Substrate-level phosphorylation US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Substrate-level phosphorylation. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Substrate-level phosphorylation US Medical PG Question 1: A 45-year-old man is brought to the emergency department by ambulance after vomiting blood. The patient reports that he only ate a small snack the morning before and had not eaten anything for over 24 hours. At the hospital, the patient is stabilized. He is admitted to a surgical floor and placed on NPO with a nasogastric tube set to intermittent suction. He has been previously diagnosed with liver cirrhosis. An esophagogastroduodenoscopy (EGD) has been planned for the next afternoon. At the time of endoscopy, some pathways were generating glucose to maintain serum glucose levels. Which of the following enzymes catalyzes the irreversible biochemical reaction of this process?
- A. Glucose-6-phosphate dehydrogenase
- B. Glycogen phosphorylase
- C. Enolase
- D. Glyceraldehyde-3-phosphate dehydrogenase
- E. Fructose-1,6-bisphosphatase (Correct Answer)
Substrate-level phosphorylation Explanation: ***Fructose-1,6-bisphosphatase***
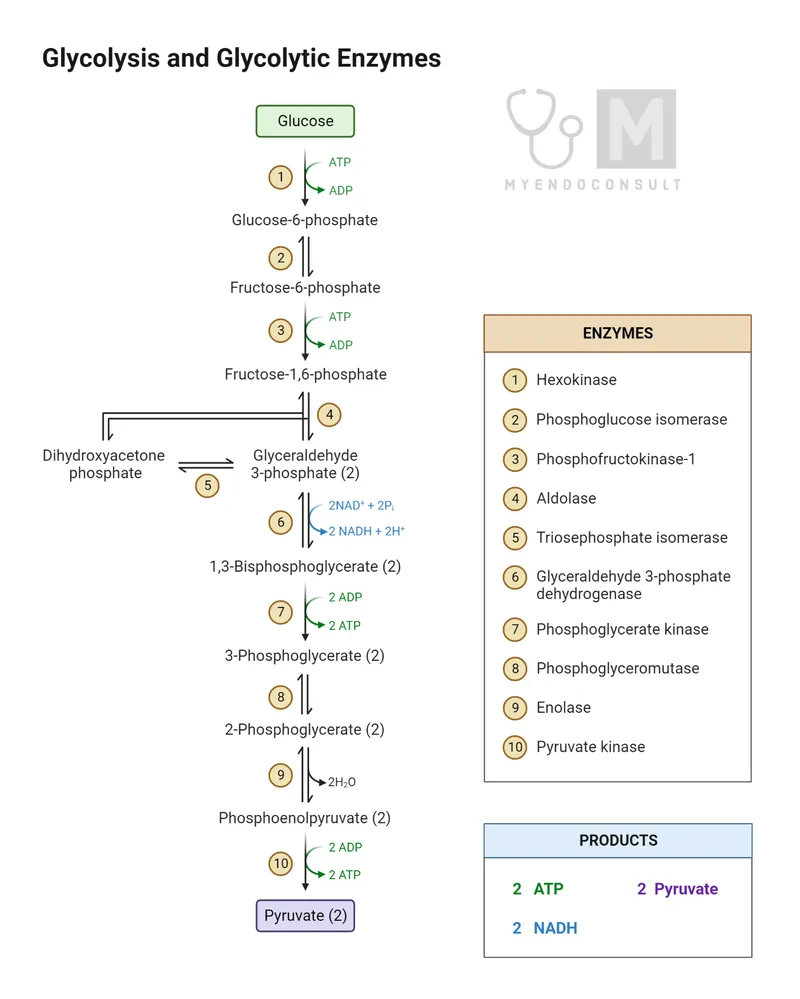
- The scenario describes a patient in a fasting state for over 24 hours, during which **gluconeogenesis** is crucial for maintaining blood glucose levels.
- **Fructose-1,6-bisphosphatase** is one of the key regulatory enzymes in gluconeogenesis, catalyzing an **irreversible reaction** that bypasses the phosphofructokinase-1 step of glycolysis.
*Glucose-6-phosphate dehydrogenase*
- This enzyme is involved in the **pentose phosphate pathway**, which generates NADPH and precursors for nucleotide synthesis.
- It does not directly participate in gluconeogenesis to produce glucose from non-carbohydrate sources.
*Glycogen phosphorylase*
- This enzyme is involved in **glycogenolysis**, the breakdown of glycogen into glucose-1-phosphate.
- While it releases glucose, the body's glycogen stores would likely be depleted after over 24 hours of fasting, making gluconeogenesis the primary pathway for glucose production.
*Enolase*
- Enolase is an enzyme in the glycolytic pathway, catalyzing the reversible conversion of 2-phosphoglycerate to phosphoenolpyruvate.
- It is not an enzyme of gluconeogenesis, nor does it catalyze an irreversible step in the glucose production process during fasting.
*Glyceraldehyde-3-phosphate dehydrogenase*
- This enzyme is also part of glycolysis, catalyzing the reversible oxidation and phosphorylation of glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate.
- Like enolase, it is not an irreversible enzyme in gluconeogenesis that would be generating glucose under fasting conditions.
Substrate-level phosphorylation US Medical PG Question 2: A 16-year-old boy presents with acute left-sided weakness. The patient is obtunded and can not provide any history other than his stomach hurts. The patient’s friend states that the patient has had episodes like this in the past and that “he has the same weird disease as his mom”. On physical examination, strength is 1 out of 5 in the left upper and lower extremities. A noncontrast CT scan of the head is normal. Laboratory tests reveal an anion gap metabolic acidosis. Which of the following is a normal function of the structure causing this patient’s condition?
- A. Regulation of blood glucose
- B. Synthesis of fatty acids
- C. Production of bile acids
- D. Metabolism of purines
- E. Conversion of ammonia to urea (Correct Answer)
Substrate-level phosphorylation Explanation: ***Conversion of ammonia to urea***
- The patient's presentation with **acute neurological deficits**, **abdominal pain**, and **anion gap metabolic acidosis** with a normal head CT, along with family history, is highly suggestive of a **urea cycle disorder (UCD)**.
- The **urea cycle** primarily functions in the **liver** to convert **toxic ammonia** into less toxic urea for excretion.
*Regulation of blood glucose*
- While regulation of blood glucose is a vital **liver function**, it does not directly relate to the primary metabolic derangement (ammonia accumulation) seen in UCDs.
- **Hypoglycemia** can occur in some UCDs, but it's not the defining feature of the neurological crisis.
*Synthesis of fatty acids*
- **Fatty acid synthesis** primarily occurs in the liver and adipose tissue, but its dysfunction is not the direct cause of the patient's acute symptoms.
- While liver dysfunction can impact lipid metabolism, it is not the central pathological process in UCDs.
*Production of bile acids*
- **Bile acid production** is a critical function of the liver for fat digestion and absorption, but it is not directly impaired in urea cycle disorders.
- Bile acid synthesis disorders would present with different clinical features, such as **cholestasis** and fat malabsorption.
*Metabolism of purines*
- **Purine metabolism** occurs in various tissues, and its dysfunction can lead to conditions like **gout** or **Lesch-Nyhan syndrome**, which differ from the presented symptoms.
- Abnormalities in purine metabolism are not the core defect in urea cycle disorders.
Substrate-level phosphorylation US Medical PG Question 3: A 26-year-old African American man comes to the physician because of a 3-day history of fatigue, back pain, and dark urine. One week ago, he developed a headache and was treated with aspirin. He does not smoke or use illicit drugs. Physical examination shows conjunctival pallor. A peripheral blood smear shows erythrocytes with inclusions of denatured hemoglobin. Which of the following enzymes is involved in providing precursors for nucleotide synthesis in this patient?
- A. Glucose-6-phosphatase
- B. Carbamoyl phosphate synthetase I
- C. Pyruvate carboxylase
- D. Transaldolase (Correct Answer)
- E. Enolase
Substrate-level phosphorylation Explanation: ***Transaldolase***
- This patient likely has **glucose-6-phosphate dehydrogenase (G6PD) deficiency**, indicated by fatigue, dark urine (hemolysis), and **Heinz bodies** (erythrocytes with inclusions of denatured hemoglobin) after aspirin exposure, which is an **oxidative stressor**.
- **Transaldolase** is an enzyme in the **non-oxidative phase of the pentose phosphate pathway (PPP)**, which produces **ribose-5-phosphate**, a precursor for nucleotide synthesis.
*Glucose-6-phosphatase*
- **Glucose-6-phosphatase** is involved in **gluconeogenesis** and glycogenolysis, primarily in the liver and kidneys, to release free glucose into the bloodstream.
- Deficiency leads to **Von Gierke disease**, characterized by hypoglycemia, hepatomegaly, lactic acidosis, and hyperlipidemia, which are not described here.
*Carbamoyl phosphate synthetase I*
- **Carbamoyl phosphate synthetase I (CPS I)** is a mitochondrial enzyme that catalyzes the first committed step in the **urea cycle**, converting ammonia and bicarbonate into carbamoyl phosphate.
- Its deficiency causes **hyperammonemia**, not hemolytic anemia or issues with nucleotide synthesis.
*Pyruvate carboxylase*
- **Pyruvate carboxylase** is a mitochondrial enzyme that converts **pyruvate to oxaloacetate**, a crucial step in **gluconeogenesis** and replenishing intermediates of the citric acid cycle.
- Deficiency can lead to lactic acidosis and hypoglycemia, which are not the primary symptoms here.
*Enolase*
- **Enolase** is an enzyme in **glycolysis** that catalyzes the dehydration of 2-phosphoglycerate to phosphoenolpyruvate.
- It is not directly involved in providing precursors for nucleotide synthesis.
Substrate-level phosphorylation US Medical PG Question 4: A 52-year-old man undergoes an exercise stress test for a 1-week history of squeezing substernal chest pain that is aggravated by exercise and relieved by rest. During the test, there is a substantial increase in the breakdown of glycogen in the muscle cells. Which of the following changes best explains this intracellular finding?
- A. Activation of phosphorylase kinase (Correct Answer)
- B. Decrease in protein kinase A
- C. Inactivation of glycogen synthase kinase
- D. Activation of protein phosphatase
- E. Increase in glucose-6-phosphate
Substrate-level phosphorylation Explanation: ***Activation of phosphorylase kinase***
- Exercise, particularly in the context of **ischemic heart disease** suggested by the patient's symptoms, triggers a rapid need for energy, leading to **glycogenolysis**.
- **Phosphorylase kinase** is the key enzyme that activates **glycogen phosphorylase**, the rate-limiting step in glycogen breakdown, to release glucose-1-phosphate from glycogen stores.
*Decrease in protein kinase A*
- **Protein kinase A (PKA)** is typically activated during exercise via **epinephrine** signaling, which in turn *activates* phosphorylase kinase and *inhibits* glycogen synthase.
- A decrease in PKA activity would lead to *reduced* glycogen breakdown, which contradicts the described increase in glycogen breakdown.
*Inactivation of glycogen synthase kinase*
- **Glycogen synthase kinase (GSK3)** phosphorylates and inactivates **glycogen synthase**, thereby *inhibiting* glycogen synthesis.
- If GSK3 were inactivated, glycogen synthesis would be *promoted*, rather than glycogen breakdown, further contradicting the clinical scenario.
*Activation of protein phosphatase*
- **Protein phosphatases** generally remove phosphate groups, which would *deactivate* glycogen phosphorylase and *activate* glycogen synthase.
- This action would promote glycogen synthesis and inhibit glycogen breakdown, which is the opposite of the observed physiological response during exercise.
*Increase in glucose-6-phosphate*
- While **glucose-6-phosphate** is an intermediate in glycogen metabolism, an increase in its concentration would primarily signal abundant glucose and tend to *inhibit* glycogen phosphorylase and *activate* glycogen synthase.
- This effect would favor glycogen synthesis and inhibit its breakdown, making it an unlikely explanation for increased glycogen breakdown during exercise.
Substrate-level phosphorylation US Medical PG Question 5: An investigator is studying a hereditary defect in the mitochondrial enzyme succinyl-CoA synthetase. In addition to succinate, the reaction catalyzed by this enzyme produces a molecule that is utilized as an energy source for protein translation. This molecule is also required for which of the following conversion reactions?
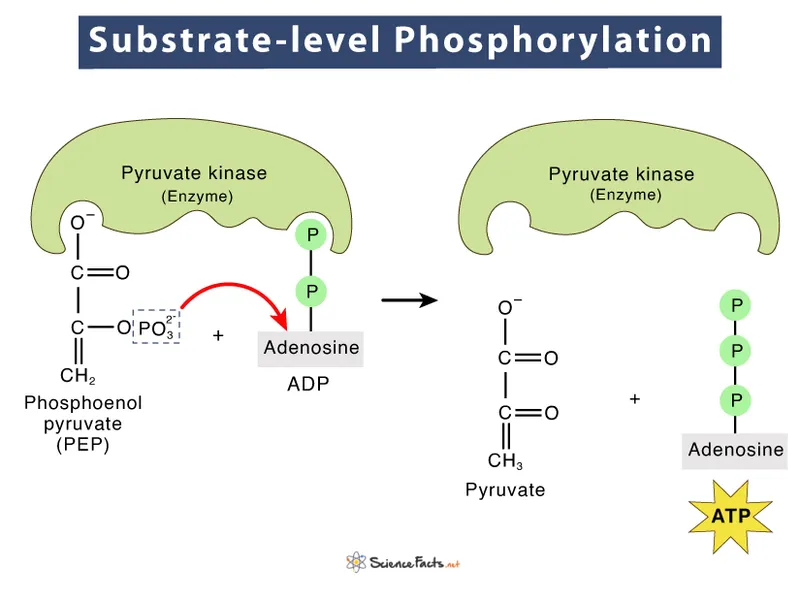
- A. Oxaloacetate to phosphoenolpyruvate (Correct Answer)
- B. Pyruvate to acetyl-CoA
- C. Acetaldehyde to acetate
- D. Glucose-6-phosphate to 6-phosphogluconolactone
- E. Fructose-6-phosphate to fructose-1,6-bisphosphate
Substrate-level phosphorylation Explanation: ***Oxaloacetate to phosphoenolpyruvate***
- The reaction catalyzed by **succinyl-CoA synthetase** (also known as succinate thiokinase) produces **GTP** (guanosine triphosphate) from GDP and Pi, in addition to succinate.
- **GTP** is required for the conversion of **oxaloacetate** to **phosphoenolpyruvate** in gluconeogenesis, catalyzed by **PEP carboxykinase**.
*Pyruvate to acetyl-CoA*
- This reaction is catalyzed by the **pyruvate dehydrogenase complex** and produces NADH, not GTP.
- It is an irreversible step linking glycolysis to the citric acid cycle.
*Acetaldehyde to acetate*
- This reaction is catalyzed by **aldehyde dehydrogenase** and uses **NAD+** as a cofactor, producing NADH.
- It is involved in alcohol metabolism.
*Glucose-6-phosphate to 6-phosphogluconolactone*
- This is the first committed step of the **pentose phosphate pathway**, catalyzed by **glucose-6-phosphate dehydrogenase**.
- It uses **NADP+** as a cofactor, producing NADPH.
*Fructose-6-phosphate to fructose-1,6-bisphosphate*
- This reaction is a key regulatory step in **glycolysis**, catalyzed by **phosphofructokinase-1 (PFK-1)**.
- It consumes **ATP**, rather than producing GTP or utilizing it as a cofactor in the context of this question.
Substrate-level phosphorylation US Medical PG Question 6: A 22-year-old medical student decides to fast for 24 hours after reading about the possible health benefits of fasting. She read that blood glucose levels are maintained by metabolic processes such as hepatic glycogenolysis and hepatic gluconeogenesis during the initial 3 days of fasting. During the day, she did not suffer from the symptoms of hypoglycemia. Which of the following signaling molecules most likely stimulated the reaction which maintained her blood glucose after all her stored glucose was broken down and used up?
- A. Adenosine diphosphate
- B. Acetyl CoA (Correct Answer)
- C. Acetate
- D. Citrate
- E. Adenosine monophosphate
Substrate-level phosphorylation Explanation: ***Acetyl CoA***
- **Acetyl CoA** is the key **allosteric activator of pyruvate carboxylase**, the first committed enzyme of gluconeogenesis that converts pyruvate to oxaloacetate.
- During prolonged fasting after glycogen stores are depleted, the body shifts to **fatty acid oxidation** (β-oxidation), which produces large amounts of **Acetyl CoA**.
- High **Acetyl CoA** levels signal that fat is being oxidized for energy, and simultaneously **activate gluconeogenesis** to maintain blood glucose for glucose-dependent tissues (brain, RBCs).
- This is the primary signaling mechanism that directly stimulates the gluconeogenic pathway after glycogen is exhausted.
*Adenosine monophosphate (AMP)*
- **AMP** levels rise during energy depletion and activate **AMP-activated protein kinase (AMPK)**.
- However, AMPK **inhibits gluconeogenesis** (not stimulates it) because gluconeogenesis is an **ATP-consuming** anabolic process (requires 6 ATP per glucose).
- AMPK promotes ATP-generating catabolic processes like fatty acid oxidation, but suppresses ATP-consuming processes like gluconeogenesis and fatty acid synthesis.
*Adenosine diphosphate (ADP)*
- **ADP** accumulates when ATP is hydrolyzed and signals moderate energy deficit.
- ADP is primarily a substrate for ATP regeneration via oxidative phosphorylation and does not directly regulate gluconeogenesis.
- Its role in metabolic regulation is less specific than allosteric activators like Acetyl CoA.
*Acetate*
- **Acetate** can be converted to Acetyl CoA but is not a direct signaling molecule for gluconeogenesis.
- It is a minor metabolite that may be produced in specific conditions (e.g., alcohol metabolism, ketoacidosis) but does not play a primary role in fasting-induced glucose homeostasis.
*Citrate*
- **Citrate** is a Krebs cycle intermediate that inhibits **phosphofructokinase-1 (PFK-1)** in glycolysis, thus reducing glucose breakdown.
- While citrate inhibition of glycolysis indirectly favors gluconeogenesis by preventing futile cycling, citrate does not **directly activate** gluconeogenic enzymes.
- Citrate primarily signals energy sufficiency and promotes fatty acid synthesis in the fed state, not fasting gluconeogenesis.
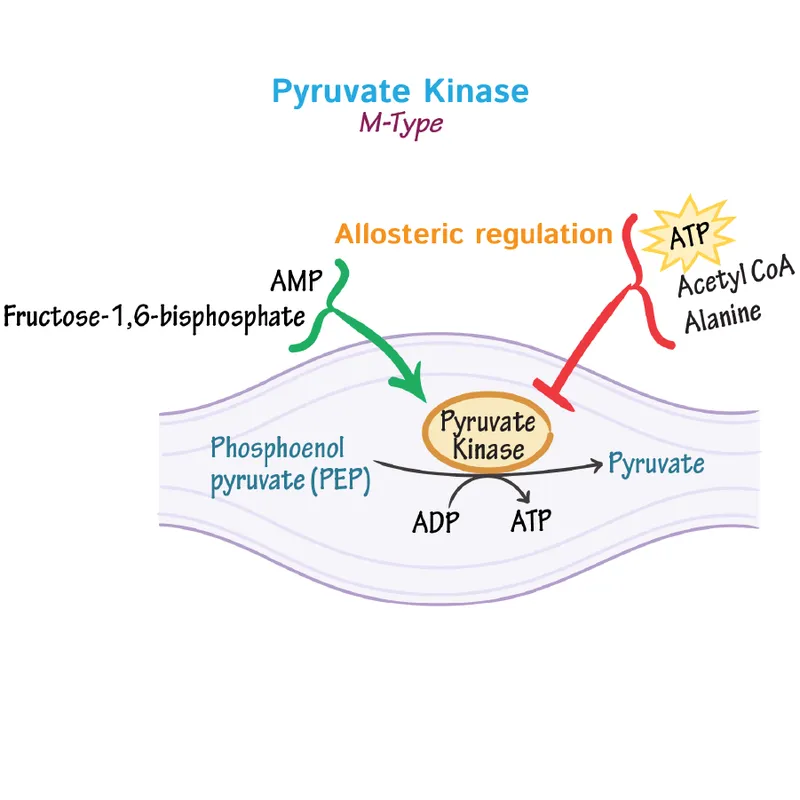
Substrate-level phosphorylation US Medical PG Question 7: A 12-year-old boy and his siblings are referred to a geneticist for evaluation of a mild but chronic hemolytic anemia that has presented with fatigue, splenomegaly, and scleral icterus. Coombs test is negative and blood smear does not show any abnormal findings. An enzymatic panel is assayed, and pyruvate kinase is found to be mutated on both alleles. The geneticist explains that pyruvate kinase functions in glycolysis and is involved in a classic example of feed-forward regulation. Which of the following metabolites is able to activate pyruvate kinase?
- A. Fructose-1,6-bisphosphate (Correct Answer)
- B. Alanine
- C. ATP
- D. Glucose-6-phosphate
- E. Glyceraldehyde-3-phosphate
Substrate-level phosphorylation Explanation: ***Fructose-1,6-bisphosphate***
- **Fructose-1,6-bisphosphate** is a potent **allosteric activator** of pyruvate kinase. This is an example of **feed-forward activation**, where a product of an early irreversible step in glycolysis (catalyzed by phosphofructokinase-1) activates a later enzyme (pyruvate kinase) in the pathway.
- This activation ensures that substrates for the later steps of glycolysis are rapidly utilized when earlier steps are highly active, matching the rate of metabolite flow and increasing the overall efficiency of glycolysis for energy production.
*Alanine*
- **Alanine** is an **inhibitor** of pyruvate kinase, not an activator. It serves as an indicator of a high cellular energy state and ample amino acid supply.
- High levels of alanine signal the cell that there is sufficient energy and building blocks, thus **shutting down** glycolysis at the pyruvate kinase step to conserve glucose for other needs like glycogen synthesis.
*ATP*
- **ATP** (adenosine triphosphate) is an **allosteric inhibitor** of pyruvate kinase. High ATP levels signal a high energy state in the cell.
- When the cell has sufficient energy, ATP binds to a regulatory site on pyruvate kinase, reducing its activity and **slowing down glycolysis** to prevent overproduction of ATP.
*Glucose-6-phosphate*
- **Glucose-6-phosphate** is an intermediate in glycolysis but does not directly activate pyruvate kinase. It can act as an allosteric inhibitor of hexokinase, the first enzyme in glycolysis, but not pyruvate kinase.
- Its accumulation typically signifies a **backup** in the glycolytic pathway (e.g., due to downstream inhibition), leading to a *reduction* in overall glucose flux rather than a direct activation of pyruvate kinase.
*Glyceraldehyde-3-phosphate*
- **Glyceraldehyde-3-phosphate** is an intermediate in glycolysis, but it does not directly activate pyruvate kinase. It is a substrate for glyceraldehyde-3-phosphate dehydrogenase.
- While its presence indicates active glycolysis, it does not exert a specific allosteric regulatory effect on pyruvate kinase in the way fructose-1,6-bisphosphate does.
Substrate-level phosphorylation US Medical PG Question 8: A 13-year-old girl is brought to the physician by her mother because of a 1-month history of abnormal movements of her muscles that she cannot control. She has a younger brother with cognitive disabilities and epilepsy. Examination shows frequent, brief, involuntary contractions of the muscle groups of the upper arms, legs, and face that can be triggered by touch. An EEG shows generalized epileptiform activity. A trichrome stain of a skeletal muscle biopsy specimen shows muscle fibers with peripheral red inclusions that disrupt the normal fiber contour. Which of the following is the most likely underlying mechanism of the patient's symptoms?
- A. CTG trinucleotide repeat expansion
- B. Mutation of the methyl-CpG binding protein 2 gene
- C. Truncated dystrophin protein
- D. Autoimmune endomysial destruction
- E. Defective oxidative phosphorylation (Correct Answer)
Substrate-level phosphorylation Explanation: ***Defective oxidative phosphorylation***
- The constellation of **uncontrolled muscle movements (myoclonus)**, **epilepsy**, and the brother's **cognitive disabilities** strongly suggests a **mitochondrial disorder**.
- **Ragged red fibers** on trichrome stain of skeletal muscle biopsy are pathognomonic for **mitochondrial myopathies**, indicating defective oxidative phosphorylation due to abnormal mitochondrial aggregates.
*CTG trinucleotide repeat expansion*
- This is characteristic of **Myotonic Dystrophy**, which primarily presents with **myotonia** (delayed muscle relaxation), progressive muscle weakness, and often cataracts, rather than prominent myoclonus and seizures.
- While muscle weakness can occur, the specific biopsy findings and prominent myoclonus point away from this diagnosis.
*Mutation of the methyl-CpG binding protein 2 gene*
- A mutation in the **MECP2 gene** causes **Rett Syndrome**, an X-linked dominant disorder seen almost exclusively in girls.
- It involves normal development for 6-18 months followed by regression, loss of purposeful hand movements, **stereotypical hand-wringing**, and microcephaly, which are not described here.
*Truncated dystrophin protein*
- A truncated dystrophin protein causes **Duchenne Muscular Dystrophy**, an X-linked recessive disorder leading to progressive muscle weakness, **Gowers' sign**, and elevated creatine kinase.
- This condition does not typically present with myoclonus or the characteristic ragged red fibers, nor does it typically involve the sibling's intellectual disability and epilepsy in this manner.
*Autoimmune endomysial destruction*
- This mechanism is characteristic of **celiac disease**, which can have neurological symptoms like ataxia or peripheral neuropathy, but not typically the severe myoclonus, epilepsy, or muscle biopsy findings seen here.
- **Inflammatory myopathies** like polymyositis may show endomysial inflammation, but the clinical picture and specific biopsy findings (ragged red fibers) are not consistent.
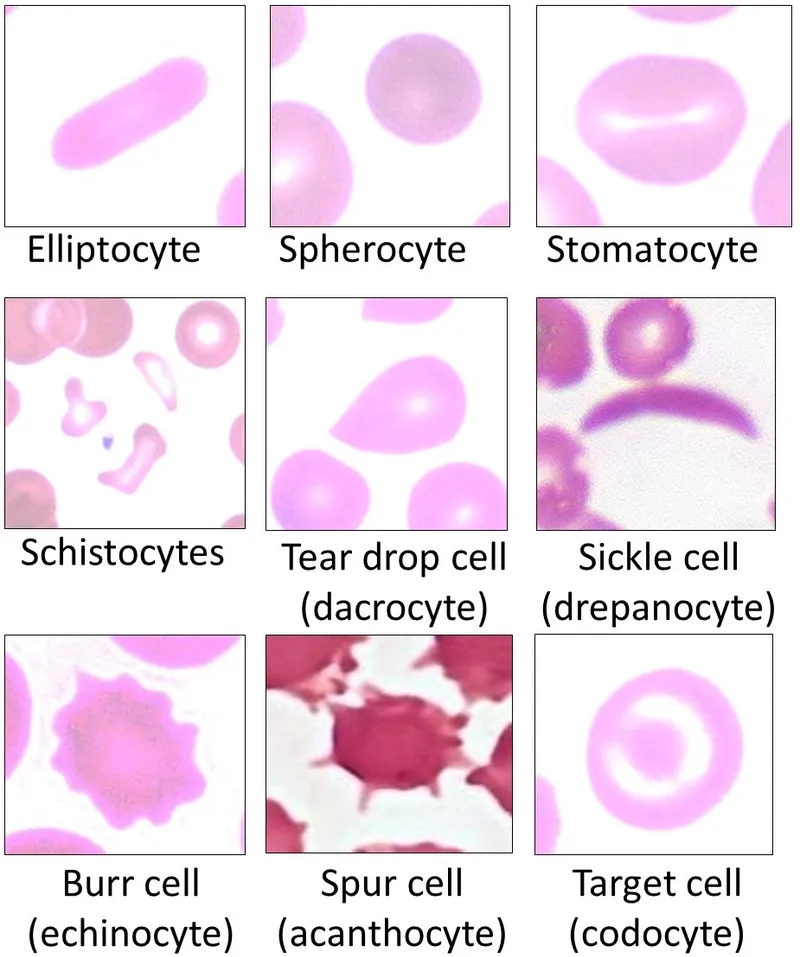
Substrate-level phosphorylation US Medical PG Question 9: An 11-year-old boy is brought to the emergency room with acute abdominal pain and hematuria. Past medical history is significant for malaria. On physical examination, he has jaundice and a generalized pallor. His hemoglobin is 5 g/dL, and his peripheral blood smear reveals fragmented RBC, microspherocytes, and eccentrocytes (bite cells). Which of the following reactions catalyzed by the enzyme is most likely deficient in this patient?
- A. Glucose-1-phosphate + UTP → UDP-glucose + pyrophosphate
- B. Glucose + ATP → Glucose-6-phosphate + ADP + H+
- C. D-glucose 6-phosphate → D-fructose-6-phosphate
- D. Glucose-6-phosphate + H2O → glucose + Pi
- E. D-glucose-6-phosphate + NADP+ → 6-phospho-D-glucono-1,5-lactone + NADPH + H+ (Correct Answer)
Substrate-level phosphorylation Explanation: ***D-glucose-6-phosphate + NADP+ → 6-phospho-D-glucono-1,5-lactone + NADPH + H+***
- This reaction is catalyzed by **glucose-6-phosphate dehydrogenase (G6PD)**, an enzyme critical for the production of **NADPH** in the **pentose phosphate pathway**.
- **NADPH** is essential for reducing **oxidative stress** in red blood cells. A deficiency in G6PD leads to increased susceptibility to hemolysis, especially under oxidative triggers like malaria, resulting in symptoms such as **acute hemolytic anemia**, jaundice, and specific morphological changes (e.g., **fragmented RBCs**, **microspherocytes**, and **eccentrocytes**, also known as **bite cells**).
*Glucose-1-phosphate + UTP → UDP-glucose + pyrophosphate*
- This reaction is catalyzed by **UDP-glucose pyrophosphorylase** and is important for **glycogen synthesis**.
- A deficiency in this enzyme would primarily affect glycogen metabolism and would not explain the **hemolytic anemia** or the characteristic red blood cell morphology seen in the patient.
*Glucose + ATP → Glucose-6-phosphate + ADP + H+*
- This reaction is catalyzed by **hexokinase**, the first committed step in **glycolysis**.
- While hexokinase deficiency can cause **hemolytic anemia**, it generally presents with chronic, moderate anemia and does not typically involve the specific red blood cell morphology (eccentrocytes/bite cells) associated with oxidative damage found in G6PD deficiency.
*D-glucose 6-phosphate → D-fructose-6-phosphate*
- This reaction is catalyzed by **phosphoglucose isomerase** (also known as phosphohexose isomerase) and is part of **glycolysis**.
- A deficiency in this enzyme would impair glycolysis and lead to **hemolytic anemia**, but its clinical presentation and RBC morphology differ from what is typically seen in G6PD deficiency, particularly the absence of oxidative stress markers like bite cells.
*Glucose-6-phosphate + H2O → glucose + Pi*
- This reaction is catalyzed by **glucose-6-phosphatase**, an enzyme found primarily in the liver and kidney, responsible for the final step in **gluconeogenesis** and glycogenolysis to release free glucose into the bloodstream.
- A deficiency in glucose-6-phosphatase leads to **glycogen storage disease type I (Von Gierke's disease)**, characterized by **hypoglycemia**, **lactic acidosis**, and hepatomegaly, not hemolytic anemia.
Substrate-level phosphorylation US Medical PG Question 10: A 3-month-old African American infant presents to the hospital with 2 days of fever, "coke"-colored urine, and jaundice. The pregnancy was uneventful except the infant was found to have hyperbilirubinemia that was treated with phototherapy. The mother explains that she breastfeeds her child and recently was treated herself for a UTI with trimethoprim-sulfamethoxazole (TMP-SMX). Which of the following diseases is similarly inherited as the disease experienced by the child?
- A. Hemophilia A (Correct Answer)
- B. Rett syndrome
- C. Beta thalassemia
- D. Sickle cell anemia
- E. Marfan syndrome
Substrate-level phosphorylation Explanation: ***Hemophilia A***
- The infant's symptoms (**fever**, **coke-colored urine**, **jaundice**, and history of **hyperbilirubinemia**) following exposure to **trimethoprim-sulfamethoxazole (TMP-SMX)** suggest **glucose-6-phosphate dehydrogenase (G6PD) deficiency**, an X-linked recessive condition.
- **Hemophilia A** is also an **X-linked recessive disorder**, making its inheritance pattern similar to G6PD deficiency.
*Rett syndrome*
- **Rett syndrome** is an **X-linked dominant** neurodevelopmental disorder, primarily affecting females severely and often embryonically lethal in males.
- Its inheritance pattern differs significantly from the X-linked recessive inheritance of G6PD deficiency.
*Beta thalassemia*
- **Beta thalassemia** is an **autosomal recessive** blood disorder, meaning it is inherited through genes located on non-sex chromosomes.
- This inheritance pattern is distinct from the X-linked recessive pattern of G6PD deficiency.
*Sickle cell anemia*
- **Sickle cell anemia** is an **autosomal recessive** hereditary blood disorder, with the gene located on chromosome 11.
- Its inheritance pathway is different from the X-linked recessive genetic inheritance seen in G6PD deficiency.
*Marfan syndrome*
- **Marfan syndrome** is an **autosomal dominant** disorder affecting connective tissue, the gene for which is located on chromosome 15.
- This mode of inheritance is distinctly different from the X-linked recessive pattern of inheritance.
More Substrate-level phosphorylation US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.