Diagnostic approaches to GSDs US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Diagnostic approaches to GSDs. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Diagnostic approaches to GSDs US Medical PG Question 1: A 15-year-old boy is sent from gym class with a chief complaint of severe muscle aches. In class today he was competing with his friends and therefore engaged in weightlifting for the first time. A few hours later he was extremely sore and found that his urine was red when he went to urinate. This concerned him and he was sent to the emergency department for evaluation.
Upon further questioning, you learn that since childhood he has always had muscle cramps with exercise. Physical exam was unremarkable. Upon testing, his creatine kinase level was elevated and his urinalysis was negative for blood and positive for myoglobin.
Thinking back to biochemistry you suspect that he may be suffering from a hereditary glycogen disorder. Given this suspicion, what would you expect to find upon examination of his cells?
- A. Normal glycogen structure (Correct Answer)
- B. Short outer glycogen chains
- C. Accumulation of glycogen in lysosomes forming dense granules
- D. Glycogen without normal branching pattern
- E. Absence of glycogen in muscles
Diagnostic approaches to GSDs Explanation: ***Normal glycogen structure***
- The patient's symptoms (exercise-induced muscle cramps, myoglobinuria, and elevated CK) are classic for **McArdle disease** (Glycogen Storage Disease Type V), caused by a deficiency in **muscle glycogen phosphorylase**.
- In McArdle disease, the enzyme responsible for breaking down glycogen (glycogen phosphorylase) is deficient, but the enzymes involved in synthesizing glycogen are normal. Therefore, the **structure of glycogen is normal**, but it accumulates in muscle cells because it cannot be catabolized.
*Short outer glycogen chains*
- **Short outer glycogen chains** are characteristic of **Cori disease** (Glycogen Storage Disease Type III), caused by a deficiency in **debranching enzyme**.
- This condition also presents with hypoglycemia and hepatomegaly, which are not described in the patient's presentation.
*Accumulation of glycogen in lysosomes forming dense granules*
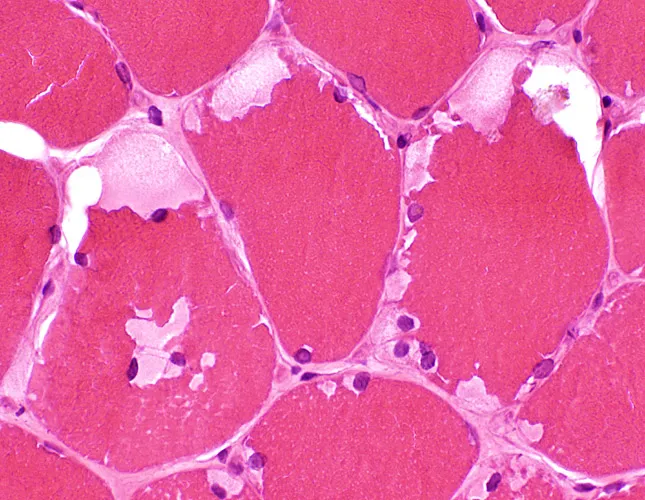
- **Accumulation of glycogen in lysosomes** and the formation of **dense granules** is characteristic of **Pompe disease** (Glycogen Storage Disease Type II), caused by a deficiency in **lysosomal alpha-glucosidase (acid maltase)**.
- Pompe disease typically presents as a severe infantile form with cardiomegaly and hypotonia, or a later-onset form with proximal muscle weakness, which differs from the patient's primary complaint of exercise intolerance and myoglobinuria.
*Glycogen without normal branching pattern*
- **Glycogen without a normal branching pattern** (very long unbranched chains) is characteristic of **Andersen disease** (Glycogen Storage Disease Type IV), caused by a deficiency in **branching enzyme**.
- This condition typically leads to cirrhosis and liver failure in infancy, which is not consistent with the patient's presentation.
*Absence of glycogen in muscles*
- While McArdle disease involves an inability to break down muscle glycogen, it does not result in the **absence of glycogen** in muscles; rather, there is an **over-accumulation** of normal-structured glycogen because it cannot be utilized.
- The defect is in **glycogenolysis**, not glycogen synthesis, so glycogen is formed but not broken down.
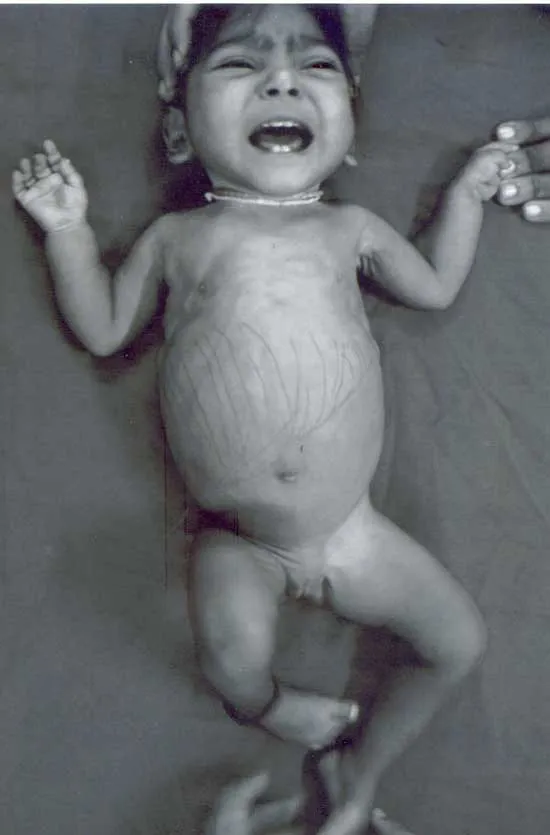
Diagnostic approaches to GSDs US Medical PG Question 2: A 6-month-old boy is referred to a geneticist after he is found to have persistent hypotonia and failure to thrive. He has also had episodes of what appears to be respiratory distress and has an enlarged heart on physical exam. There is a family history of childhood onset hypertrophic cardiomyopathy, so a biopsy is performed showing electron dense granules within the lysosomes. Genetic testing is performed showing a defect in glycogen processing. A deficiency in which of the following enzymes is most likely to be responsible for this patient's symptoms?
- A. Lysosomal alpha 1,4-glucosidase (Correct Answer)
- B. Branching enzyme
- C. Muscle phosphorylase
- D. Debranching enzyme
- E. Glucose-6-phosphatase
Diagnostic approaches to GSDs Explanation: ***Lysosomal alpha 1,4-glucosidase***
- The constellation of **hypotonia**, **failure to thrive**, **respiratory distress**, and **cardiomegaly** in an infant, along with **electron-dense granules in lysosomes** and a defect in **glycogen processing**, is characteristic of **Pompe disease (Type II glycogen storage disease)**.
- **Pompe disease** is caused by a deficiency of **lysosomal alpha 1,4-glucosidase** (also known as acid maltase), which is responsible for breaking down glycogen in lysosomes.
*Branching enzyme*
- A deficiency in **branching enzyme (amylo-alpha-1,4-to-alpha-1,6-transglucosidase)** causes **Andersen disease (Type IV glycogen storage disease)**, which typically presents with **hepatosplenomegaly**, **cirrhosis**, and **failure to thrive**.
- While it involves glycogenopathy, the specific features of **cardiomyopathy** and **lysosomal accumulation** are not primary to this disorder.
*Muscle phosphorylase*
- A deficiency in **muscle phosphorylase** causes **McArdle disease (Type V glycogen storage disease)**, which primarily affects **skeletal muscle**.
- Symptoms include **exercise intolerance**, **muscle cramps**, and **myoglobinuria**, typically presenting later in childhood or adolescence, and does not involve cardiomyopathy or lysosomal storage.
*Debranching enzyme*
- A deficiency in **debranching enzyme (alpha-1,6-glucosidase)** causes **Cori disease (Type III glycogen storage disease)**, which presents with **hepatomegaly**, **hypoglycemia**, and **muscle weakness**.
- While it can sometimes involve a milder form of cardiomyopathy, the significant **lysosomal involvement** and severe infantile onset with respiratory distress and profound hypotonia point away from Cori disease.
*Glucose-6-phosphatase*
- A deficiency in **glucose-6-phosphatase** causes **Von Gierke disease (Type I glycogen storage disease)**, characterized by **severe fasting hypoglycemia**, **lactic acidosis**, **hepatomegaly**, and **hyperlipidemia**.
- This condition primarily affects the liver and kidneys, and typically does not present with primary cardiomyopathy, hypotonia, or lysosomal glycogen accumulation.
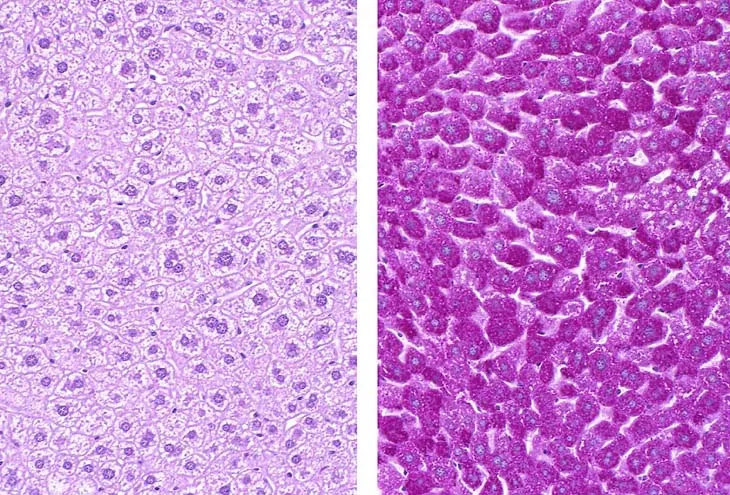
Diagnostic approaches to GSDs US Medical PG Question 3: A 12-year-old girl comes to the clinic with a grossly enlarged abdomen. She has a history of frequent episodes of weakness, sweating, and pallor that are eliminated by eating. Her development has been slow. She started to walk unassisted at 2 years and was not performing well at school. Physical examination reveals a blood pressure of 100/60 mm Hg, heart rate of 80/min, and temperature of 36.9°C (98.4℉). On physical examination, the liver is enlarged, firm, and palpable up to the pelvis. The spleen and kidney are not palpable. Laboratory investigation reveals low blood glucose and pH with high lactate, triglycerides, ketones, and free fatty acids. The liver biopsy revealed high glycogen content. Hepatic glycogen structure was normal. The enzyme assay performed on the biopsy tissue revealed very low glucose-6-phosphatase levels. What is the most likely diagnosis?
- A. Pompe's disease
- B. Cori's disease
- C. Hereditary hemochromatosis
- D. Von-Gierke's disease (Correct Answer)
- E. McArdle disease
Diagnostic approaches to GSDs Explanation: ***Von-Gierke's disease***
- The combination of **hepatomegaly**, **hypoglycemia** (causing weakness, sweating, pallor), **lactic acidosis**, **hyperlipidemia**, and elevated ketones points to a severe defect in glucose metabolism.
- **Very low glucose-6-phosphatase levels** on liver biopsy and normal hepatic glycogen structure are pathognomonic for Von-Gierke's disease (Glycogen Storage Disease Type I).
*Pompe's disease*
- This is a **lysosomal storage disease** affecting **alpha-1,4-glucosidase**, leading to glycogen accumulation in lysosomes.
- It primarily affects the **heart** and skeletal muscles and would not present with severe lactic acidosis and hyperlipidemia.
*Cori's disease*
- This is **Glycogen Storage Disease Type III**, caused by a deficiency in the **debranching enzyme** (amylo-alpha-1,6-glucosidase).
- While it can cause hepatomegaly and hypoglycemia, the hepatic glycogen structure would be abnormal due to incompletely debranched glycogen, and glucose-6-phosphatase levels would be normal.
*Hereditary hemochromatosis*
- This is an **iron overload disorder** leading to iron deposition in organs like the liver, heart, and pancreas.
- It would present with symptoms related to organ damage from iron accumulation, such as liver cirrhosis and diabetes, not the metabolic derangements seen here.
*McArdle disease*
- This is **Glycogen Storage Disease Type V**, due to a deficiency in **muscle glycogen phosphorylase**.
- It primarily causes exercise-induced muscle pain, cramping, and fatigue due to an inability to break down muscle glycogen for energy, not systemic metabolic disturbances or hepatomegaly.
Diagnostic approaches to GSDs US Medical PG Question 4: A 5-month-old boy presents with increasing weakness for the past 3 months. The patient’s mother says that the weakness is accompanied by dizziness, sweating, and vertigo early in the morning. Physical examination shows hepatomegaly. Laboratory findings show an increased amount of lactate, uric acid, and elevated triglyceride levels. Which of the following enzymes is most likely deficient in this patient?
- A. Hepatic glycogen phosphorylase
- B. Debranching enzyme
- C. Glucose-6-phosphatase (Correct Answer)
- D. Muscle glycogen phosphorylase
- E. Lysosomal α-1,4-glucosidase
Diagnostic approaches to GSDs Explanation: ***Glucose-6-phosphatase***
- The constellation of **hypoglycemia** (weakness, dizziness, sweating, vertigo, especially early morning), **hepatomegaly**, **lactic acidosis**, **hyperuricemia**, and **hypertriglyceridemia** are classic features of **Type I glycogen storage disease (von Gierke disease)**, which is caused by a deficiency of **glucose-6-phosphatase**.
- This enzyme is crucial for the final step of both **glycogenolysis** and **gluconeogenesis**, releasing free glucose into the bloodstream; its deficiency leads to an inability to maintain normal blood glucose levels during fasting and accumulation of glucose-6-phosphate, which shunts into other metabolic pathways.
*Hepatic glycogen phosphorylase*
- Deficiency in **hepatic glycogen phosphorylase** (Type VI glycogen storage disease, Hers disease) would cause **hepatomegaly** and **hypoglycemia**, but typically does not present with severe **lactic acidosis**, **hyperuricemia**, or **hypertriglyceridemia** to the same degree as von Gierke disease.
- The primary defect is in breaking down glycogen, leading to its accumulation in the liver, but the products of glycolysis can still exit the liver via gluconeogenesis.
*Debranching enzyme*
- Deficiency in **debranching enzyme** (Type III glycogen storage disease, Cori or Forbes disease) causes **hepatomegaly** and **hypoglycemia**, but usually presents with milder symptoms and less severe **lactic acidosis**, **hyperuricemia**, and **hypertriglyceridemia**.
- Patients often present with symptoms similar to Type I, but muscle involvement is also common, and **glycogen structures with short outer branches** are characteristic.
*Muscle glycogen phosphorylase*
- Deficiency in **muscle glycogen phosphorylase** (Type V glycogen storage disease, McArdle disease) primarily affects **skeletal muscle**, leading to exercise intolerance, muscle pain, and myoglobinuria.
- It does not typically cause **hypoglycemia** or **hepatomegaly**, as the liver enzyme is functional, and the symptoms described are systemic rather than muscle-specific.
*Lysosomal α-1,4-glucosidase*
- Deficiency in **lysosomal α-1,4-glucosidase** (Type II glycogen storage disease, Pompe disease) primarily affects the **heart, muscle, and liver**, causing severe **cardiomyopathy**, hypotonia, and **hepatomegaly**.
- While it involves glycogen accumulation, it typically does not present with **hypoglycemia** (as cytoplasmic glycogen metabolism is intact), **lactic acidosis**, or the specific metabolic derangements seen in this patient.
Diagnostic approaches to GSDs US Medical PG Question 5: A 3-week-old newborn is brought to the pediatrician by his mother. His mother is concerned about her son’s irritability and vomiting, particularly after breastfeeding him. The infant was born at 39 weeks via spontaneous vaginal delivery. His initial physical was benign. Today the newborn appears mildly jaundiced with palpable hepatomegaly, and his eyes appear cloudy, consistent with the development of cataracts. The newborn is also in the lower weight-age percentile. The physician considers a hereditary enzyme deficiency and orders blood work and a urinalysis to confirm his diagnosis. He recommends that milk and foods high in galactose and/or lactose be eliminated from the diet. Which of the following is the most likely deficient enzyme in this metabolic disorder?
- A. Aldose reductase
- B. Galactose-1-phosphate uridyl transferase (Correct Answer)
- C. UDP-galactose-4-epimerase
- D. Galactokinase
- E. Glucose-6-phosphate dehydrogenase
Diagnostic approaches to GSDs Explanation: ***Galactose-1-phosphate uridyl transferase***
- The constellation of symptoms including **vomiting**, **irritability**, **jaundice**, **hepatomegaly**, **cataracts**, and **failure to thrive** in a neonate, with improvement upon eliminating galactose/lactose from the diet, is highly characteristic of **classic galactosemia**.
- **Classic galactosemia** is caused by a deficiency in **galactose-1-phosphate uridyl transferase (GALT)**, leading to the accumulation of galactose-1-phosphate, which is toxic to various tissues.
*Aldose reductase*
- This enzyme converts galactose to **galactitol**, which can accumulate in the lens and cause **cataracts** in all forms of galactosemia if left untreated.
- However, isolated aldose reductase deficiency does not explain the full spectrum of severe systemic symptoms like hepatomegaly, jaundice, and failure to thrive observed in this neonate, which are indicative of classic galactosemia.
*UDP-galactose-4-epimerase*
- Deficiency in **UDP-galactose-4-epimerase (GALE)**, also known as epimerase deficiency galactosemia, has a wide range of severity.
- While it can present with similar symptoms to GALT deficiency, its severe form is rarer, and the classic, pronounced presentation described here is more commonly associated with GALT deficiency.
*Galactokinase*
- Deficiency in **galactokinase (GALK)** causes **Type II galactosemia**, which primarily manifests as **cataracts** due to galactitol accumulation.
- It typically does not present with the severe hepatic (jaundice, hepatomegaly) or systemic symptoms (vomiting, failure to thrive) seen in classic galactosemia.
*Glucose-6-phosphate dehydrogenase*
- **Glucose-6-phosphate dehydrogenase (G6PD) deficiency** primarily causes **hemolytic anemia** triggered by certain drugs, infections, or fava beans.
- It does not present with the specific constellation of symptoms related to galactose metabolism, such as cataracts, hepatomegaly, and vomiting upon milk ingestion, as described in this case.
Diagnostic approaches to GSDs US Medical PG Question 6: A 16-year-old boy comes to the physician because of muscle weakness and cramps for 5 months. He becomes easily fatigued and has severe muscle pain and swelling after 15 minutes of playing basketball with his friends. The symptoms improve after a brief period of rest. After playing, he sometimes also has episodes of reddish-brown urine. There is no family history of serious illness. Serum creatine kinase concentration is 950 U/L. Urinalysis shows:
Blood 2+
Protein negative
Glucose negative
RBC negative
WBC 1–2/hpf
Which of the following is the most likely underlying cause of this patient's symptoms?
- A. Medium-chain acyl-CoA dehydrogenase deficiency
- B. Myophosphorylase deficiency (Correct Answer)
- C. Low levels of triiodothyronine and thyroxine
- D. Acid maltase deficiency
- E. CTG repeat in the DMPK gene
Diagnostic approaches to GSDs Explanation: ***Myophosphorylase deficiency***
- This condition (McArdle disease) is an **autosomal recessive disorder** of glycogen metabolism characterized by a defect in **glycogenolysis**, specifically the breakdown of muscle glycogen. This leads to impaired energy production during exercise.
- The classic presentation includes **exercise-induced muscle pain, stiffness, cramps, fatigue**, and sometimes **myoglobinuria** (reddish-brown urine due to myoglobin release from damaged muscle), which is consistent with the patient's symptoms and elevated **creatine kinase**.
*Medium-chain acyl-CoA dehydrogenase deficiency*
- This is a disorder of **fatty acid oxidation** that primarily affects the liver, leading to episodes of **hypoketotic hypoglycemia** during fasting or illness.
- It does not typically present with isolated exercise-induced muscle pain and myoglobinuria.
*Low levels of triiodothyronine and thyroxine*
- **Hypothyroidism** can cause generalized muscle weakness, fatigue, and muscle cramps, but it is usually associated with other systemic symptoms like weight gain, cold intolerance, and constipation.
- While it can cause elevated CK, it generally does not present with acute, exercise-induced muscle pain and myoglobinuria in the manner described.
*Acid maltase deficiency*
- This (Pompe disease) is a lysosomal storage disorder affecting glycogen metabolism, but it results from a deficiency of **acid alpha-glucosidase (acid maltase)**.
- The infantile form presents with severe hypotonia and cardiomyopathy, while the juvenile and adult forms typically cause **proximal muscle weakness** and respiratory insufficiency, rather than exercise-induced muscle pain and myoglobinuria.
*CTG repeat in the DMPK gene*
- This genetic defect is associated with **myotonic dystrophy type 1 (Steinert disease)**, an autosomal dominant disorder.
- Key features include **myotonia** (delayed relaxation of muscles), muscle weakness, cataracts, and cardiac conduction abnormalities, which are distinct from the patient's presentation of exercise-induced cramps and myoglobinuria without myotonia.
Diagnostic approaches to GSDs US Medical PG Question 7: A 15-year-old boy comes to the physician because of severe muscle cramps and pain for 3 months. He first noticed these symptoms while attending tryouts for the high school football team. Since then, he becomes easily fatigued and has severe muscle pain and swelling after 10 minutes of playing. However, after a brief period of rest, the symptoms improve, and he is able to return to the game. Two days ago, he had an episode of reddish-brown urine after playing football. There is no family history of serious illness. He appears healthy. Vital signs are within normal limits. Physical and neurological examinations show no abnormalities. Serum creatine kinase concentration is 333 U/L. Urinalysis shows:
Blood 2+
Protein negative
Glucose negative
RBC negative
WBC 1–2/hpf
Which of the following is the most likely cause of this patient's symptoms?
- A. CTG repeat in the DMPK gene
- B. Myophosphorylase deficiency (Correct Answer)
- C. Dystrophin gene mutation
- D. Thyroid hormone deficiency
- E. Acid maltase deficiency
Diagnostic approaches to GSDs Explanation: ***Myophosphorylase deficiency***
- This condition (also known as **McArdle disease**) presents with **exercise-induced muscle cramps, pain, and fatigue** immediately after initiating activity, with a "second wind" phenomenon where symptoms improve after resting.
- The elevated **creatine kinase** and **reddish-brown urine** (indicating **myoglobinuria** due to rhabdomyolysis) are classic findings after strenuous activity in this glycogen storage disorder.
*CTG repeat in the DMPK gene*
- This describes **myotonic dystrophy type 1**, which presents with **myotonia** (delayed muscle relaxation), muscle weakness, and often involves multiple organ systems.
- While it causes muscle weakness, it does not typically present with acute, exercise-induced pain, cramping, and rhabdomyolysis in this manner.
*Dystrophin gene mutation*
- This is characteristic of **Duchenne or Becker muscular dystrophy**, which are progressive muscle weakness disorders.
- They typically cause **progressive proximal muscle weakness** and atrophy, not acute, intermittent, exercise-induced pain and cramping with a "second wind" phenomenon.
*Thyroid hormone deficiency*
- **Hypothyroidism** can cause muscle cramps, weakness, and elevated creatine kinase, but these symptoms are usually chronic and progressive, not acutely exercise-induced with improvement after a short rest.
- It would also present with other systemic symptoms like fatigue, weight gain, and cold intolerance, which are not described.
*Acid maltase deficiency*
- Also known as **Pompe disease**, this is a glycogen storage disorder that primarily affects infants and can present in adults with **proximal muscle weakness**, respiratory insufficiency, and cardiac involvement.
- It does not typically present with acute, exercise-induced muscle cramps, pain, and rhabdomyolysis followed by a "second wind" phenomenon like McArdle disease.
Diagnostic approaches to GSDs US Medical PG Question 8: An 8-year-old boy is brought to the pediatrician by his mother with nausea, vomiting, and decreased frequency of urination. He has acute lymphoblastic leukemia for which he received the 1st dose of chemotherapy 5 days ago. His leukocyte count was 60,000/mm3 before starting chemotherapy. The vital signs include: pulse 110/min, temperature 37.0°C (98.6°F), and blood pressure 100/70 mm Hg. The physical examination shows bilateral pedal edema. Which of the following serum studies and urinalysis findings will be helpful in confirming the diagnosis of this condition?
- A. Hyperuricemia, hyperkalemia, hyperphosphatemia, and urinary monoclonal spike
- B. Hyperkalemia, hyperphosphatemia, hypocalcemia, hyperuricemia, urine supernatant pink, and positive for heme
- C. Hyperkalemia, hyperphosphatemia, hypocalcemia, and extremely elevated creatine kinase (MM)
- D. Hyperuricemia, hyperkalemia, hyperphosphatemia, lactic acidosis, and oxalate crystals
- E. Hyperuricemia, hyperkalemia, hyperphosphatemia, hypocalcemia, and urate crystals in the urine (Correct Answer)
Diagnostic approaches to GSDs Explanation: ***Hyperuricemia, hyperkalemia, hyperphosphatemia, hypocalcemia, and urate crystals in the urine***
- This patient's presentation following chemotherapy, particularly with a high pre-treatment leukocyte count, is highly suggestive of **tumor lysis syndrome (TLS)**. TLS is characterized by rapid tumor cell breakdown, releasing intracellular contents into the bloodstream.
- The **four cardinal laboratory findings** of TLS are **hyperuricemia** (from nucleic acid breakdown), **hyperkalemia** (from intracellular potassium release), **hyperphosphatemia** (from intracellular phosphate release), and **hypocalcemia** (secondary to calcium-phosphate precipitation). The presence of **urate crystals in the urine** confirms the renal effects of uric acid overload, leading to acute kidney injury.
*Hyperuricemia, hyperkalemia, hyperphosphatemia, and urinary monoclonal spike*
- While **hyperuricemia, hyperkalemia, and hyperphosphatemia** are consistent with tumor lysis syndrome, a **urinary monoclonal spike** is typically associated with multiple myeloma or other plasma cell dyscrasias, not tumor lysis syndrome.
- The patient's history of acute lymphoblastic leukemia and recent chemotherapy points away from a monoclonal gammopathy.
- This option is also missing the key finding of **hypocalcemia**.
*Hyperkalemia, hyperphosphatemia, hypocalcemia, hyperuricemia, urine supernatant pink, and positive for heme*
- **Hyperkalemia, hyperphosphatemia, hyperuricemia, and hypocalcemia** are indeed the four cardinal metabolic abnormalities of TLS. However, a **pink urine supernatant and positive heme** indicate **hemoglobinuria** or **myoglobinuria**, pointing towards hemolysis or rhabdomyolysis, respectively.
- While TLS can lead to acute kidney injury, these specific urinalysis findings are not typical for TLS. The expected urinary finding would be **urate crystals**, not heme pigments.
*Hyperkalemia, hyperphosphatemia, hypocalcemia, and extremely elevated creatine kinase (MM)*
- **Hyperkalemia, hyperphosphatemia, and hypocalcemia** are consistent with TLS. However, **extremely elevated creatine kinase (MM)** is a hallmark of **rhabdomyolysis**, a condition involving breakdown of skeletal muscle.
- This option is also missing **hyperuricemia**, which is a cardinal feature of TLS.
- There is no clinical indication for rhabdomyolysis in this patient's presentation.
*Hyperuricemia, hyperkalemia, hyperphosphatemia, lactic acidosis, and oxalate crystals*
- While **hyperuricemia, hyperkalemia, and hyperphosphatemia** are characteristic of TLS, this option is missing **hypocalcemia**, one of the four cardinal metabolic abnormalities.
- Additionally, the presence of **oxalate crystals** in the urine is typically associated with **ethylene glycol poisoning** or primary hyperoxaluria, not tumor lysis syndrome. **Urate crystals**, not oxalate crystals, are expected due to the rapid breakdown of purines in TLS.
- **Lactic acidosis** can occur in severe TLS but is not a defining laboratory criterion.
Diagnostic approaches to GSDs US Medical PG Question 9: A 22-year-old man comes to the physician because of a fall associated with a 6-month history of increasing difficulty walking. Over the last year, his friends have also noticed his speech becoming slower. During this period, he also gave up his hobby of playing video games because he has become clumsy with his hands. His father died of esophageal varices at the age of 40 years. The patient does not smoke or drink alcohol. He takes no medications. He appears sad. His temperature is 37°C (98.6°F), pulse is 70/min, and blood pressure is 120/80 mm Hg. He is alert and oriented to person, place, and time. His speech is slurred and monotonous; his gait is unsteady. Examination shows scleral icterus and some drooling. The liver is palpated 2 to 3 cm below the right costal margin, and the spleen is palpated 1 to 2 cm below the left costal margin. Further evaluation of this patient is most likely to show which of the following findings?
- A. Increased number of CAG repeats
- B. Oligoclonal bands on CSF analysis
- C. Low serum ceruloplasmin concentration (Correct Answer)
- D. Ventriculomegaly on CT scan of the brain
- E. Increased transferrin saturation
Diagnostic approaches to GSDs Explanation: ***Low serum ceruloplasmin concentration***
- This patient presents with a constellation of symptoms including neurological (difficulty walking, clumsy hands, slurred speech, unsteady gait), hepatic (father died of esophageal varices at an early age, hepatosplenomegaly, scleral icterus), and psychiatric (sadness, slurred and monotonous speech) manifestations, all suggestive of **Wilson's disease**.
- **Wilson's disease** is an autosomal recessive disorder of copper metabolism leading to copper accumulation in various organs, predominantly the **liver**, **brain**, and **cornea**. **Low serum ceruloplasmin** is a hallmark biochemical finding, as ceruloplasmin is a copper-carrying protein, and its synthesis or copper incorporation is defective.
*Increased number of CAG repeats*
- An increased number of **CAG trinucleotide repeats** is characteristic of **Huntington's disease**.
- While Huntington's disease causes neurological and psychiatric symptoms, it does not typically involve significant **liver disease** or scleral icterus, which are prominent features in this patient's presentation.
*Oligoclonal bands on CSF analysis*
- **Oligoclonal bands** in the **cerebrospinal fluid (CSF)** are a key diagnostic finding for **multiple sclerosis**.
- Multiple sclerosis presents with neurological deficits, but it does not account for the prominent liver involvement (scleral icterus, hepatosplenomegaly, family history of varices) seen in this case.
*Ventriculomegaly on CT scan of the brain*
- **Ventriculomegaly** on a CT scan of the brain can be seen in various conditions, including **hydrocephalus** or **cerebral atrophy**.
- While some neurological degenerative diseases can lead to cerebral atrophy and resulting ventriculomegaly, it's a non-specific finding and doesn't explain the patient's severe **hepatic involvement** with scleral icterus and hepatosplenomegaly.
*Increased transferrin saturation*
- **Increased transferrin saturation** is a laboratory finding indicative of **hemochromatosis**, a disorder of iron metabolism leading to iron overload.
- While hemochromatosis can cause liver disease and neurological symptoms, the combination of **scleral icterus**, the specific neurological presentation, and the strong family history of early liver disease in this context points much more strongly towards **Wilson's disease** rather than hemochromatosis.
Diagnostic approaches to GSDs US Medical PG Question 10: A 40-year-old G1P0010 presents to the clinic with nausea and vomiting 8 weeks after a spontaneous abortion at 10 weeks gestation. She admits to heavy drinking (7–8 glasses of wine per day) for the last 20 years; however, after the pregnancy loss, she increased her drinking to 8–9 glasses per day. Hepatomegaly, right upper quadrant pain, and jaundice are noted on abdominal examination. The lungs are clear to auscultation with no abnormalities on chest X-ray. Liver function tests are obtained and a biopsy is performed. Which of the following findings is most likely to be true in her condition?
- A. ↑ NADH/NAD+; ALT:AST ≥ 2:1; β-oxidation ↓; β-hydroxybutyrate ↓; lactic acid ↓
- B. ↑ NAD+/NADH; AST:ALT ≥ 2:1; β-oxidation ↑; β-hydroxybutyrate ↓; lactic acid ↓
- C. ↑ NADH/NAD+; AST:ALT ≥ 2:1; β-oxidation ↓; β-hydroxybutyrate ↑; lactic acid ↑ (Correct Answer)
- D. ↑ NADH/NAD+; ALT:AST ≥ 2:1; β-oxidation ↓; β-hydroxybutyrate ↓; lactic acid ↑
- E. ↑ NAD+/NADH; ALT:AST ≥ 2:1; β-oxidation ↑; β-hydroxybutyrate, no change; lactic acid ↓
Diagnostic approaches to GSDs Explanation: ***↑ NADH/NAD+; AST:ALT ≥ 2:1; β-oxidation ↓; β-hydroxybutyrate ↑; lactic acid ↑***
- **Alcohol metabolism** increases the **NADH/NAD+ ratio**, diverting substrates to lipid synthesis (leading to fatty liver) and inhibiting **β-oxidation**.
- The elevated NADH also promotes **lactic acid** and **β-hydroxybutyrate** formation, while the **AST:ALT ratio ≥ 2:1** is characteristic of **alcoholic liver disease**, often due to mitochondrial damage and pyridoxal phosphate deficiency.
*↑ NADH/NAD+; ALT:AST ≥ 2:1; β-oxidation ↓; β-hydroxybutyrate ↓; lactic acid ↑*
- While a high **NADH/NAD+ ratio** and **lactic acid ↑** are consistent with alcohol metabolism, the **ALT:AST ≥ 2:1** ratio is more commonly seen in **non-alcoholic liver diseases**, and **β-hydroxybutyrate ↓** is incorrect as it should be elevated.
- **β-hydroxybutyrate** is increased in alcoholic ketoacidosis due to altered redox state, not decreased.
*↑ NAD+/NADH; AST:ALT ≥ 2:1; β-oxidation ↑; β-hydroxybutyrate ↓; lactic acid ↓*
- This option incorrectly states an **↑ NAD+/NADH ratio**, when alcohol metabolism actually increases **NADH**.
- **β-oxidation** is inhibited, not increased, and both **β-hydroxybutyrate** and **lactic acid** would be elevated.
*↑ NADH/NAD+; ALT:AST ≥ 2:1; β-oxidation ↓; β-hydroxybutyrate ↓; lactic acid ↓*
- While **↑ NADH/NAD+** and **β-oxidation ↓** are correct, the **ALT:AST ≥ 2:1** ratio is atypical for alcoholic liver disease where AST is usually higher.
- Both **β-hydroxybutyrate** and **lactic acid** should be elevated due to the increased NADH, not decreased.
*↑ NAD+/NADH; ALT:AST ≥ 2:1; β-oxidation ↑; β-hydroxybutyrate, no change; lactic acid ↓*
- This option is incorrect as **alcohol metabolism** increases **NADH**, not NAD+, and inhibits **β-oxidation**.
- **β-hydroxybutyrate** and **lactic acid** are typically elevated, not unchanged or decreased.
More Diagnostic approaches to GSDs US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.