Glycolysis

On this page
🔥 The Glucose Combustion Engine: Cellular Energy's Primary Pathway
Glycolysis is the universal currency exchange of cellular metabolism, converting every glucose molecule into usable energy and biosynthetic precursors that power everything from muscle contraction to neurotransmission. You'll master the ten-enzyme pathway that defines energy production, learn to recognize the clinical fingerprints of glycolytic disorders from hemolytic anemia to exercise intolerance, and develop the diagnostic precision to distinguish enzyme deficiencies through metabolite patterns and quantitative analysis. This foundation connects biochemistry to bedside decision-making, equipping you to interpret lab findings, predict clinical phenotypes, and apply evidence-based treatments across the metabolic network.
📌 Remember: GIFT - Glucose Invested First, Then Two ATP gained (net gain after 4 ATP produced minus 2 ATP invested in preparatory phase)
The pathway divides into two distinct phases: the preparatory phase (steps 1-5) requires 2 ATP investment to activate glucose, while the pay-off phase (steps 6-10) generates 4 ATP through substrate-level phosphorylation. This 2 ATP net gain may seem modest, but glycolysis processes glucose at rates exceeding 100 μmol/min/g tissue in active muscle.
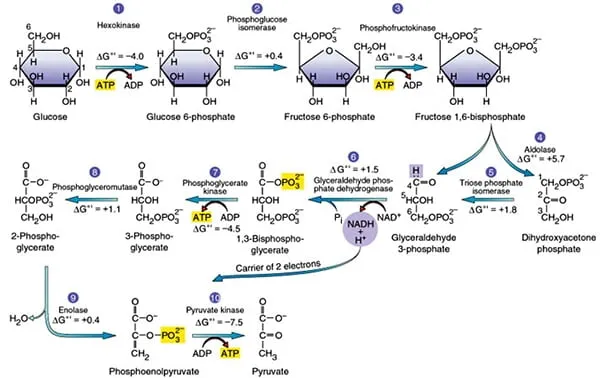
Three irreversible reactions control glycolytic flux: hexokinase (step 1), phosphofructokinase-1 (step 3), and pyruvate kinase (step 10). These enzymes respond to allosteric regulators, with PFK-1 serving as the primary rate-limiting enzyme. When cellular ATP/AMP ratio exceeds 10:1, PFK-1 activity decreases by 80%, while AMP concentrations above 0.1 mM increase activity 5-fold.
| Parameter | Preparatory Phase | Pay-off Phase | Net Result |
|---|---|---|---|
| ATP Investment | -2 ATP | 0 ATP | -2 ATP |
| ATP Generation | 0 ATP | +4 ATP | +4 ATP |
| NADH Production | 0 NADH | +2 NADH | +2 NADH |
| Carbon Products | 2 G3P | 2 Pyruvate | 2 Pyruvate |
| Reaction Steps | 5 steps | 5 steps | 10 total |
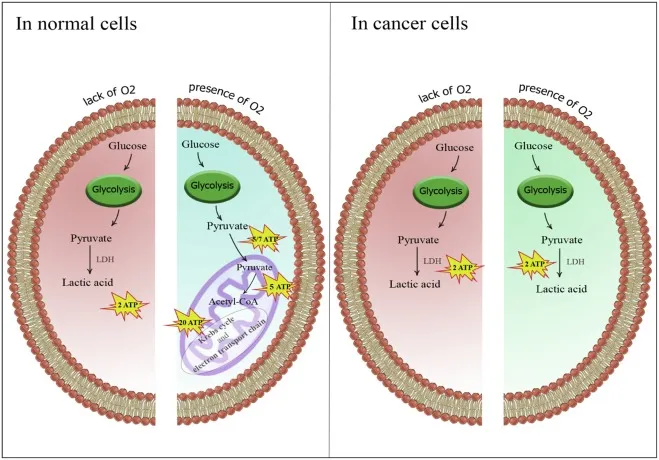
The tissue-specific regulation of glycolysis reflects metabolic demands. Brain tissue maintains glucose uptake at 0.5 μmol/g/min even during fasting, while skeletal muscle can increase glycolytic flux 100-fold during intense exercise. Cancer cells exhibit the Warburg effect, preferentially using glycolysis even in oxygen-rich conditions, consuming glucose at rates 10-20 times higher than normal cells.
💡 Master This: Glycolysis efficiency depends on NAD+ regeneration. Under aerobic conditions, the malate-aspartate shuttle transfers NADH reducing equivalents to mitochondria, yielding 2.5 ATP per NADH. During anaerobic conditions, lactate dehydrogenase regenerates NAD+ while producing lactate, maintaining glycolytic flux without additional ATP gain.
Understanding glycolytic regulation unlocks the logic behind metabolic diseases, from diabetes mellitus affecting glucose entry to pyruvate kinase deficiency causing hemolytic anemia. Connect this foundation through enzymatic mechanisms to understand how allosteric regulation fine-tunes energy production.
🔥 The Glucose Combustion Engine: Cellular Energy's Primary Pathway
⚙️ The Enzymatic Assembly Line: Precision Molecular Machinery
📌 Remember: HEX-PGI-PFK-ALD-TPI represents the first five enzymes: Hexokinase, Phosphoglucose Isomerase, PhosphoFructoKinase, ALDolase, Triose Phosphate Isomerase
Phosphofructokinase-1 represents the master regulatory enzyme, responding to 6 different allosteric effectors. ATP binding to the allosteric site decreases enzyme activity by 95% when concentrations exceed 2 mM. Conversely, AMP binding increases activity 8-fold, while fructose-2,6-bisphosphate (the most potent activator) enhances activity 20-fold at concentrations as low as 1 μM.
The pay-off phase enzymes operate through coupled reactions maintaining thermodynamic favorability. Glyceraldehyde-3-phosphate dehydrogenase couples aldehyde oxidation with phosphorylation, creating the high-energy intermediate 1,3-bisphosphoglycerate. This reaction exhibits ΔG°' = +6.3 kJ/mol but becomes favorable through product removal and NAD+ availability.
| Enzyme | Km (mM) | Vmax (μmol/min/mg) | Regulation Type | Key Effectors |
|---|---|---|---|---|
| Hexokinase | 0.1 | 150 | Product inhibition | G6P (-) |
| PFK-1 | 0.7 | 200 | Allosteric | ATP (-), AMP (+), F2,6BP (+) |
| Pyruvate Kinase | 0.5 | 300 | Allosteric | F1,6BP (+), ATP (-) |
| GAPDH | 0.2 | 100 | Substrate availability | NAD+ (+), Pi (+) |
| PGK | 0.3 | 250 | Mass action | ADP (+), ATP (-) |
Substrate channeling occurs between sequential enzymes, with aldolase and triose phosphate isomerase forming enzyme complexes that transfer intermediates without release to bulk solution. This channeling increases reaction efficiency by 40% and prevents side reactions that could deplete glycolytic intermediates.
💡 Master This: Enzyme compartmentalization determines glycolytic regulation. Hexokinase associates with mitochondrial outer membrane, positioning it near ATP synthesis sites. Pyruvate kinase exists in tissue-specific isoforms: M1 (muscle), M2 (embryonic/tumor), L (liver), and R (red blood cells), each exhibiting distinct regulatory properties.
Understanding enzymatic precision reveals how single amino acid mutations can disrupt entire metabolic pathways. Connect this mechanistic foundation through pattern recognition to understand how enzyme deficiencies manifest as distinct clinical syndromes.
⚙️ The Enzymatic Assembly Line: Precision Molecular Machinery
🎯 The Diagnostic Decoder: Pattern Recognition in Metabolic Medicine
Hemolytic anemia patterns provide the clearest diagnostic windows into glycolytic defects. Pyruvate kinase deficiency (most common glycolytic enzymopathy) presents with chronic hemolysis, reticulocytosis >15%, and 2,3-BPG levels elevated 3-4 fold above normal. The pyruvate kinase assay shows <50% normal activity, while ATP/ADP ratios in red cells drop to 2:1 (normal 8:1).
📌 Remember: HEMOLYTIC pattern recognition - High reticulocytes, Elevated bilirubin, Microcytic anemia, Osmotic fragility, Low haptoglobin, Young RBC predominance, Tissue-specific symptoms, Increased LDH, Chronic fatigue
- Pyruvate Kinase Deficiency
- Prevalence: 1:20,000 in Northern Europeans
- Hemoglobin: 6-10 g/dL (chronic stable anemia)
- Reticulocytes: 15-50% (massive compensatory response)
- 2,3-BPG elevation: Improves oxygen delivery by 25%
- ATP depletion: Causes membrane instability and spherocytosis
- Glucose-6-Phosphate Dehydrogenase Deficiency
- Prevalence: 400 million affected worldwide
- Trigger pattern: Oxidative stress from infections, drugs, foods
- Heinz bodies: >5% during hemolytic episodes
- Bite cells: Characteristic RBC morphology on smear
Exercise intolerance patterns distinguish muscle-specific glycolytic defects. Phosphofructokinase deficiency (Tarui disease) causes exercise-induced myalgia, muscle cramps within 2-5 minutes of exertion, and paradoxical improvement with continued mild exercise. Lactate levels fail to rise appropriately during ischemic exercise testing, remaining <2-fold above baseline (normal >5-fold increase).
Neonatal screening patterns identify galactokinase deficiency and hereditary fructose intolerance before irreversible damage occurs. Galactokinase deficiency presents with cataracts within days of milk feeding, galactitol accumulation in lens, and pseudotumor cerebri in 30% of cases. Hereditary fructose intolerance shows hypoglycemia within 30 minutes of fructose ingestion, hepatomegaly, and growth failure.
| Condition | Key Laboratory Finding | Diagnostic Threshold | Clinical Timeline |
|---|---|---|---|
| PK Deficiency | ATP/ADP ratio | <3:1 (normal 8:1) | Chronic anemia |
| G6PD Deficiency | Heinz bodies | >5% during crisis | Acute hemolysis |
| Tarui Disease | Lactate response | <2x rise with exercise | Minutes to hours |
| Galactokinase | Galactitol levels | >100x normal | Days after milk |
| HFI | Fructose tolerance | Hypoglycemia <60 mg/dL | 30 minutes post-fructose |
💡 Master This: Tissue-specific expression determines clinical presentation. Muscle-specific isoforms cause exercise intolerance without hemolysis, while red cell-specific defects cause hemolytic anemia without myopathy. Liver-specific defects present with hypoglycemia and hepatomegaly.
Pattern recognition transforms scattered symptoms into precise diagnoses, enabling targeted therapy and genetic counseling. Connect these diagnostic frameworks through systematic analysis to understand how quantitative thresholds distinguish similar presentations.
🎯 The Diagnostic Decoder: Pattern Recognition in Metabolic Medicine
🔬 The Metabolic Microscope: Quantitative Discrimination in Glycolytic Disorders
Enzyme activity thresholds provide definitive discrimination between carrier states, mild deficiencies, and severe deficiencies. Pyruvate kinase activity below 50% of normal indicates heterozygous carrier status, while <25% activity confirms homozygous deficiency with clinical hemolysis. G6PD activity shows X-linked inheritance patterns, with hemizygous males showing <10% normal activity and heterozygous females displaying mosaic patterns ranging 10-60%.
Metabolite accumulation patterns distinguish upstream from downstream enzyme defects. Hexokinase deficiency causes glucose accumulation with normal glucose-6-phosphate levels, while glucose-6-phosphate dehydrogenase deficiency shows G6P accumulation with reduced NADPH production. Phosphofructokinase deficiency demonstrates glucose-6-phosphate and fructose-6-phosphate elevation with normal downstream metabolites.
Stress testing protocols reveal functional enzyme capacity under physiological demands. Ischemic exercise testing measures lactate production during anaerobic conditions, with normal subjects achieving >5-fold lactate elevation within 3 minutes. Phosphofructokinase deficiency shows <2-fold lactate increase, while pyruvate kinase deficiency demonstrates intermediate impairment with 2-4 fold elevation.
| Enzyme Defect | Resting Activity | Stress Response | Metabolite Pattern | Inheritance |
|---|---|---|---|---|
| Hexokinase | <50% normal | Glucose intolerance | ↑ Glucose, normal G6P | Autosomal recessive |
| PFK-1 | <25% normal | No lactate rise | ↑ G6P, ↑ F6P | Autosomal recessive |
| Pyruvate Kinase | <50% normal | Blunted lactate | ↑ PEP, ↑ 2,3-BPG | Autosomal recessive |
| G6PD | <10% (males) | Oxidative hemolysis | ↑ G6P, ↓ NADPH | X-linked |
| Aldolase | <30% normal | Exercise intolerance | ↑ F1,6BP | Autosomal recessive |
📌 Remember: COMPENSATE for enzyme defects - Cellular adaptation, Oxygen delivery shifts, Metabolic rerouting, Pathway alternatives, Energy conservation, NADPH regeneration, Substrate accumulation, Allosteric changes, Tissue specificity, Enzyme induction
Therapeutic monitoring requires quantitative endpoints to assess treatment efficacy. Splenectomy in pyruvate kinase deficiency increases hemoglobin by 2-4 g/dL within 6 months, while reticulocyte counts decrease from >20% to <10%. Folic acid supplementation prevents megaloblastic changes in chronic hemolytic states, maintaining MCV below 100 fL.
⭐ Clinical Pearl: Neonatal jaundice in pyruvate kinase deficiency may require exchange transfusion when bilirubin exceeds 20 mg/dL. Phototherapy effectiveness decreases 50% due to ongoing hemolysis, necessitating aggressive intervention to prevent kernicterus.
Genetic counseling relies on quantitative risk assessment. Autosomal recessive glycolytic defects show 25% recurrence risk for affected offspring when both parents are carriers. Prenatal diagnosis through chorionic villus sampling at 10-12 weeks provides >99% accuracy for known familial mutations.
💡 Master This: Phenotype-genotype correlation varies significantly in glycolytic disorders. Identical mutations can produce different clinical severity based on tissue-specific expression, modifier genes, and environmental factors. Quantitative enzyme analysis provides better prognostic information than genetic testing alone.
Quantitative discrimination transforms clinical suspicion into definitive diagnosis, enabling precision medicine approaches. Connect these analytical frameworks through evidence-based treatment to understand how therapeutic algorithms optimize patient outcomes.
🔬 The Metabolic Microscope: Quantitative Discrimination in Glycolytic Disorders
⚖️ The Treatment Algorithm: Evidence-Based Therapeutic Precision
Pyruvate kinase deficiency management follows severity-stratified protocols. Mild deficiency (hemoglobin >10 g/dL) requires folic acid 5mg daily and infection prevention with annual influenza vaccination. Moderate deficiency (hemoglobin 8-10 g/dL) adds iron chelation when ferritin exceeds 1000 ng/mL from chronic transfusions. Severe deficiency (hemoglobin <8 g/dL) necessitates splenectomy consideration when transfusion requirements exceed 4 units annually.
Splenectomy outcomes in pyruvate kinase deficiency show predictable benefits. Hemoglobin increases by 2.5 ± 0.8 g/dL within 6 months, transfusion requirements decrease by 80-90%, and reticulocyte counts fall from >20% to 8-12%. However, post-splenectomy sepsis risk increases 50-fold, requiring pneumococcal, meningococcal, and Haemophilus vaccinations 2-4 weeks pre-operatively.
G6PD deficiency management emphasizes trigger avoidance and acute crisis intervention. Oxidative stress triggers include >100 medications (primaquine, sulfonamides, nitrofurantoin), fava beans, and infections. Acute hemolytic episodes require immediate trigger removal, hydration with normal saline at 1.5x maintenance, and transfusion when hemoglobin drops >3 g/dL from baseline or falls below 7 g/dL.
Hereditary fructose intolerance requires strict dietary management with <40mg fructose daily intake. Sucrose elimination prevents hypoglycemic episodes, while sorbitol avoidance (including medications and toothpaste) prevents hepatotoxicity. Vitamin C supplementation (ascorbic acid 100mg daily) compensates for reduced fruit intake, maintaining adequate antioxidant status.
| Condition | Primary Intervention | Success Rate | Monitoring Parameter | Target Value |
|---|---|---|---|---|
| PK Deficiency | Splenectomy | 85% improvement | Hemoglobin | >10 g/dL |
| G6PD Deficiency | Trigger avoidance | 95% prevention | Heinz bodies | <2% |
| HFI | Fructose restriction | 100% symptom control | Growth velocity | Normal percentile |
| Galactokinase | Galactose elimination | 90% cataract prevention | Galactitol | Undetectable |
| Tarui Disease | Exercise modification | 70% symptom reduction | CK levels | <2x normal |
⭐ Clinical Pearl: Neonatal screening for galactokinase deficiency prevents irreversible cataracts through immediate galactose restriction. Lens changes begin within 48 hours of galactose exposure, but complete reversal occurs with dietary elimination within 2 weeks if implemented early.
Gene therapy represents emerging treatment for severe glycolytic defects. Lentiviral vectors carrying normal pyruvate kinase genes show promising results in preclinical studies, achieving >50% enzyme activity restoration in transduced cells. Clinical trials target transfusion-dependent patients with >6 units annually requirements.
💡 Master This: Treatment timing determines outcome success. Early intervention in hereditary fructose intolerance prevents hepatic fibrosis and growth failure, while delayed diagnosis may result in irreversible liver damage. Genetic counseling should occur before conception in high-risk families.
Evidence-based algorithms transform complex clinical decisions into systematic approaches, improving patient outcomes while reducing complications. Connect these therapeutic frameworks through advanced integration to understand how multi-system interactions influence treatment strategies.
⚖️ The Treatment Algorithm: Evidence-Based Therapeutic Precision
🌐 The Metabolic Network: Multi-System Integration Mastery
Tissue-specific glycolytic programming reflects evolutionary optimization for distinct metabolic roles. Brain tissue maintains constitutive glucose uptake through GLUT1 transporters with Km = 1.5 mM, ensuring continuous ATP supply even during fasting states when plasma glucose falls to 3.5 mM. Skeletal muscle expresses GLUT4 transporters (Km = 15 mM) that translocate from intracellular vesicles to plasma membrane within 5 minutes of insulin stimulation, increasing glucose uptake 20-fold.
Hormonal integration networks coordinate whole-body glucose homeostasis through multi-level regulation. Insulin activates phosphofructokinase-2 through protein kinase B, increasing fructose-2,6-bisphosphate levels 10-fold within 15 minutes. This allosteric activator enhances PFK-1 activity 20-fold, while simultaneously inhibiting gluconeogenesis through fructose-1,6-bisphosphatase suppression.
📌 Remember: INSULIN integration - Increases glucose uptake, Nactivates gluconeogenesis, Stimulates glycolysis, Upregulates lipogenesis, Lowers blood glucose, Inhibits lipolysis, Nuclear transcription changes
Cancer cell reprogramming demonstrates pathological glycolytic integration. Tumor cells overexpress hexokinase II, which associates with mitochondrial outer membrane and exhibits >100-fold higher glucose affinity than normal tissues. Hypoxia-inducible factor-1α upregulates >10 glycolytic enzymes simultaneously, increasing glucose consumption 10-20 fold while suppressing mitochondrial respiration through pyruvate dehydrogenase kinase activation.
Exercise physiology reveals dynamic metabolic switching between fuel sources. Low-intensity exercise (<40% VO2max) utilizes fatty acid oxidation for >80% of energy demands, with glycolysis providing <20%. High-intensity exercise (>85% VO2max) reverses this ratio, with glycolysis supplying >90% of ATP through phosphocreatine and muscle glycogen breakdown at rates exceeding 2 mmol/kg/min.
Aging-related changes affect glycolytic efficiency through multiple mechanisms. Insulin sensitivity decreases 30-40% after age 65, reducing glucose uptake in skeletal muscle. Mitochondrial dysfunction increases reliance on glycolytic ATP production, while decreased muscle mass reduces total glycolytic capacity by 20-30% per decade after age 50.
| Physiological State | Glucose Uptake Rate | Primary Fuel | Glycolytic Contribution | Regulatory Mechanism |
|---|---|---|---|---|
| Resting Brain | 0.5 μmol/g/min | Glucose 100% | 15% (aerobic) | Constitutive GLUT1 |
| Exercising Muscle | 50 μmol/g/min | Mixed fuels | 90% (anaerobic) | Insulin + contraction |
| Tumor Tissue | 5-10 μmol/g/min | Glucose 80% | 85% (Warburg) | HIF-1α upregulation |
| Fasting Liver | 0.1 μmol/g/min | Fatty acids | 5% (gluconeogenic) | Glucagon signaling |
| RBC Circulation | 2-3 μmol/g/min | Glucose 100% | 100% (obligate) | No mitochondria |
Pharmacological modulation of glycolytic flux offers therapeutic opportunities. Metformin activates AMP-activated protein kinase, increasing glucose uptake 2-3 fold in muscle tissue while inhibiting hepatic gluconeogenesis. 2-Deoxyglucose competitively inhibits hexokinase, selectively targeting cancer cells with high glucose dependence while sparing normal tissues with lower metabolic rates.
💡 Master This: Metabolic flexibility determines physiological resilience. Healthy individuals can switch between glucose and fatty acid oxidation within 30 minutes of substrate availability changes. Metabolic inflexibility in diabetes and aging impairs this adaptive capacity, contributing to insulin resistance and metabolic dysfunction.
Multi-system integration reveals how glycolytic regulation extends beyond individual enzymes to coordinate whole-body metabolism. Connect these integration principles through rapid mastery tools to develop clinical expertise in metabolic medicine.
🌐 The Metabolic Network: Multi-System Integration Mastery
🎯 The Glycolytic Arsenal: Clinical Mastery Command Center
Essential Numbers Arsenal - memorize these clinical thresholds for instant pattern recognition:
- Normal glucose: 70-100 mg/dL (fasting), <140 mg/dL (2-hour post-meal)
- Pyruvate kinase activity: <50% normal = carrier, <25% = clinical disease
- G6PD activity: <10% normal in affected males, mosaic 10-60% in female carriers
- Lactate levels: <2 mM normal, >4 mM indicates tissue hypoxia
- 2,3-BPG elevation: 3-4 fold above normal in PK deficiency
- Reticulocyte count: >15% suggests chronic hemolysis
📌 Remember: RAPID glycolytic assessment - Reticulocytes >15%, Anemia with spherocytes, Pyruvate kinase <50%, Increased 2,3-BPG, Decreased ATP/ADP ratio
Diagnostic Decision Matrix for hemolytic anemia workup:
- Step 1: Reticulocyte count - if >15%, proceed to hemolysis workup
- Step 2: Direct Coombs test - if negative, consider intrinsic RBC defects
- Step 3: Osmotic fragility - if increased, suggests membrane defects
- Step 4: Enzyme screening - G6PD, pyruvate kinase, hexokinase assays
- Step 5: Molecular testing - if enzyme normal, consider rare defects
Treatment Protocols - evidence-based interventions with quantified outcomes:
| Clinical Scenario | Immediate Action | Target Parameter | Success Metric |
|---|---|---|---|
| PK Deficiency Crisis | Transfusion if Hgb <7 g/dL | Hemoglobin >10 g/dL | Symptom resolution |
| G6PD Hemolytic Episode | Stop trigger, hydrate | Urine output >1 mL/kg/hr | Hemolysis cessation |
| HFI Hypoglycemia | IV glucose 0.5 g/kg | Blood glucose >70 mg/dL | Neurologic recovery |
| Exercise Intolerance | Graded activity program | CK <2x normal | Improved tolerance |
| Neonatal Jaundice | Phototherapy/exchange | Bilirubin <20 mg/dL | Prevent kernicterus |
Emergency Recognition Patterns - life-threatening presentations requiring immediate intervention:
- Severe hemolytic crisis: Hemoglobin drop >4 g/dL in 24 hours
- Acute kidney injury: Hemoglobinuria with creatinine rise >50%
- Hypoglycemic coma: Glucose <40 mg/dL with altered consciousness
- Neonatal kernicterus risk: Bilirubin >20 mg/dL in first week
💡 Master This: Genetic counseling timing is critical - 25% recurrence risk for autosomal recessive defects, 50% risk for X-linked conditions in male offspring. Prenatal diagnosis available at 10-12 weeks with >99% accuracy for known mutations.
Clinical Commandments for glycolytic disorder mastery:
- Always consider enzyme deficiency in chronic hemolytic anemia
- Never give oxidizing drugs to G6PD-deficient patients
- Immediately restrict fructose in suspected HFI
- Vaccinate before splenectomy - pneumococcal, meningococcal, H. influenzae
- Monitor for iron overload in chronically transfused patients
This clinical arsenal transforms biochemical complexity into actionable medical expertise, enabling confident diagnosis and optimal patient outcomes in glycolytic disorders.
🎯 The Glycolytic Arsenal: Clinical Mastery Command Center
Practice Questions: Glycolysis
Test your understanding with these related questions
A 4-month-old boy is brought to his pediatrician for a well-child visit. His parents have noticed that he has had poor growth compared to his older siblings. The boy was delivered vaginally after a normal pregnancy. His temperature is 98.8°F (37.1°C), blood pressure is 98/68 mmHg, pulse is 88/min, and respirations are 20/min. On exam, his abdomen appears protuberant, and the boy appears to have abnormally enlarged cheeks. A finger stick reveals that the patient’s fasting blood glucose is 50 mg/dL. On further laboratory testing, the patient is found to have elevated blood lactate levels, as well as no response to a glucagon stimulation test. What enzymatic defect is most likely present?











