GSD type IV (Andersen disease) US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for GSD type IV (Andersen disease). These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
GSD type IV (Andersen disease) US Medical PG Question 1: An otherwise healthy, exclusively breastfed 4-day-old neonate is brought to the physician because of yellowing of his skin and eyes. His urine has been clear and stools have been normal. He was born at term by vacuum-assisted delivery and weighed 4000 g (8 lb 8 oz). Pregnancy was complicated by gestational diabetes mellitus. His older sibling had jaundice in the neonatal period. Vital signs are within normal limits. He appears alert and comfortable. Physical examination shows jaundice of the skin and sclerae. The liver is palpated 1 cm below the right costal margin. Laboratory studies show:
Hemoglobin 17 g/dl
Reticulocyte count 0.5 %
Total bilirubin 21.2 mg/dl
Direct bilirubin 2 mg/dl
Indirect bilirubin 19.1 mg/dl
Coombs test Negative
Which of the following is the most appropriate next step in management?
- A. Replace breast feeding with formula feeds
- B. MRI of the brain
- C. Phototherapy (Correct Answer)
- D. Increase frequency of breast feeds
- E. Intravenous immunoglobulin
GSD type IV (Andersen disease) Explanation: ***Phototherapy***
- The neonate has significantly elevated **indirect bilirubin** (19.1 mg/dL) at 4 days of age, which, along with risk factors like the older sibling's jaundice and vacuum-assisted delivery, places him at high risk for **kernicterus**. Phototherapy is the primary treatment to reduce bilirubin levels.
- The combination of **jaundice, high indirect bilirubin, normal reticulocyte count, and negative Coombs test** suggests exaggerated physiologic jaundice or possibly breastfeeding jaundice, warranting intervention at this level.
*Replace breast feeding with formula feeds*
- While temporary cessation of breastfeeding can lower bilirubin in some cases of **breast milk jaundice**, it is not the first-line treatment, especially given the high bilirubin level.
- Discontinuing breastfeeding can interfere with successful long-term breastfeeding. This option is usually considered after phototherapy has failed or in conjunction with it if bilirubin levels remain stubbornly high.
*MRI of the brain*
- An MRI of the brain is indicated to assess for **kernicterus** if there are neurological signs, but the neonate appears alert and comfortable, and there are no signs of acute bilirubin encephalopathy.
- The immediate priority is to lower the bilirubin level to prevent neurological damage, rather than evaluate for damage that may not yet be present.
*Increase frequency of breast feeds*
- This intervention is appropriate for **breastfeeding failure jaundice** (early onset jaundice related to inadequate milk intake), which typically presents earlier and with signs of dehydration or poor feeding.
- While increasing feeding frequency is generally beneficial, the high bilirubin level in this case requires more aggressive intervention like phototherapy.
*Intravenous immunoglobulin*
- **Intravenous immunoglobulin (IVIG)** is indicated for severe hyperbilirubinemia, especially when due to **isoimmune hemolytic disease** (e.g., ABO or Rh incompatibility), which is ruled out by the negative Coombs test.
- There is no evidence of hemolytic disease, making IVIG an inappropriate treatment in this scenario.
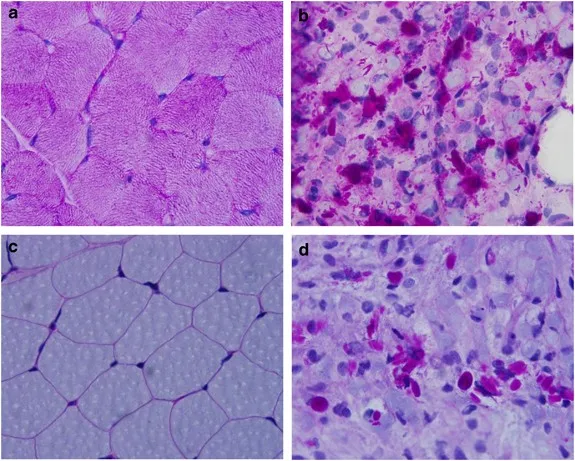
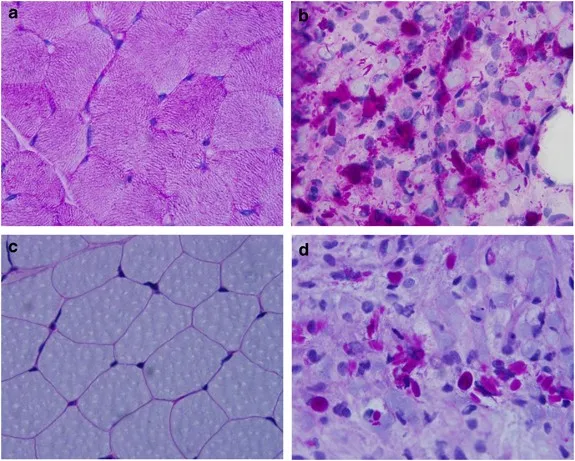
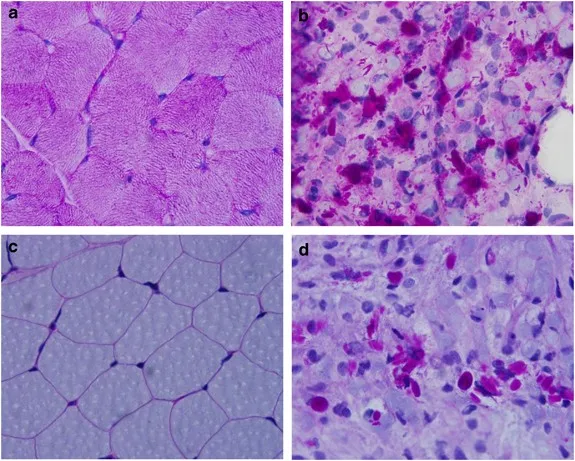
GSD type IV (Andersen disease) US Medical PG Question 2: A 12-year-old girl comes to the clinic with a grossly enlarged abdomen. She has a history of frequent episodes of weakness, sweating, and pallor that are eliminated by eating. Her development has been slow. She started to walk unassisted at 2 years and was not performing well at school. Physical examination reveals a blood pressure of 100/60 mm Hg, heart rate of 80/min, and temperature of 36.9°C (98.4℉). On physical examination, the liver is enlarged, firm, and palpable up to the pelvis. The spleen and kidney are not palpable. Laboratory investigation reveals low blood glucose and pH with high lactate, triglycerides, ketones, and free fatty acids. The liver biopsy revealed high glycogen content. Hepatic glycogen structure was normal. The enzyme assay performed on the biopsy tissue revealed very low glucose-6-phosphatase levels. What is the most likely diagnosis?
- A. Pompe's disease
- B. Cori's disease
- C. Hereditary hemochromatosis
- D. Von-Gierke's disease (Correct Answer)
- E. McArdle disease
GSD type IV (Andersen disease) Explanation: ***Von-Gierke's disease***
- The combination of **hepatomegaly**, **hypoglycemia** (causing weakness, sweating, pallor), **lactic acidosis**, **hyperlipidemia**, and elevated ketones points to a severe defect in glucose metabolism.
- **Very low glucose-6-phosphatase levels** on liver biopsy and normal hepatic glycogen structure are pathognomonic for Von-Gierke's disease (Glycogen Storage Disease Type I).
*Pompe's disease*
- This is a **lysosomal storage disease** affecting **alpha-1,4-glucosidase**, leading to glycogen accumulation in lysosomes.
- It primarily affects the **heart** and skeletal muscles and would not present with severe lactic acidosis and hyperlipidemia.
*Cori's disease*
- This is **Glycogen Storage Disease Type III**, caused by a deficiency in the **debranching enzyme** (amylo-alpha-1,6-glucosidase).
- While it can cause hepatomegaly and hypoglycemia, the hepatic glycogen structure would be abnormal due to incompletely debranched glycogen, and glucose-6-phosphatase levels would be normal.
*Hereditary hemochromatosis*
- This is an **iron overload disorder** leading to iron deposition in organs like the liver, heart, and pancreas.
- It would present with symptoms related to organ damage from iron accumulation, such as liver cirrhosis and diabetes, not the metabolic derangements seen here.
*McArdle disease*
- This is **Glycogen Storage Disease Type V**, due to a deficiency in **muscle glycogen phosphorylase**.
- It primarily causes exercise-induced muscle pain, cramping, and fatigue due to an inability to break down muscle glycogen for energy, not systemic metabolic disturbances or hepatomegaly.
GSD type IV (Andersen disease) US Medical PG Question 3: A 56-year-old male with a history of hepatitis C cirrhosis status post TIPS procedure is brought in by his wife to the emergency department because he has been acting disoriented, slurring his speech, and sleeping throughout the day. On arrival the patient is afebrile and his vital signs are pulse is 87/min, blood pressure is 137/93 mmHg, and respirations are 12/min with shallow breaths. Examination reveals a jaundiced male who appears older than stated age. Abdominal exam is positive for a fluid wave and shifting dullness to percussion. You note enlarged breasts, decreased facial hair, 3+ patellar reflexes bilaterally, and the following in the upper extremity (Image A). Paracentesis reveals ascitic fluid with neutrophil counts of < 100 cells/mcL. Serum creatinine is 1.0 and BUN is 15. Which of the following is the next best step in management?
- A. Administer neomycin and glucose
- B. IV albumin and antibiotic therapy with cefotaxime
- C. Administer rifaximin and glucose
- D. Administer lactulose (Correct Answer)
- E. Liver transplantation
GSD type IV (Andersen disease) Explanation: ***Administer lactulose***
- The patient exhibits classic symptoms of **hepatic encephalopathy** (disorientation, slurred speech, somnolence, asterixis as demonstrated by Image A), combined with findings consistent with **cirrhosis** (jaundice, ascites, gynecomastia, decreased facial hair, history of hepatitis C and TIPS).
- **Lactulose** is the first-line treatment for hepatic encephalopathy as it acidifies the colon, promoting the conversion of ammonia (a neurotoxin) to ammonium, which is then trapped and excreted in the feces.
*Administer neomycin and glucose*
- **Neomycin** is an antibiotic that can reduce ammonia-producing bacteria in the gut but is generally considered a second-line agent due to potential side effects like **ototoxicity** and **nephrotoxicity**.
- **Glucose** administration is not a primary treatment for hepatic encephalopathy; it would only be indicated if the patient were hypoglycemic, which is not suggested by the clinical picture.
*IV albumin and antibiotic therapy with cefotaxime*
- **IV albumin** is primarily used in **spontaneous bacterial peritonitis (SBP)** to prevent hepatorenal syndrome, and the paracentesis finding of < 100 cells/mcL neutrophils suggests SBP is unlikely.
- **Cefotaxime** is an appropriate antibiotic for **SBP**, but the patient's presentation is more consistent with hepatic encephalopathy, not an active infection.
*Administer rifaximin and glucose*
- **Rifaximin** is a non-absorbable antibiotic used to reduce ammonia-producing bacteria in the gut, often as an add-on or alternative to lactulose for maintenance therapy or in cases unresponsive to lactulose alone. It is not generally the initial monotherapy for an acute, severe encephalopathy episode.
- As mentioned, **glucose** is not a primary treatment for hepatic encephalopathy.
*Liver transplantation*
- **Liver transplantation** is a definitive treatment for end-stage liver disease, but it is not the **next best step** for acute management of hepatic encephalopathy.
- The immediate priority is to address the acute encephalopathy episode pharmacologically before considering long-term solutions like transplantation, which has a complex workup and waiting list.
GSD type IV (Andersen disease) US Medical PG Question 4: A 5-month-old boy presents with increasing weakness for the past 3 months. The patient’s mother says that the weakness is accompanied by dizziness, sweating, and vertigo early in the morning. Physical examination shows hepatomegaly. Laboratory findings show an increased amount of lactate, uric acid, and elevated triglyceride levels. Which of the following enzymes is most likely deficient in this patient?
- A. Hepatic glycogen phosphorylase
- B. Debranching enzyme
- C. Glucose-6-phosphatase (Correct Answer)
- D. Muscle glycogen phosphorylase
- E. Lysosomal α-1,4-glucosidase
GSD type IV (Andersen disease) Explanation: ***Glucose-6-phosphatase***
- The constellation of **hypoglycemia** (weakness, dizziness, sweating, vertigo, especially early morning), **hepatomegaly**, **lactic acidosis**, **hyperuricemia**, and **hypertriglyceridemia** are classic features of **Type I glycogen storage disease (von Gierke disease)**, which is caused by a deficiency of **glucose-6-phosphatase**.
- This enzyme is crucial for the final step of both **glycogenolysis** and **gluconeogenesis**, releasing free glucose into the bloodstream; its deficiency leads to an inability to maintain normal blood glucose levels during fasting and accumulation of glucose-6-phosphate, which shunts into other metabolic pathways.
*Hepatic glycogen phosphorylase*
- Deficiency in **hepatic glycogen phosphorylase** (Type VI glycogen storage disease, Hers disease) would cause **hepatomegaly** and **hypoglycemia**, but typically does not present with severe **lactic acidosis**, **hyperuricemia**, or **hypertriglyceridemia** to the same degree as von Gierke disease.
- The primary defect is in breaking down glycogen, leading to its accumulation in the liver, but the products of glycolysis can still exit the liver via gluconeogenesis.
*Debranching enzyme*
- Deficiency in **debranching enzyme** (Type III glycogen storage disease, Cori or Forbes disease) causes **hepatomegaly** and **hypoglycemia**, but usually presents with milder symptoms and less severe **lactic acidosis**, **hyperuricemia**, and **hypertriglyceridemia**.
- Patients often present with symptoms similar to Type I, but muscle involvement is also common, and **glycogen structures with short outer branches** are characteristic.
*Muscle glycogen phosphorylase*
- Deficiency in **muscle glycogen phosphorylase** (Type V glycogen storage disease, McArdle disease) primarily affects **skeletal muscle**, leading to exercise intolerance, muscle pain, and myoglobinuria.
- It does not typically cause **hypoglycemia** or **hepatomegaly**, as the liver enzyme is functional, and the symptoms described are systemic rather than muscle-specific.
*Lysosomal α-1,4-glucosidase*
- Deficiency in **lysosomal α-1,4-glucosidase** (Type II glycogen storage disease, Pompe disease) primarily affects the **heart, muscle, and liver**, causing severe **cardiomyopathy**, hypotonia, and **hepatomegaly**.
- While it involves glycogen accumulation, it typically does not present with **hypoglycemia** (as cytoplasmic glycogen metabolism is intact), **lactic acidosis**, or the specific metabolic derangements seen in this patient.
GSD type IV (Andersen disease) US Medical PG Question 5: A 9-month-old girl is brought to the physician because of a 1-month history of poor feeding and irritability. She is at the 15th percentile for height and 5th percentile for weight. Examination shows hypotonia and wasting of skeletal muscles. Cardiopulmonary examination shows no abnormalities. There is hepatomegaly. Her serum glucose is 61 mg/dL, creatinine kinase is 100 U/L, and lactic acid is within the reference range. Urine ketone bodies are elevated. Which of the following enzymes is most likely deficient in this patient?
- A. Glucose-6-phosphatase
- B. Muscle phosphorylase
- C. Acid alpha-glucosidase
- D. Glycogen debrancher (Correct Answer)
- E. Glucocerebrosidase
GSD type IV (Andersen disease) Explanation: ***Glycogen debrancher***
- The patient's symptoms of **hepatomegaly**, **hypoglycemia**, **poor feeding**, **growth failure**, and **elevated urine ketones** in the presence of normal lactic acid suggest Type III glycogen storage disease (Cori disease), caused by a deficiency in **glycogen debrancher enzyme**.
- **Muscle wasting** and **hypotonia** are also consistent with Type III GSD, as the debranching enzyme is present in both liver and muscle.
*Glucose-6-phosphatase*
- Deficiency in **glucose-6-phosphatase** (Type I GSD, Von Gierke disease) also presents with **hepatomegaly** and **hypoglycemia**.
- However, Type I GSD is characterized by **lactic acidosis**, which is explicitly stated as normal in this patient, and **hyperlipidemia**, which is not mentioned.
*Muscle phosphorylase*
- Deficiency in **muscle phosphorylase** (Type V GSD, McArdle disease) primarily affects skeletal muscle, causing **exercise intolerance** and **muscle pain**.
- It does not typically present with **hypoglycemia**, **hepatomegaly**, or **growth failure** in infancy.
*Acid alpha-glucosidase*
- Deficiency in **acid alpha-glucosidase** (Type II GSD, Pompe disease) causes accumulation of glycogen in lysosomes, leading to severe **cardiomyopathy**, **hypotonia**, and **muscle weakness**.
- While hypotonia is present, the absence of **cardiomegaly** and significant **liver involvement** makes this diagnosis less likely.
*Glucocerebrosidase*
- Deficiency in **glucocerebrosidase** causes Gaucher disease, a lysosomal storage disorder, not a glycogen storage disorder.
- Symptoms include **hepatosplenomegaly**, **bone crises**, and neurological symptoms, but not **hypoglycemia** or isolated muscle wasting directly related to glycogen metabolism.
GSD type IV (Andersen disease) US Medical PG Question 6: A 45-year-old woman presents to her primary care physician for knee pain. She states that she has been experiencing a discomfort and pain in her left knee that lasts for several hours but tends to improve with use. She takes ibuprofen occasionally which has been minimally helpful. She states that this pain is making it difficult for her to work as a cashier. Her temperature is 98.6°F (37.0°C), blood pressure is 117/58 mmHg, pulse is 90/min, respirations are 14/min, and oxygen saturation is 97% on room air. Physical exam reveals a stable gait that the patient claims causes her pain. The patient has a non-pulsatile, non-erythematous, palpable mass over the posterior aspect of her left knee that is roughly 3 to 4 cm in diameter and is hypoechoic on ultrasound. Which of the following is associated with this patient's condition?
- A. Venous valve failure
- B. Herniated nucleus pulposus
- C. Inflammation of the pes anserinus bursa
- D. Artery aneurysm
- E. Baker's cyst (Correct Answer)
GSD type IV (Andersen disease) Explanation: ***Baker's cyst***
- The patient's presentation of a **palpable, non-pulsatile, non-erythematous mass** in the posterior knee that is **hypoechoic on ultrasound** is highly suggestive of a Baker's cyst.
- A Baker's cyst (popliteal cyst) is often associated with **underlying knee joint pathology**, such as osteoarthritis or meniscal tears, which can cause knee pain that improves with use and is worse with activity like standing for a cashier.
*Venous valve failure*
- **Venous valve failure** leads to **chronic venous insufficiency**, presenting as varicose veins, edema, skin changes (hyperpigmentation, lipodermatosclerosis), and ulcers, typically in the lower leg and ankle.
- While it can cause leg discomfort, it does not typically manifest as a discreet, non-pulsatile mass in the posterior knee or be hypoechoic on ultrasound.
*Herniated nucleus pulposus*
- A **herniated nucleus pulposus** (slipped disc) causes **radicular pain** (sciatica) that radiates down the leg, numbness, tingling, and weakness, often exacerbated by sitting, coughing, or sneezing.
- It would not present with a palpable mass in the posterior knee and is a spinal condition, not a direct knee pathology.
*Inflammation of the pes anserine bursa*
- **Pes anserine bursitis** causes pain and tenderness specifically on the **medial aspect of the knee**, about 2-3 inches below the joint line, where the pes anserinus tendons insert.
- It would not cause a mass in the posterior knee and the pain location is distinct.
*Artery aneurysm*
- An **artery aneurysm**, particularly a popliteal artery aneurysm, would present as a **pulsatile mass** in the popliteal fossa.
- Its pulsatile nature and the risk of rupture or thrombus formation distinguish it from the described non-pulsatile mass.
GSD type IV (Andersen disease) US Medical PG Question 7: A 61-year-old man presents to the emergency department because he has developed blisters at multiple locations on his body. He says that the blisters appeared several days ago after a day of hiking in the mountains with his colleagues. When asked about potential triggering events, he says that he recently had an infection and was treated with antibiotics but he cannot recall the name of the drug that he took. In addition, he accidentally confused his medication with one of his wife's blood thinner pills several days before the blisters appeared. On examination, the blisters are flesh-colored, raised, and widespread on his skin but do not involve his mucosal surfaces. The blisters are tense to palpation and do not separate with rubbing. Pathology of the vesicles show that they continue under the level of the epidermis. Which of the following is the most likely cause of this patient's blistering?
- A. Depletion of protein C and protein S levels
- B. Antibodies to proteins connecting two sets of intermediate filaments
- C. Necrosis of skin in reaction to a drug
- D. Antibodies to proteins connecting intermediate filaments to type IV collagen (Correct Answer)
- E. Infection with an enveloped dsDNA virus
GSD type IV (Andersen disease) Explanation: ***Antibodies to proteins connecting intermediate filaments to type IV collagen***
- The patient's presentation of **tense blisters** that do not separate with rubbing (negative Nikolsky sign), **widespread distribution** on skin surfaces without **mucosal involvement**, and **subepidermal blistering** on pathology is classic for **bullous pemphigoid**.
- **Bullous pemphigoid** is caused by autoantibodies targeting hemidesmosomal proteins, specifically BP180 (type XVII collagen) and BP230, which connect **intermediate filaments to the basal lamina (containing type IV collagen)**.
*Depletion of protein C and protein S levels*
- This scenario describes **warfarin-induced skin necrosis**, which typically presents as painful, erythematous plaques that rapidly progress to **hemorrhagic bullae and necrosis**.
- While the patient might have inadvertently taken a blood thinner, the clinical presentation of widespread **tense, non-hemorrhagic blisters** is inconsistent with warfarin-induced skin necrosis.
*Antibodies to proteins connecting two sets of intermediate filaments*
- This describes the pathophysiology of **pemphigus vulgaris**, where autoantibodies target **desmogleins** (desmosomal proteins that connect keratinocytes via intermediate filaments).
- Pemphigus vulgaris typically presents with **flaccid blisters** that are easily ruptured, readily separate with rubbing (**positive Nikolsky sign**), and commonly involve **mucosal surfaces**, which contradicts this patient's findings.
*Necrosis of skin in reaction to a drug*
- While drug reactions can cause blistering conditions like **Stevens-Johnson Syndrome (SJS)** or **Toxic Epidermal Necrolysis (TEN)**, these conditions typically involve **widespread epidermal necrosis**, mucosal involvement, and often present with a **positive Nikolsky sign** and targetoid lesions for SJS.
- The description of **tense blisters** and **subepidermal cleavage** without significant epidermal necrosis makes SJS/TEN less likely.
*Infection with a enveloped dsDNA virus*
- This likely refers to **herpes simplex virus (HSV)** or **varicella-zoster virus (VZV)** infections, which cause vesicular eruptions.
- Viral blisters are typically **grouped vesicles on an erythematous base** (e.g., herpes zoster), often painful, and **rarely widespread tense bullae** as described in this patient.
GSD type IV (Andersen disease) US Medical PG Question 8: A 6-year-old boy is brought to the pediatrician by his mother after he reported having red urine. He has never experienced this before and did not eat anything unusual before the episode. His past medical history is notable for sensorineural deafness requiring hearing aids. He is otherwise healthy and enjoys being in the 1st grade. His birth history was unremarkable. His temperature is 98.8°F (37.1°C), blood pressure is 145/85 mmHg, pulse is 86/min, and respirations are 18/min. On examination, he is a well-appearing boy in no acute distress. Cardiac, respiratory, and abdominal exams are normal. A urinalysis is notable for microscopic hematuria and mild proteinuria. This patient’s condition is most commonly caused by which of the following inheritance patterns?
- A. X-linked dominant
- B. Autosomal recessive
- C. Autosomal dominant
- D. X-linked recessive (Correct Answer)
- E. Mitochondrial inheritance
GSD type IV (Andersen disease) Explanation: ***X-linked recessive***
- This is the **most common inheritance pattern** for **Alport syndrome**, accounting for approximately **80-85% of all cases**.
- This inheritance pattern is characteristic of **Alport syndrome**, which classically presents with **hematuria**, **sensorineural hearing loss**, and ocular abnormalities.
- The patient's presentation with **red urine (hematuria)** and a history of **sensorineural deafness** strongly points to **Alport syndrome**, which is primarily caused by **X-linked recessive** inheritance due to mutations in the *COL4A5* gene encoding the alpha-5 chain of type IV collagen.
- Males with the mutation are typically more severely affected, while female carriers may have variable manifestations.
*X-linked dominant*
- While Alport syndrome can manifest in females with an X-linked dominant-like pattern (due to mosaicism or severely affected carrier females), the **classic and most common inheritance** for severe forms in males is **X-linked recessive**.
- Conditions like **Fragile X syndrome** or **Vitamin D-resistant rickets** are examples of X-linked dominant conditions, but they do not typically present with the specific triad observed here.
*Autosomal recessive*
- An autosomal recessive form of **Alport syndrome** exists, but it accounts for only approximately **10-15% of cases** compared to the X-linked recessive form.
- While other conditions with **renal and auditory involvement** can be autosomal recessive (e.g., specific forms of branchio-oto-renal syndrome), the **combination of hematuria and sensorineural deafness with significant renal progression risk** in a male points most strongly to the X-linked form.
*Autosomal dominant*
- Alport syndrome also has an **autosomal dominant** form, which is typically due to mutations in *COL4A3* or *COL4A4* genes, but it accounts for only approximately **5% of cases** and is **less common** than the X-linked recessive form.
- The autosomal dominant form often presents with a **later onset** and a **more variable phenotype** with milder disease progression.
- While some forms of **polycystic kidney disease** are autosomal dominant and can cause hematuria, they typically involve cyst formation, which is not suggested by the clinical picture here.
*Mitochondrial inheritance*
- This inheritance pattern is associated with disorders affecting **energy production**, commonly involving multiple organ systems, including muscle, brain, and eye.
- While some mitochondrial disorders can affect the kidneys or cause hearing impairment, the **specific combination of hematuria and sensorineural deafness** as the primary presentation in this context is not characteristic of mitochondrial inheritance.
GSD type IV (Andersen disease) US Medical PG Question 9: A 24-year-old man is brought to the emergency department by his brother because of a 3-hour history of lethargy and confusion. The brother says that 2 days ago, the patient ate several large-capped mushrooms he had foraged in the woods. After eating the mushrooms, he developed severe, bloody diarrhea that has since resolved. His pulse is 140/min, respirations are 26/min, and blood pressure is 98/62 mm Hg. Examination shows dry mucous membranes and tenderness to deep palpation in the right upper quadrant. Serum studies show a serum AST concentration of 2335 U/L and ALT concentration of 2294 U/L. Inhibition of which of the following processes is the most likely cause of this patient's condition?
- A. Parasympathetic activation
- B. Messenger RNA synthesis (Correct Answer)
- C. Microtubule polymerization
- D. Cell depolarization
- E. ATP production
GSD type IV (Andersen disease) Explanation: ***Messenger RNA synthesis***
- This patient's symptoms, including **severe gastrointestinal upset** followed by apparent recovery and then **hepatic encephalopathy** (lethargy, confusion, elevated AST/ALT), are classic for **Amanita phalloides (death cap mushroom) poisoning**.
- The primary toxin, **α-amanitin**, specifically inhibits **RNA polymerase II**, thereby blocking **mRNA synthesis** and leading to hepatocyte death and liver failure.
*Parasympathetic activation*
- This is characteristic of poisoning by muscarinic agonists (e.g., *Inocybe* or *Clitocybe* mushrooms), causing symptoms like **salivation, lacrimation, urination, defecation, gastrointestinal cramping, and emesis (SLUDGE)**.
- While initial GI symptoms might overlap, the severe liver damage and delayed presentation of encephalopathy are inconsistent with sole parasympathetic overactivation.
*Microtubule polymerization*
- Inhibition of microtubule polymerization is associated with toxins like **colchicine** or **vincristine**, which can cause gastrointestinal toxicity and myelosuppression.
- It does not directly explain the severe hepatotoxicity and delayed onset of liver failure seen in this patient.
*Cell depolarization*
- This mechanism is associated with neurotoxins that affect ion channels, such as those found in some species of *Gyromitra* mushrooms (producing **monomethylhydrazine**) or *Psilocybe* (containing **psilocybin**).
- While neurotoxicity can occur, the prominent and severe liver failure points away from cell depolarization as the primary mechanism in this case.
*ATP production*
- Toxins that inhibit ATP production (e.g., cyanide, carbon monoxide, some mitochondrial poisons) cause widespread cellular dysfunction and can lead to multi-organ failure.
- While severe liver failure will eventually impair ATP production, α-amanitin's direct mechanism is earlier in the protein synthesis pathway (mRNA synthesis), leading to a delayed, but profound, impact on cellular function and viability.
GSD type IV (Andersen disease) US Medical PG Question 10: A 4-year-old girl is brought to the physician by her mother because of fatigue and generalized weakness for 4 months. Examination shows decreased muscle tone. Her fasting serum glucose concentration is 41 mg/dL. The physician suspects a defect in one of the enzymes involved in the carnitine shuttle. Increased serum concentration of which of the following should most raise suspicion of a different diagnosis?
- A. Ammonia (Correct Answer)
- B. Creatine kinase
- C. Alanine aminotransferase
- D. Uric acid
- E. β-hydroxybutyrate
GSD type IV (Andersen disease) Explanation: ***Ammonia***
- An elevated **ammonia** level in the context of hypoglycemia and muscle weakness in a child suggests an **inborn error of metabolism** that affects the **urea cycle** or **organic acidemia**, not primarily the carnitine shuttle.
- Urea cycle disorders lead to **hyperammonemia**, which can cause neurological symptoms, fatigue, and muscle weakness, often exacerbated by catabolic states.
- This finding would **strongly suggest a different diagnosis** from a carnitine shuttle defect.
*Creatine kinase*
- **Creatine kinase (CK)** levels are typically **elevated in carnitine shuttle defects** due to muscle damage and myopathy.
- Elevated CK would **support** the suspected diagnosis of a carnitine shuttle defect rather than suggest an alternative.
- This is an **expected finding** in fatty acid oxidation disorders.
*Alanine aminotransferase*
- **Alanine aminotransferase (ALT)** can be elevated in **carnitine shuttle defects** due to liver involvement and hepatic dysfunction.
- While elevated ALT indicates liver damage, it can occur in fatty acid oxidation disorders and would not necessarily point away from a carnitine shuttle defect.
- This finding is **consistent with** rather than against the suspected diagnosis.
*Uric acid*
- **Uric acid** levels are not directly affected by defects in the **carnitine shuttle**.
- While an elevated uric acid level might prompt investigation into conditions like **glycogen storage diseases** or purine metabolism disorders, it is not a strong discriminator for alternative diagnoses in this clinical context.
*β-hydroxybutyrate*
- **β-hydroxybutyrate** is a **ketone body** produced from fatty acid oxidation during fasting states.
- In carnitine shuttle defects, the body **cannot effectively oxidize fatty acids** to produce ketones, resulting in **hypoketotic hypoglycemia** (low or inappropriately low ketones despite low glucose).
- If β-hydroxybutyrate is **elevated** during fasting hypoglycemia, this indicates **intact fatty acid oxidation** and would suggest a different diagnosis such as **hyperinsulinism**, **glycogen storage disease**, or other causes of hypoglycemia where ketogenesis is preserved.
- However, **ammonia elevation** is a stronger indicator of an alternative diagnosis (urea cycle disorder) compared to the scenario presented.
More GSD type IV (Andersen disease) US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.