storage Syndromes
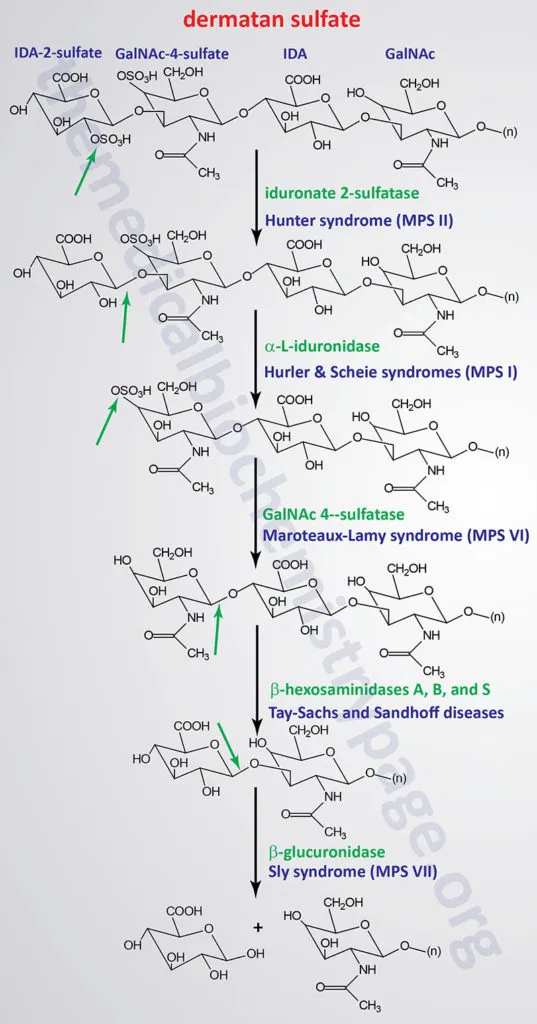
The MPS Classification: Seven Syndromes, Distinct Phenotypes
MPS disorders demonstrate genotype-phenotype correlation with enzyme activity levels determining clinical severity and age of onset:
- MPS I (Hurler/Scheie spectrum): α-L-iduronidase deficiency
- Hurler syndrome: <1% enzyme activity, onset 6-12 months
- Hurler-Scheie: 1-5% activity, onset 3-8 years
- Scheie syndrome: 5-10% activity, onset 5-15 years
- MPS II (Hunter syndrome): Iduronate-2-sulfatase deficiency
- X-linked inheritance (only MPS with male predominance)
- Severe form: <5% activity, death by 10-15 years
- Mild form: 5-20% activity, normal lifespan possible
- MPS III (Sanfilippo syndrome): Four enzyme defects (A, B, C, D)
- Predominant CNS involvement with behavioral problems
- Heparan sulfate accumulation 10-50x normal in brain
- Relatively mild somatic features compared to severe neurodegeneration
📌 Remember: Hunter syndrome is X-linked - Hunter hunts on the X chromosome. All other MPS are autosomal recessive.
| MPS Type | Enzyme Defect | Primary GAG | CNS Involvement | Corneal Clouding | Survival |
|---|---|---|---|---|---|
| I (Hurler) | α-L-iduronidase | Dermatan/Heparan | Severe | Present | <10 years |
| I (Scheie) | α-L-iduronidase | Dermatan/Heparan | Minimal | Present | Normal |
| II (Hunter) | Iduronate-2-sulfatase | Dermatan/Heparan | Variable | Absent | 10-60 years |
| III (Sanfilippo) | 4 different enzymes | Heparan | Severe | Absent | 10-20 years |
| IV (Morquio) | GALNS or β-galactosidase | Keratan/Chondroitin | Minimal | Present | 20-40 years |
| VI (Maroteaux-Lamy) | Arylsulfatase B | Dermatan | Absent | Present | 20-40 years |
| VII (Sly) | β-glucuronidase | Multiple GAGs | Variable | Variable | Variable |
Skeletal Dysplasia: The Dysostosis Multiplex Pattern
MPS disorders create pathognomonic skeletal changes called dysostosis multiplex, resulting from GAG accumulation in cartilage and bone matrix:
- Skull abnormalities: Macrocephaly with frontal bossing
- Head circumference >97th percentile by age 2
- Premature suture closure in 30-50% of patients
- J-shaped sella turcica on lateral skull X-ray
- Spinal deformities: Progressive kyphoscoliosis and instability
- Atlantoaxial instability in 20-40% requiring surgical fusion
- Thoracolumbar kyphosis with vertebral beaking
- Spinal cord compression risk increases with age
- Long bone changes: Metaphyseal widening and joint contractures
- Coxa valga with hip dysplasia in 60-80%
- Genu valgum requiring osteotomy in severe cases
- Joint contractures affecting 90% by adolescence
⭐ Clinical Pearl: Bullet-shaped vertebrae with anterior beaking on lateral spine X-ray is pathognomonic for MPS, seen in >90% of patients by age 5.
- Cardiac involvement: Progressive valvular disease and cardiomyopathy
- Mitral regurgitation in 70-90% by age 10
- Aortic stenosis developing in 40-60% by adolescence
- Coronary artery narrowing from GAG deposition
- Sudden cardiac death risk 5-10% in severe phenotypes
Neurological Manifestations: The CNS Storage Spectrum
CNS involvement varies dramatically among MPS types, correlating with blood-brain barrier penetration and neural GAG accumulation:
- Severe CNS disease (MPS I Hurler, MPS III Sanfilippo)
- Developmental regression beginning 18-36 months
- Seizures in 60-80% by school age
- Hydrocephalus requiring shunting in 40-70%
- Sleep apnea in 80-90% from airway GAG deposition
- Minimal CNS involvement (MPS VI, MPS IV)
- Normal intelligence maintained throughout life
- Hearing loss in 60-90% from middle ear GAG accumulation
- Cervical myelopathy from spinal instability
- Variable CNS disease (MPS II Hunter)
- Severe form: Progressive dementia with death by teens
- Mild form: Normal cognition with behavioral issues
💡 Master This: Heparan sulfate accumulation correlates with CNS severity - MPS III (pure heparan sulfate storage) shows severe neurodegeneration while MPS VI (dermatan sulfate only) spares cognition completely.
The mucopolysaccharidoses demonstrate how substrate-specific storage creates predictable clinical patterns, with GAG type and tissue distribution determining organ system involvement and disease progression.
Connect these GAG storage patterns through diagnostic recognition frameworks to understand how clinical presentations guide specific testing strategies.
🏗️ Mucopolysaccharidosis Architecture: The GAG Storage Syndromes
🔍 Clinical Recognition Mastery: The Diagnostic Pattern Matrix
Age-Based Recognition Patterns: The Temporal Diagnostic Framework
LSD presentations cluster into distinct age groups with characteristic features that guide differential diagnosis:
- Neonatal presentation (0-1 month)
- Hydrops fetalis: Gaucher type 2, GM1 gangliosidosis, I-cell disease
- Hepatosplenomegaly: >3 cm below costal margin at birth
- Coarse facial features: Evident within first weeks
- Cardiac involvement: Hypertrophic cardiomyopathy in Pompe disease
- Infantile onset (1-12 months)
- Developmental regression: Loss of milestones after normal early development
- Organomegaly: Progressive hepatosplenomegaly with growth failure
- Neurological signs: Hypotonia, seizures, visual impairment
- Cherry-red spot: Tay-Sachs, Niemann-Pick A, GM1 gangliosidosis
- Childhood onset (1-10 years)
- Behavioral changes: Hyperactivity, aggression in MPS III
- Skeletal deformities: Progressive dysostosis multiplex
- Corneal clouding: MPS I, MPS IV, MPS VI (not MPS II or III)
- Hearing loss: Progressive sensorineural in 70-90%
📌 Remember: CORNEAL clouding pattern - Cloudy in MPS I, IV, VI; Clear in MPS II, III (Hunter and Sanfilippo have clear corneas).
| Age Group | Key Features | High-Yield Diagnoses | Diagnostic Tests |
|---|---|---|---|
| Neonatal | Hydrops, hepatomegaly | Gaucher type 2, I-cell | Enzyme assays, urine GAGs |
| Infantile | Regression, cherry-red spot | Tay-Sachs, Niemann-Pick A | Hex A, sphingomyelinase |
| Childhood | Coarse features, organomegaly | MPS I, II, VI | Urine GAGs, enzyme assays |
| Adolescent | Behavioral, skeletal | MPS III, Gaucher type 1 | Heparan sulfate, β-glucosidase |
| Adult | Psychiatric, movement | Gaucher, Fabry, Pompe | Targeted enzyme testing |
Systematic organ system evaluation reveals characteristic patterns that narrow differential diagnosis:
- Neurological patterns: "See this, think that" correlations
- Cherry-red spot + regression = GM2/GM1 gangliosidosis or Niemann-Pick A
- Horizontal gaze palsy = Niemann-Pick C (90% specificity)
- Myoclonus + ataxia = Sialidosis, neuronal ceroid lipofuscinosis
- Peripheral neuropathy + angiokeratoma = Fabry disease
- Skeletal recognition patterns: Dysostosis multiplex variants
- Bullet vertebrae + coarse features = MPS (any type)
- Platyspondyly + normal intelligence = MPS IV (Morquio)
- Atlantoaxial instability = MPS I, II, IV (high surgical risk)
- Cardiac pattern recognition: Substrate-specific involvement
- Hypertrophic cardiomyopathy + muscle weakness = Pompe disease
- Valvular thickening + skeletal dysplasia = MPS I, II, VI
- Coronary narrowing + angiokeratoma = Fabry disease
⭐ Clinical Pearl: Angiokeratoma in a male with neuropathic pain and renal disease = Fabry disease until proven otherwise. α-galactosidase A deficiency affects 1 in 40,000 males.
- Ophthalmological patterns: Eye findings as diagnostic clues
- Corneal clouding: MPS I, IV, VI (mucopolysaccharidoses with keratan sulfate)
- Cherry-red spot: Gangliosidoses and Niemann-Pick A
- Corneal verticillata: Fabry disease (pathognomonic in males)
- Retinal degeneration: Neuronal ceroid lipofuscinosis
Laboratory-Guided Diagnostic Algorithms: The Biochemical Roadmap
Systematic laboratory evaluation follows clinical suspicion with tiered testing approaches:
- First-tier screening: High-yield, accessible tests
- Urine GAGs: Elevated >3x normal in all MPS except MPS III variants
- Plasma chitotriosidase: Elevated 100-1000x in Gaucher disease
- Lyso-Gb3: Elevated 5-50x in Fabry disease (males >females)
- CK elevation: >1000 U/L suggests Pompe disease in infants
- Second-tier confirmation: Specific enzyme assays
- Leukocyte enzymes: Reliable for most LSDs except Fabry (use plasma)
- Dried blood spots: Available for 15+ LSDs with >95% sensitivity
- Fibroblast enzymes: Gold standard when leukocyte assays inconclusive
- Third-tier molecular: Genetic confirmation and counseling
- Targeted sequencing: >400 known mutations for common LSDs
- Whole exome sequencing: For atypical presentations or negative enzyme assays
- Prenatal testing: Available for all major LSDs with >99% accuracy
💡 Master This: Enzyme activity <10% of normal typically causes severe phenotypes, while 10-30% activity may present as attenuated forms with later onset and slower progression.
The clinical recognition framework transforms rare disease diagnosis from random testing to systematic pattern recognition, enabling early detection and appropriate management of these complex disorders.
Connect these recognition patterns through differential diagnosis frameworks to understand how systematic comparison distinguishes similar presentations.
🔍 Clinical Recognition Mastery: The Diagnostic Pattern Matrix
⚖️ Differential Diagnosis Mastery: The Systematic Discrimination Framework
Hepatosplenomegaly-Predominant Presentations: The Visceral Storage Spectrum
Massive organomegaly narrows differential diagnosis to specific LSD categories with characteristic patterns:
- Gaucher disease variants: Glucocerebroside accumulation patterns
- Type 1: Spleen 5-20x normal, thrombocytopenia <100,000/μL
- Chitotriosidase: Elevated 100-1000x in 95% of patients
- Bone involvement: Erlenmeyer flask deformity, bone crises
- No neurological involvement in Type 1 (key discriminator)
- Niemann-Pick A/B: Sphingomyelin storage with foam cells
- Type A: Neurodegeneration + hepatosplenomegaly + death <3 years
- Type B: Pulmonary involvement + hepatosplenomegaly + normal CNS
- Foam cells: >20% in bone marrow with sea-blue histiocytes
- Glycogen storage disease type II (Pompe): α-glucosidase deficiency
- Infantile: Cardiomegaly + hepatomegaly + hypotonia
- CK elevation: >1000 U/L (vs normal in other LSDs)
- Muscle biopsy: Vacuolar myopathy with acid phosphatase positivity
📌 Remember: GAUCHER vs NIEMANN-PICK - Gaucher has Great bone disease, Niemann-Pick has Pulmonary and Neurological disease.
| Disease | Spleen Size | CNS Involvement | Bone Disease | Biomarker | Prognosis |
|---|---|---|---|---|---|
| Gaucher Type 1 | 5-20x normal | None | Severe | Chitotriosidase ↑↑↑ | Normal lifespan |
| Niemann-Pick A | 3-10x normal | Severe | Minimal | Sphingomyelinase ↓↓↓ | Death <3 years |
| Niemann-Pick B | 5-15x normal | None | Minimal | Sphingomyelinase ↓↓ | Adulthood |
| Pompe infantile | 2-5x normal | Minimal | None | CK ↑↑↑, α-glucosidase ↓↓↓ | Death <2 years |
| MPS I Hurler | 3-8x normal | Severe | Severe | GAGs ↑↑↑, α-iduronidase ↓↓↓ | Death <10 years |
Neurodegeneration-Predominant Presentations: The CNS Storage Hierarchy
Progressive neurological decline requires systematic evaluation of age-specific patterns and associated features:
- Infantile neurodegeneration (6-18 months onset)
- Tay-Sachs: Cherry-red spot + hyperacusis + macrocephaly
- Krabbe disease: Peripheral neuropathy + optic atrophy + irritability
- Metachromatic leukodystrophy: Gait abnormalities + white matter changes
- GM1 gangliosidosis: Coarse features + cherry-red spot + skeletal dysplasia
- Childhood neurodegeneration (2-10 years onset)
- MPS III (Sanfilippo): Behavioral problems + hyperactivity + sleep disturbance
- Neuronal ceroid lipofuscinosis: Seizures + visual loss + motor decline
- Niemann-Pick C: Vertical gaze palsy + cataplexy + hepatosplenomegaly
- Adult neurodegeneration (>20 years onset)
- Gaucher type 3: Horizontal gaze palsy + myoclonus + organomegaly
- Fabry disease: Neuropathic pain + angiokeratoma + renal disease
⭐ Clinical Pearl: Vertical gaze palsy is pathognomonic for Niemann-Pick C, present in 90% by age 10. Horizontal gaze palsy suggests Gaucher type 3.
- Biochemical discriminators: Substrate-specific accumulation patterns
- GM2 ganglioside: Tay-Sachs (hexosaminidase A <5%)
- Galactocerebroside: Krabbe (galactocerebrosidase <10%)
- Sulfatide: Metachromatic leukodystrophy (arylsulfatase A <10%)
- Heparan sulfate: MPS III (urine GAGs >10x normal)
Skeletal Dysplasia-Predominant Presentations: The Bone Storage Matrix
Progressive skeletal deformities with characteristic radiological patterns enable systematic differentiation:
- MPS with severe skeletal involvement
- MPS I (Hurler): Dysostosis multiplex + corneal clouding + CNS involvement
- MPS II (Hunter): Similar to Hurler but X-linked + no corneal clouding
- MPS VI (Maroteaux-Lamy): Severe skeletal + corneal clouding + normal intelligence
- MPS with unique skeletal patterns
- MPS IV (Morquio): Platyspondyly + odontoid hypoplasia + normal intelligence
- Short stature: <3rd percentile by age 5
- Atlantoaxial instability: >40% require surgical stabilization
- Non-MPS skeletal storage diseases
- Mucolipidosis II (I-cell): Severe dysostosis + gingival hyperplasia + early death
- Multiple sulfatase deficiency: Combined MPS features + ichthyosis
💡 Master This: Odontoid hypoplasia with atlantoaxial instability is characteristic of MPS IV and requires careful anesthetic management due to spinal cord compression risk.
The systematic differential diagnosis framework transforms complex clinical presentations into manageable diagnostic algorithms, enabling accurate identification and appropriate treatment of these challenging disorders.
Connect these differential frameworks through treatment decision algorithms to understand how accurate diagnosis guides therapeutic interventions.
⚖️ Differential Diagnosis Mastery: The Systematic Discrimination Framework
🎯 Treatment Revolution: The Therapeutic Precision Matrix
Enzyme Replacement Therapy: The Protein Precision Platform
ERT provides functional enzyme to replace deficient activity, achieving substrate clearance and clinical improvement in systemic manifestations:
- Gaucher disease ERT: Imiglucerase (recombinant β-glucocerebrosidase)
- Dosing: 60 units/kg IV every 2 weeks (standard dose)
- High-dose: 120 units/kg for severe bone disease
- Response monitoring: Hemoglobin ↑ 1-2 g/dL, platelet count ↑ 50-100%
- Spleen reduction: 20-50% within 6-12 months
- Bone improvement: T-score improvement over 2-4 years
- Fabry disease ERT: Agalsidase alfa/beta (α-galactosidase A)
- Dosing: 1.0 mg/kg (agalsidase beta) or 0.2 mg/kg (agalsidase alfa) IV every 2 weeks
- Renal protection: Stabilizes GFR decline when started early
- Cardiac benefits: LV mass reduction 10-20% over 1-2 years
- Pain reduction: >50% improvement in neuropathic pain scores
- Pompe disease ERT: Alglucosidase alfa (acid α-glucosidase)
- Dosing: 20 mg/kg IV every 2 weeks
- Infantile form: Survival improvement from <2 years to >10 years
- Late-onset: Stabilizes muscle function and respiratory capacity
- Biomarker response: CK reduction 50-80% within 6 months
📌 Remember: ERT LIMITS - Effective for Reversible manifestations, Tissue penetration Limited (especially CNS), Immune reactions possible, Monthly Infusions required, Treatment Starts early for best results.
| Disease | ERT Drug | Dose | Frequency | Primary Endpoints | CNS Penetration |
|---|---|---|---|---|---|
| Gaucher | Imiglucerase | 60 U/kg | Q2 weeks | Hgb, platelets, spleen | None |
| Fabry | Agalsidase beta | 1.0 mg/kg | Q2 weeks | GFR, LV mass, pain | Minimal |
| Pompe | Alglucosidase alfa | 20 mg/kg | Q2 weeks | Muscle function, survival | Limited |
| MPS I | Laronidase | 0.58 mg/kg | Weekly | GAGs, liver, joints | None |
| MPS II | Idursulfase | 0.5 mg/kg | Weekly | GAGs, liver, respiratory | None |
| MPS VI | Galsulfase | 1.0 mg/kg | Weekly | GAGs, endurance, joints | None |
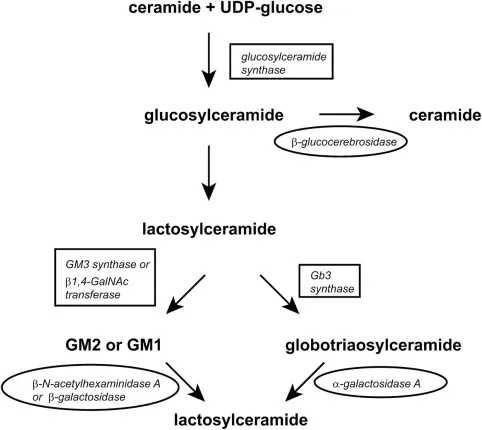
SRT reduces substrate production to match residual enzyme capacity, providing oral therapy option with different risk-benefit profile:
- Miglustat (Zavesca): Glucosylceramide synthase inhibitor
- Indications: Gaucher type 1 (mild-moderate), Niemann-Pick C
- Dosing: 100 mg TID (adults), weight-based (pediatric)
- Efficacy: Slower onset than ERT but sustained improvement
- Side effects: Diarrhea (85%), weight loss (60%), tremor (30%)
- Monitoring: Neurological exams every 6 months for peripheral neuropathy
- Eliglustat (Cerdelga): Selective glucosylceramide synthase inhibitor
- Indications: Gaucher type 1 in CYP2D6 extensive/intermediate metabolizers
- Dosing: 84 mg BID (extensive metabolizers), 84 mg daily (intermediate)
- Advantages: Better tolerability than miglustat, oral convenience
- Contraindications: CYP2D6 poor metabolizers, severe hepatic impairment
⭐ Clinical Pearl: CYP2D6 genotyping is mandatory before eliglustat initiation. Poor metabolizers (7% of population) have dramatically increased drug exposure and cardiac arrhythmia risk.
- Combination therapy strategies: ERT + SRT for enhanced efficacy
- Dose reduction: ERT dose can be reduced 25-50% when combined with SRT
- Synergistic effects: Greater substrate clearance than either therapy alone
- Cost considerations: Combination may be cost-neutral due to reduced ERT dosing
Pharmacological Chaperone Therapy: The Protein Folding Revolution
Chaperone therapy stabilizes misfolded enzymes, restoring partial function in amenable mutations:
- Migalastat (Galafold): α-galactosidase A pharmacological chaperone
- Mechanism: Binds and stabilizes mutant α-gal A, facilitating proper folding
- Amenable mutations: ~35% of Fabry mutations respond to chaperone therapy
- Dosing: 123 mg every other day (oral)
- Patient selection: In vitro amenability testing required
- Efficacy: Comparable to ERT in amenable patients with oral convenience
- Future chaperones: Multiple compounds in development
- Gaucher chaperones: Ambroxol, NCGC607 showing promising results
- Pompe chaperones: AT2220 (miglustat analog) in clinical trials
- Combination approaches: Chaperone + ERT for enhanced enzyme activity
💡 Master This: Pharmacological chaperones work only for missense mutations that produce misfolded but potentially functional enzyme. Nonsense mutations or large deletions are not amenable to chaperone therapy.
The therapeutic revolution in LSDs demonstrates how precision medicine approaches can transform devastating genetic diseases into manageable conditions, with ongoing research promising even more effective treatments including gene therapy and advanced enzyme technologies.
Connect these treatment strategies through advanced therapeutic integration to understand how emerging technologies will further revolutionize LSD management.
🎯 Treatment Revolution: The Therapeutic Precision Matrix
🚀 Advanced Therapeutic Integration: The Next-Generation Treatment Paradigm
Gene Therapy Revolution: The Genetic Correction Platform
Gene therapy addresses root cause enzyme deficiency through sustained transgene expression, potentially providing lifelong treatment with single administration:
- AAV-mediated liver gene therapy: Hepatocyte-targeted enzyme production
- Gaucher disease: AAV2/8-GBA achieving >50% normal enzyme activity
- Fabry disease: AAV-GLA producing sustained α-gal A levels for >2 years
- Pompe disease: AAV-GAA with liver-specific promoters avoiding immune responses
- Advantages: Single treatment, sustained expression, reduced immunogenicity
- CNS-directed gene therapy: Breaking the blood-brain barrier
- Intrathecal AAV9: Targets spinal cord and brain parenchyma
- MPS IIIA: AAV9-SGSH showing cognitive improvement in clinical trials
- Late infantile NCL: AAV2-CLN2 demonstrating slowed progression
- Delivery optimization: Enhanced AAV vectors with >10x CNS tropism
- Hematopoietic stem cell gene therapy: Systemic enzyme delivery
- Lentiviral vectors: Self-inactivating design with enhanced safety
- MPS I: HSC-gene therapy achieving enzyme cross-correction
- Advantages: Autologous cells, no immunosuppression, lifelong expression
📌 Remember: AAV ADVANTAGES - Autologous (no rejection), Adeno-associated (low immunogenicity), Vector integration rare (safer), Administration single dose, Durable expression, Versatile tissue targeting, Accessible manufacturing, Non-pathogenic wild-type virus, Tissue-specific promoters, Adjustable expression levels, Good safety profile, Effective CNS penetration, Stable episomal persistence.
| Gene Therapy Approach | Target Tissue | Delivery Route | Expression Duration | Clinical Status |
|---|---|---|---|---|
| AAV-liver | Hepatocytes | IV injection | >5 years | Phase I/II trials |
| AAV-CNS | Brain/spinal cord | Intrathecal | >3 years | Phase I/II trials |
| HSC-lentiviral | Hematopoietic | Ex vivo transduction | Lifelong | Phase II/III trials |
| AAV-muscle | Skeletal muscle | IM injection | >2 years | Preclinical |
| AAV-retinal | Retinal cells | Subretinal | >4 years | Phase I trials |
Advanced Enzyme Engineering: The Protein Optimization Revolution
Next-generation enzymes overcome current ERT limitations through enhanced properties and targeted delivery:
- Enhanced enzyme stability: Thermostable variants with extended half-life
- Modified glycosylation: Reduced clearance extending circulation time 2-5x
- PEGylation strategies: Polymer conjugation reducing immunogenicity 50-80%
- Fc fusion proteins: Antibody domains extending half-life 10-20x
- Improved tissue targeting: Receptor-mediated uptake enhancement
- Mannose-6-phosphate optimization: Enhanced lysosomal targeting
- Transferrin receptor targeting: Blood-brain barrier penetration
- Insulin-like growth factor targeting: Muscle-specific delivery
- Substrate-specific optimization: Tailored enzyme properties
- pH optimization: Enhanced activity at lysosomal pH 4.5-5.0
- Substrate affinity: Reduced Km values for improved efficiency
- Allosteric modulators: Small molecules enhancing enzyme activity 2-10x
⭐ Clinical Pearl: Blood-brain barrier penetration remains the major limitation for CNS manifestations. Transferrin receptor-targeted enzymes achieve 10-50x higher brain uptake compared to conventional ERT.
- Combination enzyme strategies: Multi-enzyme approaches for complex substrates
- Enzyme cocktails: Multiple deficient enzymes in single formulation
- Bifunctional enzymes: Engineered proteins with dual enzymatic activities
- Sequential therapy: Optimized timing of different enzyme administrations
Precision Monitoring and Biomarker Integration: The Therapeutic Optimization Matrix
Advanced biomarker strategies enable personalized dosing and outcome prediction:
- Substrate-specific biomarkers: Quantitative monitoring of therapeutic response
- Lyso-Gb3: Fabry disease monitoring with >95% correlation to clinical improvement
- Glucosylsphingosine: Gaucher disease biomarker more sensitive than chitotriosidase
- Heparan sulfate oligosaccharides: MPS III progression monitoring
- Pharmacokinetic optimization: Individualized dosing strategies
- Population PK modeling: Dose adjustment based on patient characteristics
- Therapeutic drug monitoring: Enzyme activity levels guiding dose optimization
- Antibody monitoring: Neutralizing antibodies requiring immunomodulation
- Imaging biomarkers: Non-invasive assessment of tissue improvement
- Cardiac MRI: T1 mapping detecting Fabry cardiomyopathy improvement
- Brain MRI: DTI changes monitoring white matter in MPS disorders
- Bone imaging: DEXA and MRI tracking Gaucher bone disease
💡 Master This: Biomarker-guided therapy can reduce treatment costs 20-40% while improving outcomes through personalized dosing and early intervention strategies.
Emerging Therapeutic Modalities: The Innovation Pipeline
Revolutionary approaches address fundamental limitations of current therapies:
- CRISPR gene editing: In vivo genetic correction
- Base editing: Single nucleotide correction without double-strand breaks
- Prime editing: Precise insertions/deletions with minimal off-targets
- Delivery challenges: Tissue-specific targeting and editing efficiency
- Induced pluripotent stem cells: Personalized cell therapy
- Patient-specific iPSCs: Genetic correction followed by differentiation
- Organoid models: Disease modeling and drug screening
- Clinical applications: Neuronal replacement for CNS manifestations
- Nanotechnology platforms: Targeted drug delivery
- Liposomal enzymes: Enhanced stability and tissue targeting
- Nanoparticle carriers: Blood-brain barrier penetration
- Smart delivery systems: pH-responsive and enzyme-triggered release
The advanced therapeutic integration represents a paradigm shift from symptomatic management to curative approaches, with multiple breakthrough technologies converging to offer unprecedented hope for patients and families affected by these devastating genetic disorders.
🚀 Advanced Therapeutic Integration: The Next-Generation Treatment Paradigm
Practice Questions: Lysosomal storage diseases
Test your understanding with these related questions
An 18-month-old boy is brought in by his parents for a routine check-up. The parents state that the patient still has not had any language development, and they are concerned about developmental delay. Of note, they have also noticed that the patient’s facial features have changed significantly in the last year. The patient also seems to have trouble visually focusing on objects or on the television. On exam, the patient's temperature is 98.2°F (36.8°C), blood pressure is 108/72 mmHg, pulse is 86/min, and respirations are 14/min. Of interest, the patient has not increased much in length or weight in the past 3 months. He is now in the 25th percentile for weight but is in the 90th percentile for head circumference. The patient does not appear to have any gross or fine motor deficiencies. Of note, he has coarse facial features that were not previously noted, including a long face, prominent forehead, and protruding eyes. The patient has corneal clouding bilaterally. At rest, the patient keeps his mouth hanging open. After extensive workup, the patient is found to have 2 mutated copies of the IDUA gene, with no production of the protein iduronidase. Which of the following is the likely mutation found in this disease?













