Fates of pyruvate US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Fates of pyruvate. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Fates of pyruvate US Medical PG Question 1: A 49-year-old man is brought to the emergency department after being discovered unconscious in a field near the county fair. Several empty bottles of vodka were found near him. On arrival, he is mumbling incoherently. He appears malodorous and disheveled. Serum studies show:
Na+ 150 mEq/L
K+ 3.3 mEq/L
Cl- 115 mEq/L
HCO3- 13 mEq/L
Urea nitrogen 30 mg/dL
Glucose 75 mg/dL
Creatinine 1.4 mg/dL
Lactic acid 6 mmol/L (N < 2)
Which of the following changes to enzyme activity best explains this patient's laboratory findings?
- A. Increased activity of phenylalanine hydroxylase
- B. Decreased activity of glucose-6-phosphate dehydrogenase
- C. Decreased activity of phosphofructokinase-2
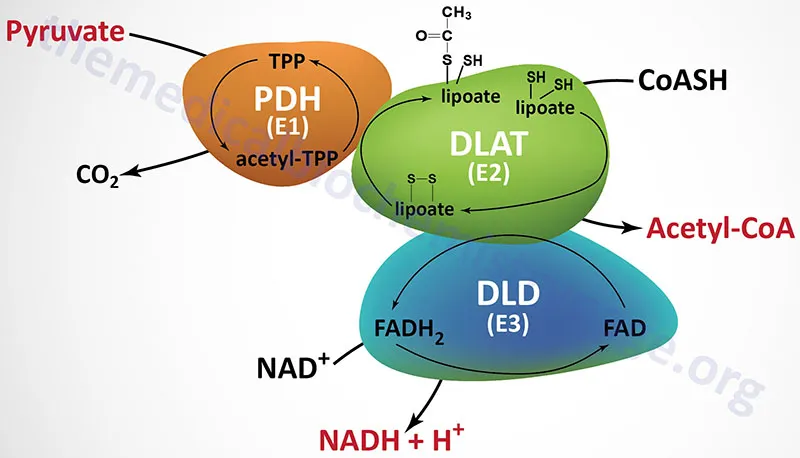
- D. Decreased activity of pyruvate dehydrogenase (Correct Answer)
- E. Increased activity of α-ketoglutarate dehydrogenase
Fates of pyruvate Explanation: ***Decreased activity of pyruvate dehydrogenase***
- The patient presents with **lactic acidosis** (lactic acid 6 mmol/L) and a high anion gap metabolic acidosis (AG = Na - Cl - HCO3 = 150 - 115 - 13 = 22 mEq/L). **Alcohol consumption** can lead to an increased NADH/NAD+ ratio, which inhibits pyruvate dehydrogenase.
- Inhibition of **pyruvate dehydrogenase** prevents the conversion of pyruvate to acetyl-CoA, shunting pyruvate towards lactate via lactate dehydrogenase, which is enhanced by the high NADH levels.
*Increased activity of phenylalanine hydroxylase*
- **Phenylalanine hydroxylase** is involved in the metabolism of phenylalanine to tyrosine, and its increased activity is not associated with lactic acidosis.
- Defective phenylalanine hydroxylase leads to **phenylketonuria**, which presents differently.
*Decreased activity of glucose-6-phosphate dehydrogenase*
- **Glucose-6-phosphate dehydrogenase (G6PD)** deficiency leads to hemolytic anemia due to impaired NADPH production, making red blood cells susceptible to oxidative stress, and is not directly linked to lactic acidosis.
- It does not explain the observed electrolyte imbalances or elevated lactate levels.
*Decreased activity of phosphofructokinase-2*
- **Phosphofructokinase-2 (PFK-2)** regulates glycolysis by producing fructose-2,6-bisphosphate, which activates PFK-1. Decreased activity would generally *reduce* glycolysis rather than promote lactic acid buildup.
- A decrease in PFK-2 activity is not a primary cause of lactic acidosis in the context of alcohol intoxication.
*Increased activity of α-ketoglutarate dehydrogenase*
- **α-ketoglutarate dehydrogenase** is a key enzyme in the citric acid cycle. Increased activity would enhance the citric acid cycle, potentially *reducing* the accumulation of glycolytic intermediates, rather than causing lactic acidosis.
- Its increased activity would generally lead to more efficient energy production, not the metabolic derangements seen here.
Fates of pyruvate US Medical PG Question 2: An investigator is studying nutritional deficiencies in humans. A group of healthy volunteers are started on a diet deficient in pantothenic acid. After 4 weeks, several of the volunteers develop irritability, abdominal cramps, and burning paresthesias of their feet. These symptoms are fully reversed after reintroduction of pantothenic acid to their diet. The function of which of the following enzymes was most likely impaired in the volunteers during the study?
- A. Gamma-glutamyl carboxylase
- B. Alpha-ketoglutarate dehydrogenase (Correct Answer)
- C. Dopamine beta-hydroxylase
- D. Methionine synthase
- E. Glutathione reductase
Fates of pyruvate Explanation: ***Alpha-ketoglutarate dehydrogenase***
- **Pantothenic acid** (vitamin B5) is a precursor of **coenzyme A (CoA)**, which is essential for the function of alpha-ketoglutarate dehydrogenase in the **Krebs cycle**.
- Impairment of this enzyme, critical for energy production, can lead to widespread metabolic dysfunction, manifesting as neurological and gastrointestinal symptoms like **irritability, abdominal cramps**, and **burning paresthesias**, which are classic signs of pantothenic acid deficiency.
*Gamma-glutamyl carboxylase*
- This enzyme is involved in the post-translational modification of several proteins, including **clotting factors**, and requires **vitamin K** as a cofactor.
- Its deficiency leads to bleeding disorders, not the neurological and GI symptoms described.
*Dopamine beta-hydroxylase*
- This enzyme converts **dopamine to norepinephrine** and requires vitamin C and copper.
- Its impairment can affect neurotransmitter synthesis but is not directly linked to pantothenic acid deficiency.
*Methionine synthase*
- This enzyme is crucial for the metabolism of **homocysteine** and requires **vitamin B12** and **folate** as cofactors.
- Its deficiency is associated with megaloblastic anemia and neurological symptoms, but not the specific presentation seen with pantothenic acid deficiency.
*Glutathione reductase*
- This enzyme is essential for maintaining the reduced state of **glutathione**, an antioxidant, and requires **riboflavin** (vitamin B2) in its coenzyme form, FAD.
- Dysfunction typically leads to oxidative stress, hemolytic anemia, and other symptoms different from those described.
Fates of pyruvate US Medical PG Question 3: A 4-month-old boy is brought to the physician because of a seizure. He was delivered at term after an uncomplicated pregnancy. He is currently at the 10th percentile for height, 5th percentile for weight, and 15th percentile for head circumference. Examination shows muscle hypotonia. His serum lactic acid and alanine are elevated. A functional assay of pyruvate dehydrogenase complex in serum leukocytes shows decreased enzyme activity. Supplementation with which of the following substances should be avoided in this patient?
- A. Arachidonic acid
- B. Riboflavin
- C. Thiamine
- D. Lysine
- E. Valine (Correct Answer)
Fates of pyruvate Explanation: ***Valine***
- While this is marked as the answer requiring avoidance, it's important to note that **branched-chain amino acids (BCAAs)** like valine, leucine, and isoleucine do NOT directly involve the pyruvate dehydrogenase complex in their metabolism.
- BCAAs are metabolized via **branched-chain α-keto acid dehydrogenase** (a separate enzyme complex) to produce acetyl-CoA and succinyl-CoA, **bypassing pyruvate dehydrogenase entirely**.
- In fact, ketogenic substrates that produce acetyl-CoA without generating pyruvate may be beneficial in PDC deficiency, as they provide energy without requiring PDC activity.
- **Note:** Among the listed options, none are classically contraindicated in PDC deficiency. The primary dietary modification in PDC deficiency is **carbohydrate restriction** (which produces pyruvate), not BCAA avoidance.
*Arachidonic acid*
- **Arachidonic acid** is an **omega-6 fatty acid** that serves as a precursor to eicosanoids and is important for normal development.
- Fatty acids are metabolized to acetyl-CoA via **β-oxidation**, which bypasses the pyruvate dehydrogenase complex entirely.
- High-fat, ketogenic diets are often **beneficial** in PDC deficiency, making fatty acid supplementation appropriate.
*Riboflavin*
- **Riboflavin (Vitamin B2)** is a precursor to **FAD and FMN**, coenzymes essential for the electron transport chain and various metabolic pathways.
- Riboflavin supplementation is sometimes beneficial in mitochondrial disorders and does not worsen PDC deficiency.
- It is considered safe and potentially therapeutic.
*Thiamine*
- **Thiamine (Vitamin B1)** is a crucial cofactor for **pyruvate dehydrogenase complex**, making it the most relevant therapeutic supplement for PDC deficiency.
- High-dose thiamine supplementation is often the **first-line treatment** in PDC deficiency, as it can enhance residual enzyme activity in some patients (particularly those with thiamine-responsive variants).
- Thiamine should be supplemented, not avoided.
*Lysine*
- **Lysine** is an essential amino acid that is exclusively **ketogenic**, being metabolized to **acetyl-CoA** without producing pyruvate.
- Since lysine metabolism bypasses PDC, it is safe and does not contribute to lactic acidosis.
- Lysine supplementation is not contraindicated in PDC deficiency.
Fates of pyruvate US Medical PG Question 4: A 10-year-old boy is brought to the emergency department due to vomiting and weakness. He is attending a summer camp and was on a hike with the other kids and a camp counselor. His friends say that the boy skipped breakfast, and the counselor says he forgot to pack snacks for the kids during the hike. The child’s parents are contacted and report that the child has been completely healthy since birth. They also say there is an uncle who would have to eat regularly or he would have similar symptoms. At the hospital, his heart rate is 90/min, respiratory rate is 17/min, blood pressure is 110/65 mm Hg, and temperature is 37.0°C (98.6°F). Physical examination reveals a visibly lethargic child with slight disorientation to time and place. Mild hepatosplenomegaly is observed but no signs of dehydration are noted. A blood sample is drawn, and fluids are started via an intravenous line.
Lab report
Serum glucose 44 mg/dL
Serum ketones absent
Serum creatinine 1.0 mg/dL
Blood urea nitrogen 32 mg/dL
Alanine aminotransferase (ALT) 425 U/L
Aspartate aminotransferase (AST) 372 U/L
Hemoglobin (Hb%) 12.5 g/dL
Mean corpuscular volume (MCV) 80 fl
Reticulocyte count 1%
Erythrocyte count 5.1 million/mm3
Which of the following is most likely deficient in this patient?
- A. Acyl-CoA dehydrogenase (Correct Answer)
- B. α-glucosidase
- C. Glucose-6-phosphatase
- D. Acetyl-CoA carboxylase
- E. Nicotinic acid
Fates of pyruvate Explanation: ***Acyl-CoA dehydrogenase***
- This patient presents with **hypoglycemia** (44 mg/dL) and **absent ketone bodies** after prolonged fasting, along with elevated **liver transaminases** and **hepatosplenomegaly**, which are classic signs of a **fatty acid oxidation disorder**.
- A deficiency in **acyl-CoA dehydrogenase**, particularly **medium-chain acyl-CoA dehydrogenase (MCAD)**, prevents adequate fatty acid breakdown for energy and ketone production, leading to **hypoketotic hypoglycemia** during periods of fasting.
*α-glucosidase*
- A deficiency in **α-glucosidase** (Pompe disease) leads to the accumulation of **glycogen** in lysosomes, primarily affecting muscles, heart, and liver.
- While it can cause hepatomegaly and muscle weakness, it typically presents with **cardiomyopathy** and does not directly cause hypoketotic hypoglycemia.
*Glucose-6-phosphatase*
- A deficiency in **glucose-6-phosphatase** (Von Gierke disease) is a type of **glycogen storage disease** characterized by severe **fasting hypoglycemia with lactic acidosis**, **massive hepatomegaly**, and **hyperlipidemia**.
- Unlike fatty acid oxidation disorders, Von Gierke disease typically presents with **lactic acidosis** as the predominant metabolic derangement, and patients often have a **doll-like face** and **growth retardation** from chronic presentation.
*Acetyl-CoA carboxylase*
- **Acetyl-CoA carboxylase** is a key enzyme in **fatty acid synthesis**, not fatty acid oxidation.
- A deficiency would primarily impair the body's ability to synthesize fatty acids, which is not consistent with the hypoketotic hypoglycemia observed here.
*Nicotinic acid*
- **Nicotinic acid** (niacin or vitamin B3) is a precursor to **NAD+** and **NADP+**, coenzymes involved in various metabolic reactions, including fatty acid synthesis and breakdown.
- While a deficiency (pellagra) can cause dermatitis, diarrhea, and dementia, it does not directly lead to **hypoketotic hypoglycemia** or fatty liver disease.
Fates of pyruvate US Medical PG Question 5: A 3-day-old female infant presents with poor feeding, lethargy, vomiting after feeding, and seizures. Labs revealed ketoacidosis and elevated hydroxypropionic acid levels. Upon administration of parenteral glucose and protein devoid of valine, isoleucine, methionine, and threonine, and carnitine, the infant began to recover. Which of the following enzymes is most likely deficient in this infant?
- A. Branched-chain ketoacid dehydrogenase
- B. Propionyl-CoA carboxylase (Correct Answer)
- C. Cystathionine synthase
- D. Phenylalanine hydroxylase
- E. Homogentisate oxidase
Fates of pyruvate Explanation: ***Propionyl-CoA carboxylase***
- The presence of **ketoacidosis** and elevated **hydroxypropionic acid** levels is characteristic of propionic acidemia, which is caused by a deficiency in **propionyl-CoA carboxylase**.
- The therapeutic benefit from a diet restricted in **valine, methionine, threonine**, and **isoleucine** (precursors of propionyl-CoA) along with carnitine supplementation further supports this diagnosis.
*Branched-chain ketoacid dehydrogenase*
- A deficiency in this enzyme leads to **Maple Syrup Urine Disease**, characterized by elevated **branched-chain ketoacids** and associated with a distinctive sweet odor in urine.
- While it causes neurotoxicity and poor feeding, the specific finding of elevated **hydroxypropionic acid** points away from this diagnosis.
*Cystathionine synthase*
- Deficiency in **cystathionine synthase** causes **homocystinuria**, leading to elevated **homocysteine** levels.
- Symptoms include developmental delay, ectopia lentis, and skeletal abnormalities, but not typically elevated **hydroxypropionic acid** or severe neonatal ketoacidosis in this manner.
*Phenylalanine hydroxylase*
- This enzyme is deficient in **phenylketonuria (PKU)**, resulting in high levels of **phenylalanine** and its metabolites.
- PKU is typically associated with intellectual disability, seizures, and a musty odor, but not ketoacidosis or elevated **hydroxypropionic acid**.
*Homogentisate oxidase*
- A deficiency in this enzyme causes **alkaptonuria**, characterized by the accumulation of **homogentisic acid**.
- This condition is usually benign in infancy, primarily manifesting as dark urine upon standing and later developing into ochronosis and arthritis, without acute neonatal ketoacidosis or elevated **hydroxypropionic acid**.
Fates of pyruvate US Medical PG Question 6: An investigator is studying a hereditary defect in the mitochondrial enzyme succinyl-CoA synthetase. In addition to succinate, the reaction catalyzed by this enzyme produces a molecule that is utilized as an energy source for protein translation. This molecule is also required for which of the following conversion reactions?
- A. Oxaloacetate to phosphoenolpyruvate (Correct Answer)
- B. Pyruvate to acetyl-CoA
- C. Acetaldehyde to acetate
- D. Glucose-6-phosphate to 6-phosphogluconolactone
- E. Fructose-6-phosphate to fructose-1,6-bisphosphate
Fates of pyruvate Explanation: ***Oxaloacetate to phosphoenolpyruvate***
- The reaction catalyzed by **succinyl-CoA synthetase** (also known as succinate thiokinase) produces **GTP** (guanosine triphosphate) from GDP and Pi, in addition to succinate.
- **GTP** is required for the conversion of **oxaloacetate** to **phosphoenolpyruvate** in gluconeogenesis, catalyzed by **PEP carboxykinase**.
*Pyruvate to acetyl-CoA*
- This reaction is catalyzed by the **pyruvate dehydrogenase complex** and produces NADH, not GTP.
- It is an irreversible step linking glycolysis to the citric acid cycle.
*Acetaldehyde to acetate*
- This reaction is catalyzed by **aldehyde dehydrogenase** and uses **NAD+** as a cofactor, producing NADH.
- It is involved in alcohol metabolism.
*Glucose-6-phosphate to 6-phosphogluconolactone*
- This is the first committed step of the **pentose phosphate pathway**, catalyzed by **glucose-6-phosphate dehydrogenase**.
- It uses **NADP+** as a cofactor, producing NADPH.
*Fructose-6-phosphate to fructose-1,6-bisphosphate*
- This reaction is a key regulatory step in **glycolysis**, catalyzed by **phosphofructokinase-1 (PFK-1)**.
- It consumes **ATP**, rather than producing GTP or utilizing it as a cofactor in the context of this question.
Fates of pyruvate US Medical PG Question 7: An investigator is studying muscle tissue in high-performance athletes. He obtains blood samples from athletes before and after a workout session consisting of short, fast sprints. Which of the following findings is most likely upon evaluation of blood obtained after the workout session?
- A. Decreased concentration of NADH
- B. Increased concentration of H+ (Correct Answer)
- C. Decreased concentration of lactate
- D. Increased concentration of insulin
- E. Increased concentration of ATP
Fates of pyruvate Explanation: ***Increased concentration of H+***
- During **anaerobic metabolism** in high-intensity exercise like sprints, pyruvate is converted to **lactate** by **lactate dehydrogenase** to regenerate NAD+. This process produces H+, leading to a decrease in pH and an increase in H+ concentration in the blood.
- The accumulation of **hydrogen ions (H+)** contributes to metabolic acidosis, muscle fatigue, and the burning sensation experienced during intense exertion.
- Blood gas analysis would show **decreased pH** and **increased H+ concentration**.
*Decreased concentration of NADH*
- NADH is primarily an **intracellular metabolite** and is not typically measured in blood samples as it does not circulate freely in significant concentrations.
- Within muscle cells during anaerobic glycolysis, NADH is consumed by lactate dehydrogenase to convert pyruvate to lactate, regenerating NAD+ for continued glycolysis.
- This option is not a realistic blood finding from a clinical laboratory perspective.
*Decreased concentration of lactate*
- **High-intensity sprints** primarily rely on **anaerobic metabolism**, which rapidly produces **lactate** from pyruvate.
- Therefore, the concentration of lactate in the blood would significantly **increase** after such a workout, not decrease.
- Elevated blood lactate is a hallmark finding after intense anaerobic exercise.
*Increased concentration of insulin*
- **Insulin** levels typically **decrease** during exercise, especially high-intensity exercise, due to **sympathetic nervous system activation** and the body's need to mobilize glucose from liver glycogen and fatty acids.
- Exercise promotes glucose uptake through **insulin-independent mechanisms** (GLUT4 translocation via AMP-activated protein kinase).
- Increased insulin would be counterproductive during intense exercise when glucose mobilization is needed.
*Increased concentration of ATP*
- ATP does not circulate in blood in measurable concentrations as a typical laboratory finding.
- Within muscle cells, ATP is rapidly **consumed** during intense exercise to fuel muscle contraction.
- While cells work to maintain ATP levels through anaerobic glycolysis and the creatine phosphate system, net ATP does not accumulate in the blood.
Fates of pyruvate US Medical PG Question 8: The balance between glycolysis and gluconeogenesis is regulated at several steps, and accumulation of one or more products/chemicals can either promote or inhibit one or more enzymes in either pathway. Which of the following molecules if increased in concentration can promote gluconeogenesis?
- A. ADP
- B. Acetyl-CoA (Correct Answer)
- C. AMP
- D. Fructose-2,6-bisphosphate
- E. Insulin
Fates of pyruvate Explanation: ***Acetyl-CoA***
- **Acetyl-CoA** promotes gluconeogenesis by activating **pyruvate carboxylase**, the enzyme that converts pyruvate to oxaloacetate, effectively pushing the pathway forward.
- High levels of **Acetyl-CoA** generally signal a state of abundant energy from fatty acid oxidation, indicating that glucose is not immediately needed for energy and can be synthesized for storage or use elsewhere.
*ADP*
- **ADP** is a key indicator of low cellular energy and **stimulates** glycolysis while **inhibiting** gluconeogenesis to produce ATP.
- Its presence signals a need for energy synthesis rather than glucose production.
*AMP*
- **AMP** also signals low energy status and is a powerful **allosteric activator** of **phosphofructokinase-1 (PFK-1)**, the rate-limiting enzyme in glycolysis.
- Activates **AMP-activated protein kinase (AMPK)**, which promotes catabolic processes like glycolysis and inhibits anabolic processes like gluconeogenesis.
*Fructose-2,6-bisphosphate*
- **Fructose-2,6-bisphosphate** is a potent **allosteric activator** of **PFK-1** in glycolysis and a strong **inhibitor** of **fructose-1,6-bisphosphatase** in gluconeogenesis.
- Its levels increase in response to insulin, promoting glucose utilization and inhibiting glucose production.
*Insulin*
- **Insulin** is a hormone that **promotes glucose uptake** and utilization by tissues and **inhibits gluconeogenesis**.
- It achieves this by activating enzymes involved in glycolysis and glycogen synthesis while inhibiting key enzymes in gluconeogenesis, such as **fructose-1,6-bisphosphatase**.
Fates of pyruvate US Medical PG Question 9: A research group is investigating an allosteric modulator to improve exercise resistance and tolerance at low-oxygen conditions. The group has created cultures of myocytes derived from high-performance college athletes. The application of this compound to these cultures in a low-oxygen environment and during vigorous contraction leads to longer utilization of glucose before reaching a plateau and cell death; however, the culture medium is significantly acidified in this experiment. An activating effect on which of the following enzymes would explain these results?
- A. Bisphosphoglycerate mutase
- B. Lactate dehydrogenase (Correct Answer)
- C. Enolase
- D. Malate dehydrogenase
- E. Pyruvate dehydrogenase
Fates of pyruvate Explanation: ***Lactate dehydrogenase***
- Enhanced **lactate dehydrogenase** activity would lead to increased conversion of **pyruvate to lactate**, regenerating **NAD+** for glycolysis to continue under **anaerobic conditions**.
- This process explains the **longer glucose utilization** and the significant **acidification of the medium** due to lactate production.
*Bisphosphoglycerate mutase*
- This enzyme is involved in the synthesis of **2,3-bisphosphoglycerate (2,3-BPG)** in red blood cells, which affects **hemoglobin's oxygen affinity**, not direct glucose utilization in myocytes under anaerobic conditions.
- While important for oxygen delivery, its activation would not primarily explain the observed **increased glucose utilization** and **lactic acid accumulation** in myocyte cultures.
*Enolase*
- **Enolase** catalyzes the conversion of **2-phosphoglycerate to phosphoenolpyruvate** in glycolysis.
- While crucial for glycolysis, its activation alone without an efficient disposal pathway for **pyruvate** (like lactate formation) would not sustain glucose metabolism and lead to such pronounced acidification under anaerobic stress.
*Malate dehydrogenase*
- **Malate dehydrogenase** is primarily involved in the **citric acid cycle** and the **malate-aspartate shuttle**, operating under **aerobic conditions** to convert malate to oxaloacetate.
- Its activation would not sustain glycolysis or lead to the observed **acidification** in a low-oxygen environment, where the citric acid cycle is inhibited.
*Pyruvate dehydrogenase*
- **Pyruvate dehydrogenase** converts **pyruvate to acetyl-CoA**, shunting carbons into the **citric acid cycle** for **aerobic respiration**.
- In a **low-oxygen environment**, this enzyme's activity would be limited due to reduced oxygen, and its activation would not explain the sustained glucose utilization or the significant **lactic acid accumulation** from anaerobic metabolism.
Fates of pyruvate US Medical PG Question 10: You are culturing bacteria on lactose-rich and glucose-free media. These bacteria regulate gene expression via the lac operon to ferment lactose into glucose and galactose for their metabolic needs. You add free glucose to the media. The addition of glucose reduces lactose fermentation secondary to which of the following changes?
- A. Increased level of cAMP
- B. Increased binding by the repressor to the operator
- C. Decreased binding by the repressor to the operator
- D. Decreased level of cAMP (Correct Answer)
- E. Increased binding of CAP to DNA
Fates of pyruvate Explanation: ***Decreased level of cAMP***
- The addition of **glucose** leads to a **decrease in intracellular cAMP levels**, which is a key component in catabolite repression.
- Reduced cAMP means less cAMP-CAP complex formation, thus **decreasing the positive regulation** of the *lac* operon.
*Increased level of cAMP*
- An **increased level of cAMP** would occur in the **absence of glucose**, which would then promote the formation of the **cAMP-CAP complex** necessary for *lac* operon activation.
- This would lead to **increased lactose fermentation**, which is the opposite of the scenario described.
*Increased binding by the repressor to the operator*
- The **repressor protein** binds to the operator in the **absence of lactose** to inhibit transcription.
- Lactose's presence (even with glucose) would lead to the conversion into **allolactose**, which binds to the repressor and *prevents* its binding to the operator.
*Decreased binding by the repressor to the operator*
- This scenario would happen in the **presence of lactose**, as **allolactose** would bind to the repressor and cause it to dissociate from the operator.
- While lactose is present in the initial setup, the question focuses on the *inhibitory effect of glucose*, which is independent of repressor binding related to lactose.
*Increased binding of CAP to DNA*
- **CAP (catabolite activator protein)** binding to DNA is *stimulated* by its association with **cAMP**.
- Since glucose leads to decreased cAMP, it would result in **decreased CAP binding to DNA**, thereby reducing *lac* operon transcription.
More Fates of pyruvate US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.