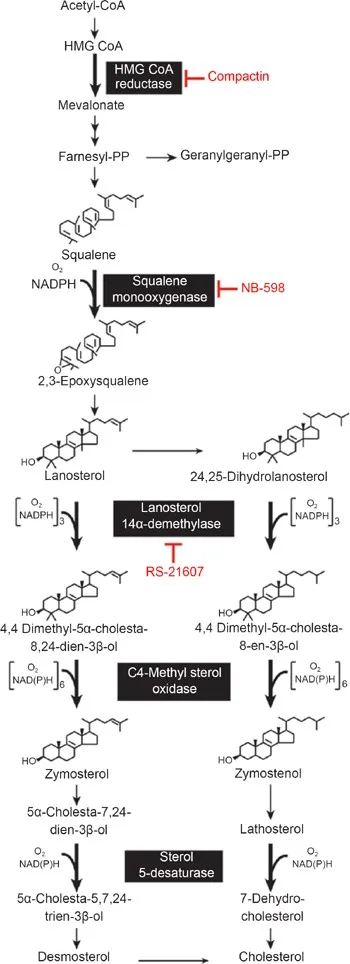
Cholesterol synthesis and regulation US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Cholesterol synthesis and regulation. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Cholesterol synthesis and regulation US Medical PG Question 1: A 54-year-old man comes to the physician for a health maintenance examination. He feels well. He is 173 cm (5 ft 8 in) tall and weighs 84 kg (185 lb); BMI is 28 kg/m2. His vital signs are within normal limits. Physical examination shows no abnormalities. Serum lipid studies show:
Total cholesterol 280 mg/dL
HDL-cholesterol 30 mg/dL
LDL-cholesterol 195 mg/dL
Triglycerides 275 mg/dL
Treatment with atorvastatin and cholestyramine is initiated. Which of the following changes is most likely induced by both agents?
- A. Increased hepatic bile salt synthesis
- B. Increased LDL receptor expression (Correct Answer)
- C. Increased lipoprotein lipase activity
- D. Decreased hepatic de novo cholesterol synthesis
- E. Increased cholesterol levels in hepatocytes
Cholesterol synthesis and regulation Explanation: ***Increased LDL receptor expression***
- **Statins** (e.g., atorvastatin) inhibit **HMG-CoA reductase**, leading to decreased intracellular cholesterol and thus upregulating hepatic **LDL receptors** to scavenge more cholesterol from the blood.
- **Bile acid sequestrants** (e.g., cholestyramine) bind bile acids in the gut, interrupting their enterohepatic circulation and increasing their fecal excretion. This increased demand for new bile acid synthesis by the liver depletes hepatocyte cholesterol, causing an upregulation of hepatic **LDL receptors** to acquire more cholesterol.
*Increased hepatic bile salt synthesis*
- **Cholestyramine** (a bile acid sequestrant) directly increases bile salt synthesis by the liver as it depletes the enterohepatic pool of bile acids, but **atorvastatin** (a statin) does not directly increase bile salt synthesis.
- Statins primarily reduce cholesterol synthesis, which is a precursor for bile acids, so their direct effect on bile acid synthesis is not an increase.
*Increased lipoprotein lipase activity*
- Neither **atorvastatin** nor **cholestyramine** primarily function by increasing **lipoprotein lipase (LPL)** activity.
- Fibrates are known to increase LPL activity, which enhances the clearance of **triglyceride-rich lipoproteins**.
*Decreased hepatic de novo cholesterol synthesis*
- **Atorvastatin** directly inhibits **HMG-CoA reductase**, the rate-limiting enzyme in **cholesterol synthesis**, thereby decreasing *de novo* cholesterol production.
- **Cholestyramine** does not directly decrease hepatic *de novo* cholesterol synthesis; instead, it increases cholesterol utilization for bile acid synthesis, which can secondarily influence cholesterol synthesis rates.
*Increased cholesterol levels in hepatocytes*
- Both **atorvastatin** and **cholestyramine** work to *decrease* intracellular **hepatocyte cholesterol levels**.
- Decreased intracellular cholesterol is the trigger for the upregulation of **LDL receptors**, which is the primary mechanism of action for both drugs in reducing circulating LDL.
Cholesterol synthesis and regulation US Medical PG Question 2: A 57-year-old presents to your clinic complaining of baldness. He is overweight, has been diagnosed with BPH, and is currently taking atorvastatin for hyperlipidemia. The patient has tried several over-the-counter products for hair-loss; however, none have been effective. After discussing several options, the patient is prescribed a medication to treat his baldness that has the additional benefit of treating symptoms of BPH as well. Synthesis of which of the following compounds would be expected to decrease in response to this therapy?
- A. Testosterone
- B. FSH
- C. LH
- D. GnRH
- E. DHT (Correct Answer)
Cholesterol synthesis and regulation Explanation: ***DHT***
- The medication described is likely **finasteride**, a **5-alpha-reductase inhibitor**. This enzyme converts **testosterone** to **dihydrotestosterone (DHT)**.
- Decreased DHT levels are beneficial for treating both **androgenetic alopecia (baldness)** and **benign prostatic hyperplasia (BPH)** due to its potent androgenic effects on hair follicles and prostate growth.
*Testosterone*
- While finasteride inhibits the conversion of testosterone to DHT, it does not directly decrease testosterone synthesis. In fact, **testosterone levels may slightly increase** as its conversion to DHT is blocked.
- Testosterone itself is not the primary androgen responsible for male pattern baldness or BPH; it's its more potent metabolite, DHT.
*FSH*
- **Follicle-stimulating hormone (FSH)** is a gonadotropin released from the anterior pituitary that stimulates sperm production and ovarian follicle development.
- The medication prescribed does not directly affect FSH synthesis or release; its action is peripheral, affecting androgen metabolism.
*LH*
- **Luteinizing hormone (LH)** is another gonadotropin that stimulates testosterone production in Leydig cells.
- The drug's mechanism of action is local inhibition of an enzyme, not a central regulation of pituitary hormones like LH.
*GnRH*
- **Gonadotropin-releasing hormone (GnRH)** is released from the hypothalamus and stimulates the anterior pituitary to release FSH and LH.
- This therapy specifically targets the conversion of an androgen and does not impact the hypothalamic-pituitary-gonadal axis at the level of GnRH.
Cholesterol synthesis and regulation US Medical PG Question 3: An investigator is studying vitamin D metabolism in mice. He induces a gene mutation that interferes with the function of an enzyme in the renal proximal tubules that is required for vitamin D activation. He then measures serum levels of various metabolites. Production of which of the following will be impaired in this mouse?
- A. Ergocalciferol
- B. Cholecalciferol
- C. 7-dehydrocholesterol
- D. 25-hydroxyvitamin D
- E. 1,25-dihydroxyvitamin D (calcitriol) (Correct Answer)
Cholesterol synthesis and regulation Explanation: ***1,25-dihydroxyvitamin D (calcitriol)***
- This is the **biologically active form** of vitamin D, produced in the kidney by the **1α-hydroxylase enzyme** in the renal proximal tubules.
- A mutation interfering with this enzyme would directly impair the conversion of 25-hydroxyvitamin D to **1,25-dihydroxyvitamin D**, the active form.
- This enzyme adds the second hydroxyl group at position 1, creating the dihydroxy form (calcitriol).
*Ergocalciferol*
- This is **vitamin D2**, obtained from dietary sources (plants, supplements) and is not produced endogenously in the body.
- Its production would not be directly affected by a renal tubular enzyme defect.
*Cholecalciferol*
- This is **vitamin D3**, primarily synthesized in the skin upon exposure to sunlight or obtained from animal-based dietary sources.
- Its production occurs before any renal activation steps, so it would not be impaired.
*7-dehydrocholesterol*
- This is a **precursor molecule** in the skin that is converted to cholecalciferol (vitamin D3) upon UV radiation.
- Its levels would not be directly affected by a defect in renal vitamin D activation.
*25-hydroxyvitamin D*
- This is the **storage form** of vitamin D, produced in the liver from cholecalciferol or ergocalciferol by the 25-hydroxylase enzyme.
- Its production occurs prior to the renal activation step and would therefore not be impaired by a defect in the renal tubules.
Cholesterol synthesis and regulation US Medical PG Question 4: A 62-year-old man comes to the physician for an annual health maintenance examination. He has a history of stable angina, gout, and hypertension. His medications include lisinopril and aspirin. He has smoked a pack of cigarettes daily for 20 years. He drinks 5–6 beers on the weekends. His blood pressure is 150/85 mm Hg. Laboratory studies show a total cholesterol of 276 mg/dL with an elevated low-density lipoprotein (LDL) concentration and low high-density lipoprotein (HDL) concentration. Administration of which of the following agents is the most appropriate next step in management?
- A. Peroxisome proliferator-activated receptor alpha activator
- B. Cholesterol absorption inhibitor
- C. HMG-CoA reductase inhibitor (Correct Answer)
- D. Bile acid resin
- E. Proprotein convertase subtilisin kexin 9 inhibitor
Cholesterol synthesis and regulation Explanation: ***HMG-CoA reductase inhibitor***
- This patient has a history of **stable angina**, **hypertension**, and **dyslipidemia** (elevated LDL, low HDL), placing him at high risk for cardiovascular events. **Statins** (HMG-CoA reductase inhibitors) are first-line therapy for reducing LDL cholesterol and cardiovascular risk in such patients.
- They work by **inhibiting the rate-limiting step of cholesterol synthesis** in the liver, leading to an upregulation of LDL receptors and increased clearance of LDL from the blood.
*Peroxisome proliferator-activated receptor alpha activator*
- These agents, like **fibrates**, primarily reduce **triglycerides** and can increase HDL, but they are less effective at lowering LDL compared to statins.
- They are typically used for patients with **severe hypertriglyceridemia** or in combination with statins if significant HDL or triglyceride issues persist.
*Cholesterol absorption inhibitor*
- **Ezetimibe** works by preventing the absorption of cholesterol in the small intestine.
- While effective at lowering LDL, it is generally used as an **add-on therapy to statins** or for patients unable to tolerate statins, rather than as a first-line agent in high-risk patients.
*Bile acid resin*
- **Bile acid sequestrants** (e.g., cholestyramine) work by binding bile acids in the intestine, preventing their reabsorption and increasing their excretion.
- This leads to increased hepatic synthesis of bile acids from cholesterol, lowering LDL, but they can cause **gastrointestinal side effects** and are generally less potent and less tolerated than statins.
*Proprotein convertase subtilisin kexin 9 inhibitor*
- **PCSK9 inhibitors** are highly effective at lowering LDL cholesterol by preventing the degradation of LDL receptors, thereby increasing LDL clearance.
- They are typically reserved for patients with **familial hypercholesterolemia** or those with established cardiovascular disease who have not achieved adequate LDL lowering with maximally tolerated statin therapy, often due to their high cost and subcutaneous administration.
Cholesterol synthesis and regulation US Medical PG Question 5: An investigator is studying a hereditary defect in the mitochondrial enzyme succinyl-CoA synthetase. In addition to succinate, the reaction catalyzed by this enzyme produces a molecule that is utilized as an energy source for protein translation. This molecule is also required for which of the following conversion reactions?
- A. Oxaloacetate to phosphoenolpyruvate (Correct Answer)
- B. Pyruvate to acetyl-CoA
- C. Acetaldehyde to acetate
- D. Glucose-6-phosphate to 6-phosphogluconolactone
- E. Fructose-6-phosphate to fructose-1,6-bisphosphate
Cholesterol synthesis and regulation Explanation: ***Oxaloacetate to phosphoenolpyruvate***
- The reaction catalyzed by **succinyl-CoA synthetase** (also known as succinate thiokinase) produces **GTP** (guanosine triphosphate) from GDP and Pi, in addition to succinate.
- **GTP** is required for the conversion of **oxaloacetate** to **phosphoenolpyruvate** in gluconeogenesis, catalyzed by **PEP carboxykinase**.
*Pyruvate to acetyl-CoA*
- This reaction is catalyzed by the **pyruvate dehydrogenase complex** and produces NADH, not GTP.
- It is an irreversible step linking glycolysis to the citric acid cycle.
*Acetaldehyde to acetate*
- This reaction is catalyzed by **aldehyde dehydrogenase** and uses **NAD+** as a cofactor, producing NADH.
- It is involved in alcohol metabolism.
*Glucose-6-phosphate to 6-phosphogluconolactone*
- This is the first committed step of the **pentose phosphate pathway**, catalyzed by **glucose-6-phosphate dehydrogenase**.
- It uses **NADP+** as a cofactor, producing NADPH.
*Fructose-6-phosphate to fructose-1,6-bisphosphate*
- This reaction is a key regulatory step in **glycolysis**, catalyzed by **phosphofructokinase-1 (PFK-1)**.
- It consumes **ATP**, rather than producing GTP or utilizing it as a cofactor in the context of this question.
Cholesterol synthesis and regulation US Medical PG Question 6: A startup is working on a novel project in which they claim they can replicate the organelle that is defective in MELAS syndrome. Which of the following metabolic processes must they be able to replicate if their project is to mimic the metabolic processes of this organelle?
- A. Hexose monophosphate shunt
- B. Cholesterol synthesis
- C. Glycolysis
- D. Fatty acid (beta) oxidation (Correct Answer)
- E. Fatty acid synthesis
Cholesterol synthesis and regulation Explanation: ***Fatty acid (beta) oxidation***
- **MELAS syndrome** (Mitochondrial Encephalomyopathy, Lactic Acidosis, and Stroke-like episodes) is caused by defects in **mitochondrial function**.
- **Beta-oxidation of fatty acids** is a crucial metabolic process that occurs within the mitochondria, generating ATP.
*Hexose monophosphate shunt*
- The **hexose monophosphate shunt** (pentose phosphate pathway) occurs in the **cytosol** and is primarily involved in producing NADPH and synthesizing nucleotides, not a primary mitochondrial function.
- Its dysfunction is not directly linked to the core metabolic defects seen in MELAS syndrome.
*Cholesterol synthesis*
- **Cholesterol synthesis** primarily occurs in the **cytosol** and the **endoplasmic reticulum**, not within the mitochondria.
- While cholesterol metabolism can be indirectly affected by mitochondrial health, it is not a direct mitochondrial metabolic pathway.
*Glycolysis*
- **Glycolysis** is the metabolic pathway that converts glucose into pyruvate, occurring in the **cytosol**.
- Although it precedes mitochondrial oxidative phosphorylation, glycolysis itself does not occur within the mitochondria.
*Fatty acid synthesis*
- **Fatty acid synthesis** primarily takes place in the **cytosol** and endoplasmic reticulum, utilizing NADPH from the hexose monophosphate shunt.
- It is an anabolic process, while MELAS typically involves defects in catabolic/energy-producing mitochondrial pathways.
Cholesterol synthesis and regulation US Medical PG Question 7: A 60-year-old patient is at his physician’s office for a routine health maintenance exam. The patient has a past medical history of osteoarthritis in his right knee and GERD that is well-controlled with over the counter medication. On a fasting lipid profile, he is found to have high cholesterol. The patient is started on daily atorvastatin to reduce his risk of cardiovascular disease. What is the major apolipoprotein found on the lipoprotein most directly affected by his statin medication?
- A. Apolipoprotein C-II
- B. Apolipoprotein B-100 (Correct Answer)
- C. Apolipoprotein A-I
- D. Apolipoprotein B-48
- E. Apolipoprotein E
Cholesterol synthesis and regulation Explanation: ***Apolipoprotein B-100***
- Statins primarily reduce **LDL-C** levels by inhibiting **HMG-CoA reductase**, leading to increased LDL receptor expression and clearance of LDL particles from the blood.
- **Apolipoprotein B-100** is the main apolipoprotein found on **LDL** and is crucial for its binding to the LDL receptor.
*Apolipoprotein C-II*
- This apolipoprotein activates **lipoprotein lipase**, which is involved in the hydrolysis of triglycerides in **chylomicrons** and **VLDL**, not directly targeted by statins.
- While statins can indirectly affect VLDL, ApoC-II is not the major apolipoprotein of the lipoprotein most directly affected by statins.
*Apolipoprotein A-I*
- **Apolipoprotein A-I** is the primary apolipoprotein found on **HDL**, which is involved in **reverse cholesterol transport**.
- While statins can have a modest effect on increasing HDL, their primary action is on reducing LDL.
*Apolipoprotein B-48*
- **Apolipoprotein B-48** is found exclusively on **chylomicrons**, which are responsible for the transport of exogenous dietary fats from the intestines.
- Chylomicrons are not the primary target of statin therapy, which focuses on endogenous cholesterol metabolism.
*Apolipoprotein E*
- **Apolipoprotein E** is found on chylomicrons, VLDL, and HDL and plays a role in receptor binding for their clearance from circulation.
- While important for lipoprotein metabolism, it is not the *major* apolipoprotein of the lipoprotein most *directly* affected by statins (LDL).
Cholesterol synthesis and regulation US Medical PG Question 8: Steroid hormone synthesis, lipid synthesis, and chemical detoxification are activities of which of the following?
- A. Peroxisomes
- B. Nucleolus
- C. Rough Endoplasmic Reticulum
- D. Smooth Endoplasmic Reticulum (Correct Answer)
- E. Golgi bodies
Cholesterol synthesis and regulation Explanation: ***Smooth Endoplasmic Reticulum***
- The **smooth endoplasmic reticulum (SER)** is rich in enzymes that catalyze the synthesis of **lipids**, including steroid hormones, and is crucial for the detoxification of drugs and poisons, particularly in liver cells.
- Its tubular structure, devoid of ribosomes, differentiates its functions from the rough ER, focusing on metabolic processes like **calcium ion storage** and carbohydrate metabolism.
*Peroxisomes*
- Peroxisomes are primarily involved in the breakdown of **fatty acids** and amino acids, producing hydrogen peroxide as a byproduct.
- They also play a role in detoxification but are not the primary site for steroid hormone or general lipid synthesis.
*Nucleolus*
- The **nucleolus** is a dense structure within the nucleus responsible for synthesizing **ribosomal RNA (rRNA)** and assembling ribosomes.
- It has no direct role in steroid hormone synthesis, lipid metabolism, or chemical detoxification.
*Rough Endoplasmic Reticulum*
- The **rough endoplasmic reticulum (RER)** is studded with **ribosomes** and is primarily involved in the synthesis and modification of **proteins** destined for secretion or insertion into membranes.
- While it's part of the endomembrane system, it does not directly perform lipid synthesis or chemical detoxification as its main functions.
*Golgi bodies*
- **Golgi bodies (or Golgi apparatus)** are responsible for modifying, sorting, and packaging **proteins and lipids** synthesized in the ER into vesicles for secretion or delivery to other organelles.
- They do not perform the initial synthesis of steroid hormones or lipids, nor are they the primary site for chemical detoxification.
Cholesterol synthesis and regulation US Medical PG Question 9: The human body obtains vitamin D either from diet or from sun exposure. Darker-skinned individuals require more sunlight to create adequate vitamin D stores as the increased melanin in their skin acts like sunscreen; thus, it blocks the necessary UV required for vitamin D synthesis. Therefore, if these individuals spend inadequate time in the light, dietary sources of vitamin D are necessary. Which of the following requires sunlight for its formation?
- A. 1,25-dihydroxyvitamin D
- B. 25-hydroxyvitamin D
- C. 7-dehydrocholesterol
- D. Cholecalciferol (D3) (Correct Answer)
- E. Ergocalciferol (D2)
Cholesterol synthesis and regulation Explanation: ***Cholecalciferol (D3)***
- **Cholecalciferol** (vitamin D3) is synthesized in the skin when **7-dehydrocholesterol** is exposed to **ultraviolet B (UVB) radiation** from sunlight.
- This is the initial step in the body's natural production of vitamin D, which then undergoes further hydroxylation in the liver and kidneys to become its active form.
*1,25-dihydroxyvitamin D*
- This is the **active form of vitamin D**, also known as **calcitriol**, produced in the **kidneys** from 25-hydroxyvitamin D via 1-alpha-hydroxylase.
- Its formation requires prior synthesis of cholecalciferol and subsequent hydroxylation, but it does not directly require sunlight.
*25-hydroxyvitamin D*
- This compound, also known as **calcidiol**, is formed in the **liver** from cholecalciferol (or ergocalciferol) through **25-hydroxylation**.
- While its precursor, cholecalciferol, is sunlight-dependent, 25-hydroxyvitamin D itself is not directly formed by sunlight.
*7-dehydrocholesterol*
- **7-dehydrocholesterol** is a **precursor molecule** found in the skin that is converted to cholecalciferol upon exposure to sunlight.
- It is not "formed" by sunlight; rather, it's the substrate upon which sunlight acts.
*Ergocalciferol (D2)*
- **Ergocalciferol** (vitamin D2) is primarily obtained from **plant-based sources** and fortified foods.
- It is not synthesized in the human skin through exposure to sunlight.
Cholesterol synthesis and regulation US Medical PG Question 10: A 52-year-old man comes to the physician because of a 4-month history of fatigue, weakness, constipation, decreased appetite, and intermittent flank pain. He takes ibuprofen for knee and shoulder pain. Physical examination shows mild tenderness bilaterally in the costovertebral areas. His serum calcium concentration is 11.2 mg/dL, phosphorus concentration is 2.5 mg/dL, and N-terminal parathyroid hormone concentration is 830 pg/mL. Which of the following steps in vitamin D metabolism is most likely increased in this patient?
- A. Ergocalciferol → 25-hydroxyergocalciferol
- B. 7-dehydrocholesterol → cholecalciferol
- C. 25-hydroxycholecalciferol → 1,25-dihydroxycholecalciferol (Correct Answer)
- D. 25-hydroxycholecalciferol → 24,25-dihydroxycholecalciferol
- E. Cholecalciferol → 25-hydroxycholecalciferol
Cholesterol synthesis and regulation Explanation: ***25-hydroxycholecalciferol → 1,25-dihydroxycholecalciferol***
- This patient presents with **hypercalcemia** (11.2 mg/dL), **hypophosphatemia** (2.5 mg/dL), and a markedly **elevated N-terminal parathyroid hormone (PTH)** concentration (830 pg/mL), which are classic findings for **primary hyperparathyroidism**.
- In primary hyperparathyroidism, elevated PTH directly stimulates the **renal 1-alpha-hydroxylase enzyme**, increasing the conversion of **25-hydroxycholecalciferol** (calcidiol) to its active form, **1,25-dihydroxycholecalciferol** (calcitriol), which raises calcium levels.
*Ergocalciferol → 25-hydroxyergocalciferol*
- This step involves the **hepatic 25-hydroxylase enzyme** converting dietary vitamin D2 (ergocalciferol) to its storage form, which is not primarily regulated by PTH in the context of hyperparathyroidism.
- While essential for vitamin D activation, this conversion rate is usually adequate and not the primary increased step responsible for the hypercalcemic state in this patient's presentation.
*7-dehydrocholesterol → cholecalciferol*
- This process is the **cutaneous synthesis of vitamin D3 (cholecalciferol)**, which is dependent on UV light exposure and is not directly regulated by PTH.
- This initial step of vitamin D synthesis occurs in the skin and is upstream of the metabolic pathway influenced by PTH.
*25-hydroxycholecalciferol → 24,25-dihydroxycholecalciferol*
- This conversion produces an **inactive form of vitamin D** and is catalyzed by the **24-hydroxylase enzyme**.
- This enzyme activity is typically **suppressed by high PTH** and **increased by high levels of 1,25-dihydroxycholecalciferol**, serving to degrade excess active vitamin D; therefore, this step would likely be decreased, not increased, in primary hyperparathyroidism.
*Cholecalciferol → 25-hydroxycholecalciferol*
- This is the **hepatic 25-hydroxylation** of vitamin D3, producing 25-hydroxycholecalciferol (calcidiol), the major circulating form of vitamin D.
- While critical for producing the substrate for further activation, this step is not the *most likely increased* step in response to high PTH in primary hyperparathyroidism.
More Cholesterol synthesis and regulation US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.