Lipid metabolism

On this page
🧬 The Lipid Universe: Your Body's Energy Empire
Lipids power your body's longest journeys, insulate its most vital structures, and signal between distant organs-yet their metabolism remains invisible until it fails spectacularly as atherosclerosis, fatty liver, or ketoacidosis. You'll master how enzymes choreograph fat synthesis and breakdown, how insulin and glucagon command these pathways, and why the liver, adipose tissue, and muscle each play distinct metabolic roles. By understanding both the elegant biochemistry and its clinical derailments, you'll recognize disease patterns early and deploy targeted therapies with precision, transforming abstract pathways into diagnostic and therapeutic power at the bedside.

Lipid metabolism represents the body's most sophisticated energy management system, processing 9 kcal/g compared to carbohydrates' 4 kcal/g. This metabolic empire spans multiple organ systems, from adipose tissue storing 15-20% of body weight as triglycerides to the liver synthesizing 800-1200 mg of cholesterol daily.
📌 Remember: FLICK - Fatty acid synthesis, Lipolysis, Integration with glucose, Cholesterol synthesis, Ketone production. These five pillars support the entire lipid metabolic framework, each requiring distinct enzymatic machinery and regulatory mechanisms.
The metabolic flexibility between fed and fasted states demonstrates lipid metabolism's central role. During 12-hour fasting, the body transitions from glucose oxidation to 60-70% fat oxidation, mobilizing stored triglycerides through hormone-sensitive lipase activation. This metabolic switch involves coordinated regulation of 15+ key enzymes across multiple pathways.
-
Energy Storage Systems
- Triglycerides: 135,000 kcal in average adult
- Phospholipids: 850-950 mg daily turnover
- Cholesterol: 300-500 mg dietary absorption
- Endogenous synthesis: 800-1200 mg/day
- Bile acid conversion: 400-500 mg/day
-
Metabolic Integration Points
- Acetyl-CoA: Central hub connecting carbohydrate and fat metabolism
- Malonyl-CoA: Reciprocal regulation of synthesis vs. oxidation
- Citrate: Allosteric activator of acetyl-CoA carboxylase
- Concentration threshold: 0.1-0.3 mM for lipogenesis activation

⭐ Clinical Pearl: Patients with familial hypercholesterolemia have 2-4x elevated LDL levels from birth, developing coronary artery disease by age 30-40 in heterozygotes and 10-20 in homozygotes. Early statin therapy reduces cardiovascular events by 25-35%.
| Lipid Class | Daily Turnover | Energy Yield | Clinical Threshold | Pathological Range |
|---|---|---|---|---|
| Triglycerides | 300-400g | 9 kcal/g | >150 mg/dL | >500 mg/dL |
| Cholesterol | 1.2-1.5g | 0 kcal/g | >200 mg/dL | >300 mg/dL |
| Phospholipids | 850-950mg | Variable | N/A | Deficiency states |
| Free Fatty Acids | 50-100g | 9 kcal/g | >0.4 mEq/L | >2.0 mEq/L |
| Ketone Bodies | 20-50g | 4.5 kcal/g | >0.5 mM | >3.0 mM |
The integration of lipid metabolism with hormonal regulation creates a sophisticated control system. Insulin promotes lipogenesis while inhibiting lipolysis, whereas glucagon and epinephrine reverse these processes within 5-10 minutes of hormonal stimulation. This rapid metabolic switching enables survival during feast-famine cycles that shaped human evolution.
Connect these foundational principles through enzymatic machinery to understand how specific proteins orchestrate the lipid metabolic symphony.
🧬 The Lipid Universe: Your Body's Energy Empire
⚙️ The Enzymatic Orchestra: Molecular Machines of Fat Metabolism
📌 Remember: CARB-PALM - Citrate activates, AMP inhibits, Regulated by phosphorylation, Biotinylated enzyme. Palmitoyl-CoA inhibits, Acetyl-CoA carboxylase, Lipogenesis control, Malonyl-CoA production. This mnemonic captures the complex regulation of the lipogenesis gateway enzyme.
-
Rate-Limiting Enzymes and Regulation
- Acetyl-CoA carboxylase: Biotin-dependent, citrate activation
- HMG-CoA reductase: Sterol-regulated, SREBP-controlled transcription
- Hormone-sensitive lipase: PKA phosphorylation, cAMP-dependent
- Phosphorylation increases activity 10-fold
- Dephosphorylation by PP2A within 2-5 minutes
-
Allosteric Control Mechanisms
- Citrate: Positive effector for ACC at 0.1-0.3 mM
- Palmitoyl-CoA: Negative feedback inhibitor
- AMP: Energy sensor, inhibits anabolic pathways
- AMP:ATP ratio >0.1 triggers metabolic switching
⭐ Clinical Pearl: Statins inhibit HMG-CoA reductase, reducing cholesterol synthesis by 30-50% and upregulating LDL receptors by 2-3 fold. This dual mechanism explains their 25-35% reduction in cardiovascular events across multiple clinical trials.
The compartmentalization of lipid metabolism creates distinct enzymatic environments. Fatty acid synthesis occurs in the cytoplasm using NADPH from the pentose phosphate pathway, while β-oxidation proceeds in mitochondria using NAD+ and FAD. This spatial separation prevents futile cycling and enables independent regulation.
| Enzyme | Location | Km Value | Vmax | Regulatory Mechanism |
|---|---|---|---|---|
| ACC | Cytoplasm | 20 μM | Variable | Allosteric + Phosphorylation |
| FAS | Cytoplasm | 50 μM | 100 nmol/min/mg | Transcriptional |
| CPT-1 | Mitochondria | 30 μM | 80 nmol/min/mg | Malonyl-CoA inhibition |
| HSL | Adipocytes | 10 μM | 200 nmol/min/mg | cAMP-dependent phosphorylation |
| HMG-CoA Reductase | ER | 5 μM | 50 nmol/min/mg | Sterol feedback |
Enzyme induction and degradation provide longer-term metabolic control. Fatty acid synthase mRNA increases 5-10 fold during high-carbohydrate feeding, mediated by SREBP-1c and ChREBP transcription factors. Conversely, fasting induces 3-5 fold increases in CPT-1 and β-oxidation enzymes through PPAR-α activation.
The precision of enzymatic control extends to subcellular localization. Fatty acid synthase associates with the cytoskeleton, while ACC forms 20-40 nm filamentous polymers visible by electron microscopy. This spatial organization optimizes substrate channeling and metabolic efficiency.
Connect enzymatic precision through regulatory networks to understand how hormonal signals coordinate lipid metabolism across multiple tissues.
⚙️ The Enzymatic Orchestra: Molecular Machines of Fat Metabolism
🎛️ The Metabolic Control Tower: Hormonal Command Systems
📌 Remember: INSULIN-LIPS - Inhibits HSL, No lipolysis, Stimulates lipogenesis, Upregulates FAS, Lowers cAMP, Increases ACC activity, No ketogenesis. Lipid storage, Increased glucose uptake, Phosphatase activation, Synthesis promotion.
-
Insulin's Anabolic Actions (Fed State)
- ACC dephosphorylation: Activation within 5 minutes
- HSL phosphorylation: Inactivation, 70% reduction in lipolysis
- SREBP-1c activation: 2-3 fold increase in lipogenic enzymes
- FAS mRNA increases 5-10 fold within 4-6 hours
- ACC mRNA increases 3-5 fold within 2-4 hours
-
Counter-Regulatory Hormones (Fasted State)
- Glucagon: cAMP elevation, PKA activation
- Epinephrine: β3-adrenergic stimulation in adipose tissue
- Cortisol: Permissive effect, enhances lipolytic sensitivity
- HSL phosphorylation increases activity 8-10 fold
- Adipose triglyceride lipase activation 3-4 fold
⭐ Clinical Pearl: Type 2 diabetics with insulin resistance show 40-60% impaired suppression of lipolysis, leading to elevated free fatty acids that worsen hepatic insulin resistance. This creates a vicious cycle where lipid metabolism dysfunction perpetuates glucose intolerance.
The temporal dynamics of hormonal regulation create distinct metabolic phases. During the absorptive phase (0-4 hours post-meal), insulin dominates with 10-50 fold increases above basal levels. The post-absorptive phase (4-12 hours) shows declining insulin and rising glucagon, while prolonged fasting (>12 hours) activates cortisol and growth hormone.
| Hormone | Peak Response Time | Duration | Primary Lipid Effect | Magnitude |
|---|---|---|---|---|
| Insulin | 30-60 minutes | 2-4 hours | ↑ Lipogenesis, ↓ Lipolysis | 5-10 fold |
| Glucagon | 15-30 minutes | 1-2 hours | ↑ Ketogenesis | 3-5 fold |
| Epinephrine | 2-5 minutes | 30-60 minutes | ↑ Lipolysis | 10-20 fold |
| Cortisol | 2-4 hours | 6-12 hours | Permissive lipolysis | 2-3 fold |
| Growth Hormone | 1-2 hours | 4-8 hours | ↑ Lipolysis | 3-4 fold |
💡 Master This: The insulin:glucagon ratio determines metabolic state more than absolute hormone levels. A ratio >2.0 promotes anabolism, while <0.5 triggers catabolism. This explains why diabetic ketoacidosis occurs despite normal or elevated insulin levels when glucagon predominates.
Chronic hormonal adaptations reshape lipid metabolism over days to weeks. Prolonged insulin resistance upregulates SREBP-1c and ChREBP, paradoxically increasing hepatic lipogenesis despite impaired glucose uptake. This mechanism contributes to non-alcoholic fatty liver disease affecting 25-30% of adults in developed countries.
Connect hormonal coordination through tissue-specific responses to understand how different organs contribute to whole-body lipid homeostasis.
🎛️ The Metabolic Control Tower: Hormonal Command Systems
🏭 The Metabolic Factory Network: Tissue-Specific Lipid Operations
📌 Remember: ADIPOSE-ROLES - Adiponectin secretion, Dynamic storage, Insulin sensitivity, Plasminogen activator inhibitor, Osteocalcin regulation, Steroid metabolism, Energy buffering. Resistin production, Oxidative metabolism, Leptin signaling, Endocrine functions, Systemic inflammation.
-
Adipose Tissue Specialization
- White adipose: Energy storage, endocrine function
- Brown adipose: Thermogenesis, glucose disposal
- Beige adipose: Inducible thermogenic capacity
- UCP1 expression increases 50-100 fold with cold exposure
- Glucose uptake increases 10-15 fold during activation
-
Hepatic Lipid Processing Hub
- VLDL synthesis: 20-40g triglycerides daily
- Cholesterol synthesis: 800-1200mg daily production
- Ketone production: 150-200g during prolonged fasting
- β-hydroxybutyrate: Primary ketone (70-80% of total)
- Acetoacetate: Secondary ketone (15-20% of total)
The liver's metabolic zonation creates specialized microenvironments for lipid processing. Periportal hepatocytes (Zone 1) excel in β-oxidation and gluconeogenesis, while pericentral hepatocytes (Zone 3) specialize in lipogenesis and ketogenesis. This spatial organization optimizes metabolic efficiency based on oxygen and substrate gradients.
⭐ Clinical Pearl: Non-alcoholic fatty liver disease progresses from simple steatosis (>5% hepatic fat) to steatohepatitis when oxidative stress overwhelms antioxidant capacity. Patients with >10% hepatic fat content show 2-3 fold increased cardiovascular risk independent of other metabolic factors.
Skeletal muscle demonstrates remarkable metabolic flexibility, switching between glucose and fat oxidation based on substrate availability and energy demands. During moderate exercise (50-65% VO2 max), muscle derives 50-60% of energy from fat oxidation, increasing to 85-90% during prolonged endurance activities.
| Tissue | Primary Function | Daily Throughput | Key Enzymes | Regulatory Factors |
|---|---|---|---|---|
| Adipose | Energy Storage | 300-400g FA flux | HSL, ATGL, LPL | Insulin, Catecholamines |
| Liver | Processing Hub | 100-150g FA oxidation | CPT-1, HMG-CoA | Glucagon, Insulin |
| Muscle | Energy Consumer | 200-300g FA oxidation | CPT-1, HAD | Contraction, Insulin |
| Brain | Ketone Consumer | 50-100g ketone use | SCOT, ACAT | Ketone availability |
| Heart | Fat Specialist | 60-80g FA oxidation | CPT-1, MCAD | Fatty acid supply |
💡 Master This: Understanding tissue-specific metabolic specialization explains disease patterns. Muscle insulin resistance primarily affects glucose uptake, while adipose insulin resistance increases lipolysis and free fatty acid release, contributing to hepatic steatosis and systemic inflammation.
Cardiac muscle shows unique metabolic characteristics, deriving 60-70% of energy from fatty acid oxidation under normal conditions. This preference reflects high mitochondrial density (30-35% of cell volume) and abundant CPT-1 expression. During ischemia, the heart rapidly switches to glucose metabolism, but prolonged fatty acid exposure can impair this flexibility.
The integration of tissue-specific functions creates whole-body metabolic homeostasis. Adipose tissue releases free fatty acids during fasting, liver converts excess to ketones for brain utilization, and muscle adjusts oxidative capacity based on training status. This coordinated response maintains energy availability across diverse physiological states.
Connect tissue specialization through pathological disruptions to understand how metabolic diseases affect lipid homeostasis.
🏭 The Metabolic Factory Network: Tissue-Specific Lipid Operations
🚨 The Metabolic Breakdown: When Lipid Systems Fail
📌 Remember: LIPID-CHAOS - LDL receptor defects, Increased cholesterol synthesis, Premature atherosclerosis, Impaired clearance, Dysregulated feedback. Cardiovascular events, High cholesterol levels, ApoB mutations, Oxidative stress, Statin resistance patterns.
-
Primary Lipid Disorders
- Familial hypercholesterolemia: LDL >190 mg/dL in heterozygotes
- Familial combined hyperlipidemia: Multiple lipoprotein elevation
- Lipoprotein lipase deficiency: Triglycerides >1000 mg/dL
- Chylomicronemia syndrome: Pancreatitis risk when TG >500 mg/dL
- Eruptive xanthomas: Pathognomonic skin lesions
-
Secondary Metabolic Disruptions
- Type 2 diabetes: Insulin resistance affects multiple pathways
- Hypothyroidism: Reduced LDL clearance, elevated cholesterol
- Nephrotic syndrome: Increased VLDL synthesis, dyslipidemia
- Proteinuria >3.5g/day triggers compensatory lipoprotein synthesis
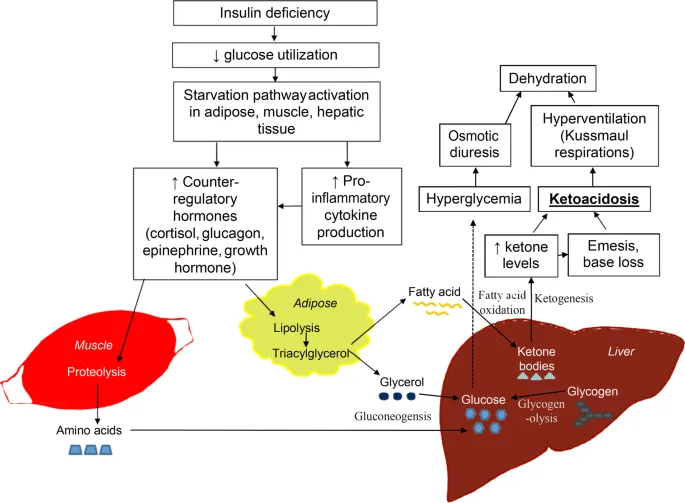
Diabetic ketoacidosis represents acute metabolic decompensation where insulin deficiency triggers uncontrolled lipolysis and ketogenesis. Free fatty acid levels increase 5-10 fold, overwhelming hepatic oxidative capacity and driving ketone production to >20 mM (normal <0.5 mM). The resulting metabolic acidosis (pH <7.3) and dehydration create life-threatening complications.
⭐ Clinical Pearl: The "ketone gap" helps distinguish diabetic ketoacidosis from other causes of metabolic acidosis. When serum ketones exceed 3 mM with anion gap >12 mEq/L, DKA is virtually certain. Treatment requires insulin therapy plus aggressive fluid resuscitation to reverse lipolysis.
Non-alcoholic fatty liver disease demonstrates how metabolic flexibility becomes pathological rigidity. Hepatic steatosis begins when triglyceride synthesis exceeds VLDL export capacity, typically when hepatic fat content exceeds 5% of liver weight. Progression to steatohepatitis involves oxidative stress, inflammation, and fibrosis development.
| Disorder | Prevalence | Key Defect | Clinical Threshold | Complications |
|---|---|---|---|---|
| Familial Hypercholesterolemia | 1:250 | LDL receptor | >190 mg/dL | CAD by age 40 |
| MCAD Deficiency | 1:10,000 | β-oxidation | Fasting intolerance | Hypoglycemia, coma |
| Gaucher Disease | 1:40,000 | Glucocerebrosidase | Organomegaly | Bone disease |
| NAFLD | 25-30% adults | Insulin resistance | >5% hepatic fat | Cirrhosis risk |
| Diabetic Ketoacidosis | 4-8% diabetics/year | Insulin deficiency | Ketones >3 mM | Cerebral edema |
💡 Master This: Metabolic disorders often show tissue-specific manifestations based on metabolic demands. Brain relies heavily on glucose and ketones, making it vulnerable to fatty acid oxidation defects. Heart depends on fat oxidation, explaining cardiomyopathy in MCAD deficiency.
The concept of metabolic inflexibility explains many acquired lipid disorders. Normal metabolism requires rapid switching between fed and fasted states, but chronic overnutrition or insulin resistance impairs this flexibility. Muscle becomes glucose-dependent even during fasting, while liver continues lipogenesis despite energy excess.
Therapeutic interventions target specific metabolic bottlenecks. Statins inhibit cholesterol synthesis, fibrates activate PPAR-α to enhance fat oxidation, and SGLT2 inhibitors promote ketogenesis by reducing insulin levels. Understanding the underlying metabolic defects guides rational therapeutic selection.
Connect pathological mechanisms through therapeutic interventions to understand how modern treatments restore metabolic balance.
🚨 The Metabolic Breakdown: When Lipid Systems Fail
🎯 The Therapeutic Arsenal: Precision Metabolic Interventions
📌 Remember: STATIN-POWER - Synthesis inhibition, Triglyceride reduction, Atherosclerosis regression, Thrombosis prevention, Inflammation reduction, Nitric oxide enhancement. Plaque stabilization, Oxidation prevention, Wall thickness reduction, Endothelial function, Receptor upregulation.
-
Statin Mechanisms and Outcomes
- HMG-CoA reductase inhibition: IC50 values 1-50 nM
- LDL receptor upregulation: 2-3 fold increase
- Cardiovascular risk reduction: 25-35% across trials
- Primary prevention: 1.2-1.5% absolute risk reduction
- Secondary prevention: 3-5% absolute risk reduction
-
Novel Therapeutic Targets
- PCSK9 inhibitors: Monoclonal antibodies, 60-70% LDL reduction
- Bempedoic acid: ATP citrate lyase inhibition
- Inclisiran: siRNA targeting PCSK9 synthesis
- Duration: 6-month dosing intervals
- Efficacy: 50-60% sustained LDL reduction

The emergence of metabolic modulators represents a paradigm shift toward multi-pathway targeting. GLP-1 receptor agonists not only improve glucose control but also slow gastric emptying, reduce appetite, and promote weight loss of 5-15% in clinical trials. These agents demonstrate how incretin-based therapy addresses multiple metabolic abnormalities simultaneously.
⭐ Clinical Pearl: Combination therapy targeting multiple pathways often provides synergistic benefits. Statin plus ezetimibe reduces LDL by 50-60% compared to 30-40% with statin alone, while adding PCSK9 inhibitors can achieve LDL levels <30 mg/dL in high-risk patients.
Precision medicine approaches tailor therapy based on genetic polymorphisms and metabolic phenotypes. CYP2C19 variants affect clopidogrel metabolism, while SLCO1B1 polymorphisms influence statin-induced myopathy risk. Pharmacogenomic testing guides drug selection and dosing in 10-15% of patients with adverse reactions.
| Therapeutic Class | Primary Target | Efficacy | Onset | Duration |
|---|---|---|---|---|
| Statins | HMG-CoA Reductase | 30-50% LDL ↓ | 2-4 weeks | Daily dosing |
| PCSK9 Inhibitors | PCSK9 Protein | 60-70% LDL ↓ | 1-2 weeks | 2-4 weeks |
| Fibrates | PPAR-α | 30-50% TG ↓ | 1-2 weeks | Daily dosing |
| Ezetimibe | NPC1L1 | 18-25% LDL ↓ | 1-2 weeks | Daily dosing |
| GLP-1 Agonists | GLP-1 Receptor | 5-15% weight ↓ | 4-8 weeks | Weekly dosing |
💡 Master This: Therapeutic success requires matching drug mechanisms to patient phenotypes. High triglycerides respond best to fibrates or omega-3 fatty acids, while elevated LDL requires statins or PCSK9 inhibitors. Understanding metabolic pathways guides rational drug selection.
Lifestyle interventions remain foundational, with Mediterranean diet reducing cardiovascular events by 30% in primary prevention trials. Exercise training improves insulin sensitivity, increases HDL by 5-10%, and reduces triglycerides by 20-30%. These interventions work through multiple pathways that complement pharmacological approaches.
The future of lipid therapeutics involves personalized medicine based on genetic profiles, metabolomic signatures, and artificial intelligence algorithms. Machine learning models incorporating >100 biomarkers can predict cardiovascular risk with 85-90% accuracy, enabling precision prevention strategies.
Connect therapeutic precision through clinical mastery frameworks to develop systematic approaches for optimal patient care.
🎯 The Therapeutic Arsenal: Precision Metabolic Interventions
🏆 The Clinical Mastery Toolkit: Rapid-Fire Lipid Expertise
📌 Remember: LIPID-MASTER - Lab interpretation, Identify patterns, Phenotype recognition, Intervention selection, Dose optimization. Monitor response, Adjust therapy, Side effect management, Target achievement, Evidence application, Risk stratification.
Essential Clinical Thresholds for Immediate Recognition:
-
Cardiovascular Risk Stratification
- Very High Risk: LDL goal <70 mg/dL (or <55 mg/dL in Europe)
- High Risk: LDL goal <100 mg/dL
- Moderate Risk: LDL goal <130 mg/dL
- 10-year ASCVD risk >20% = very high risk
- Diabetes + additional risk factor = high risk
-
Emergency Recognition Patterns
- Triglycerides >500 mg/dL: Pancreatitis risk, immediate intervention
- Total cholesterol >300 mg/dL: Consider familial hypercholesterolemia
- HDL <40 mg/dL (men) or <50 mg/dL (women): Metabolic syndrome component
⭐ Clinical Pearl: The "Rule of 7s" for statin intensity: High-intensity statins reduce LDL by ≥50%, moderate-intensity by 30-49%, low-intensity by <30%. Each doubling of statin dose provides only 6-7% additional LDL reduction, explaining why combination therapy often surpasses dose escalation.
| Clinical Scenario | First-Line Therapy | Target LDL | Monitoring Interval | Add-On Options |
|---|---|---|---|---|
| Primary Prevention | Moderate statin | <130 mg/dL | 6-8 weeks | Ezetimibe |
| Secondary Prevention | High-intensity statin | <70 mg/dL | 4-6 weeks | PCSK9 inhibitor |
| Familial Hypercholesterolemia | High-intensity statin | <70 mg/dL | 4-6 weeks | Combination therapy |
| Hypertriglyceridemia | Fibrate/Omega-3 | <150 mg/dL | 6-8 weeks | Lifestyle modification |
| Mixed Dyslipidemia | Statin + fibrate | Multiple targets | 4-6 weeks | GLP-1 agonist |
💡 Master This: "See This, Think That" - Eruptive xanthomas = triglycerides >1000 mg/dL; Corneal arcus <45 years = familial hypercholesterolemia; Hepatomegaly + dyslipidemia = NAFLD; Recurrent pancreatitis = lipoprotein lipase deficiency.
Advanced Clinical Integration Points:
-
Statin Intolerance Management (affects 10-15% of patients)
- True myopathy: CK elevation >10x upper limit normal
- Statin-associated muscle symptoms: Often nocebo effect
- Alternative approaches: Ezetimibe, bempedoic acid, PCSK9 inhibitors
-
Combination Therapy Optimization
- Statin + Ezetimibe: Additional 18-25% LDL reduction
- Triple therapy: Can achieve LDL <30 mg/dL in refractory cases
- Cost-effectiveness: Generic statins $50-100/year vs PCSK9i $5000-6000/year
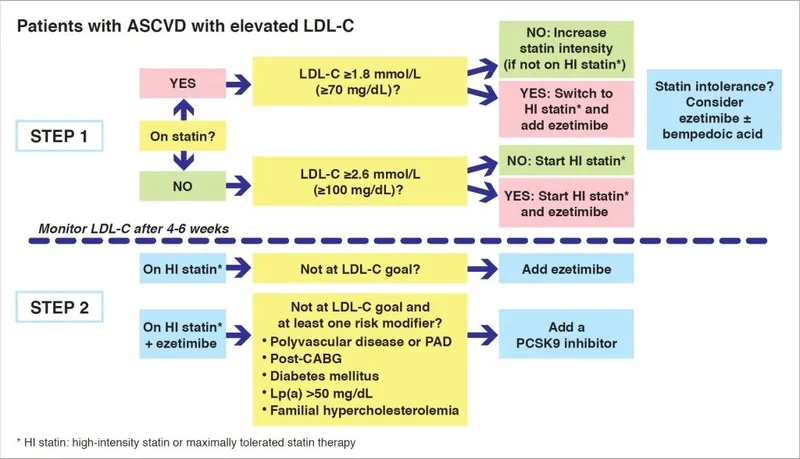
The integration of emerging biomarkers enhances risk prediction beyond traditional lipid panels. Lipoprotein(a) >50 mg/dL confers 2-3 fold increased cardiovascular risk, while ApoB levels better reflect atherogenic particle number than LDL cholesterol alone. Coronary calcium scoring reclassifies 25-30% of intermediate-risk patients.
⭐ Clinical Pearl: Therapeutic lifestyle changes remain foundational, reducing LDL by 10-15% and providing cardiovascular benefits independent of medications. Mediterranean diet + 150 minutes/week exercise + weight loss >5% can eliminate need for medications in 30-40% of moderate-risk patients.
Quality Metrics for Clinical Excellence:
- Time to target: 80% of patients reach LDL goals within 12 weeks
- Adherence optimization: Medication synchronization improves compliance by 15-20%
- Shared decision-making: Patient engagement increases long-term adherence by 25-30%
- Cardiovascular outcomes: Number needed to treat = 50-100 for primary prevention, 25-50 for secondary prevention
🏆 The Clinical Mastery Toolkit: Rapid-Fire Lipid Expertise
Practice Questions: Lipid metabolism
Test your understanding with these related questions
A 15-year-old boy is brought to the emergency department by his parents because of lethargy, repeated vomiting, and abdominal pain for 6 hours. Over the past 2 weeks, he has reported increased urinary frequency to his parents that they attributed to his increased oral fluid intake. Examination shows dry mucous membranes and rapid, deep breathing. Laboratory studies show the presence of acetoacetate in the urine. Which of the following cells is unable to use this molecule for energy production?












