Glycogen storage diseases

On this page
🧬 The Metabolic Maze: Decoding Glycogen Storage Diseases
Glycogen storage diseases transform a seemingly simple metabolic pathway into a diagnostic puzzle with profound multi-system consequences. You'll master how specific enzyme defects create distinct clinical signatures-from the massive hepatomegaly of Von Gierke disease to the exercise intolerance of McArdle disease-then learn to recognize laboratory patterns that point toward each subtype. By integrating biochemical logic with clinical presentation, you'll build systematic approaches to diagnosis and evidence-based management that prevent life-threatening complications while optimizing long-term outcomes across hepatic, cardiac, and skeletal muscle manifestations.
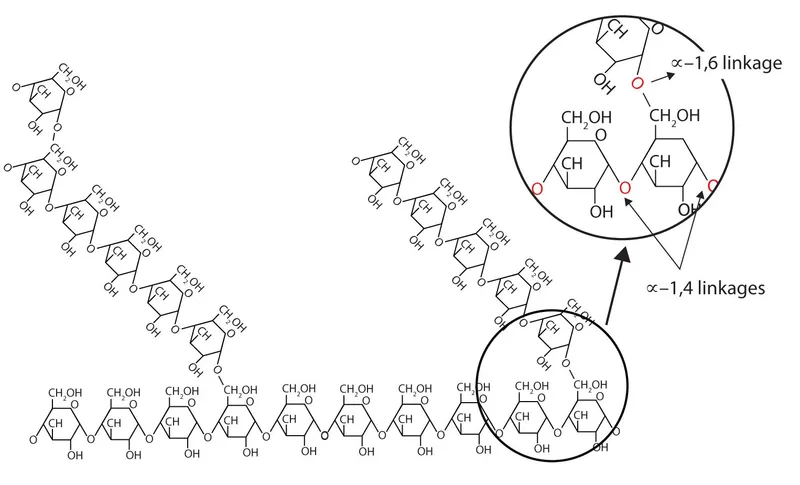
📌 Remember: GLYCOGEN - Glucose Linked Yielding Cellular Optimal Glucose Energy Needs. Glycogen serves as the body's immediate glucose reserve, storing 300-600g in liver and muscle with 4 kcal/g energy density.
The Enzymatic Orchestra: Glycogen Metabolism Fundamentals
Glycogen metabolism operates through two primary pathways with opposing functions:
-
Glycogenesis (Storage Pathway)
- Glucose → Glucose-6-phosphate via hexokinase
- G6P → Glucose-1-phosphate via phosphoglucomutase
- G1P → UDP-glucose via UDP-glucose pyrophosphorylase
- UDP-glucose incorporation via glycogen synthase
- Rate-limiting enzyme: Glycogen synthase
- Regulation: Insulin ↑, glucagon/epinephrine ↓
- Branch points: Every 8-12 glucose residues via branching enzyme
-
Glycogenolysis (Breakdown Pathway)
- Glycogen → Glucose-1-phosphate via glycogen phosphorylase
- G1P → Glucose-6-phosphate via phosphoglucomutase
- G6P → Glucose via glucose-6-phosphatase (liver only)
- Muscle limitation: No G6Pase = glucose trapped as G6P
- Liver advantage: G6Pase enables glucose release to circulation
| Enzyme | Location | Function | GSD Type | Clinical Pattern |
|---|---|---|---|---|
| Glucose-6-phosphatase | Liver, kidney | G6P → Glucose | Type I | Severe hypoglycemia, hepatomegaly |
| Acid α-glucosidase | Lysosomes | Glycogen breakdown | Type II | Cardiomyopathy, muscle weakness |
| Debranching enzyme | Liver, muscle | Branch point cleavage | Type III | Hepatomegaly + myopathy |
| Branching enzyme | Liver, muscle | Branch formation | Type IV | Cirrhosis, early death |
| Muscle phosphorylase | Skeletal muscle | Muscle glycogenolysis | Type V | Exercise intolerance |
💡 Master This: Glycogen contains 6-10% water by weight and provides immediate glucose within seconds, while gluconeogenesis requires minutes to hours. This explains why GSD patients develop rapid-onset hypoglycemia during fasting states.
Understanding glycogen's branched structure with α-1,4 glycosidic bonds (linear) and α-1,6 bonds (branch points every 8-12 residues) predicts which enzyme defects cause specific clinical patterns. Connect this foundation through enzymatic precision to understand how single molecular defects create predictable organ-specific manifestations.
🧬 The Metabolic Maze: Decoding Glycogen Storage Diseases
⚡ The Clinical Spectrum: GSD Classification Mastery
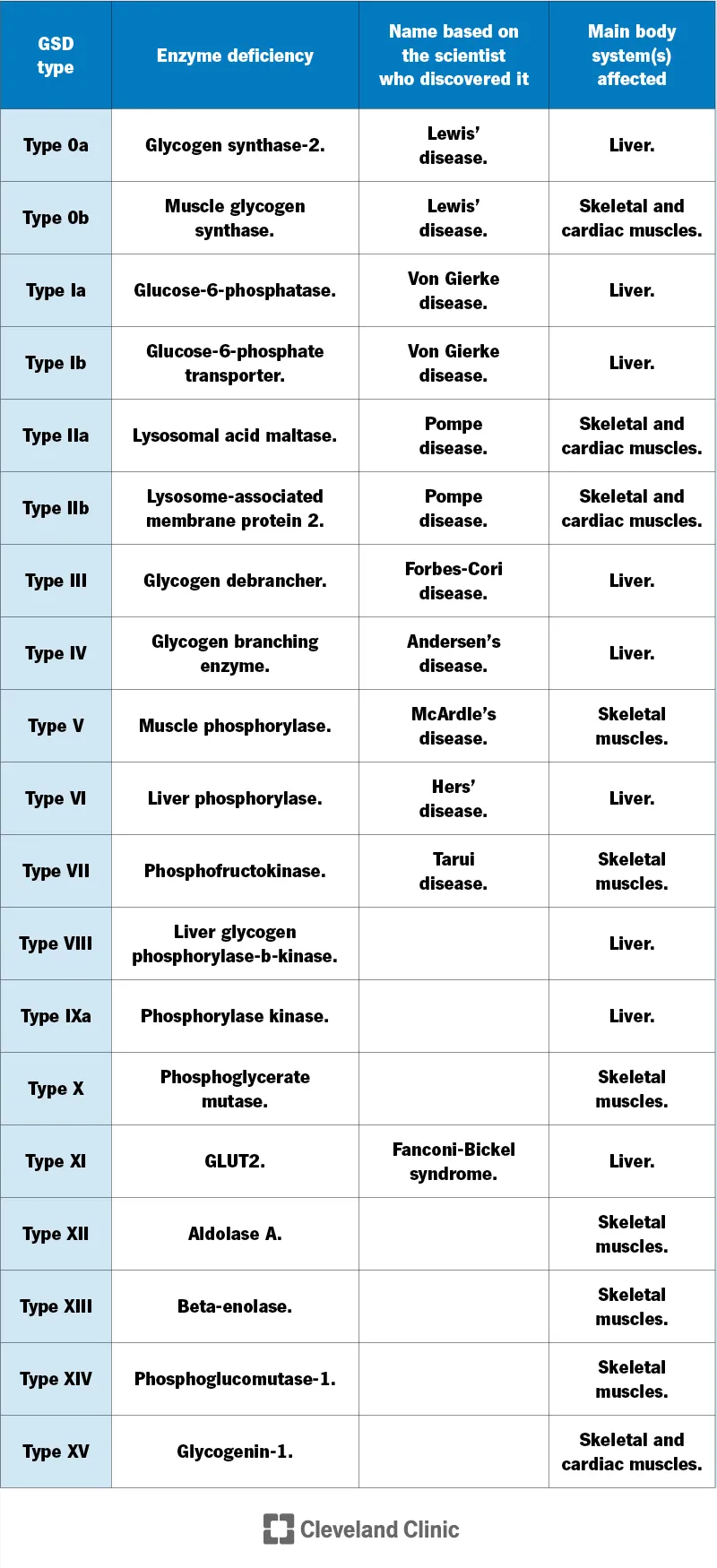
📌 Remember: LIVER FIRST - Liver GSDs (Types I, III, IV, VI) cause Immediate Vascular Emergencies Requiring Feeding Interventions Rapidly Sustained To prevent hypoglycemic crises.
Hepatic vs Myopathic GSD Patterns
The anatomical distribution of enzyme defects creates two primary clinical patterns:
-
Hepatic GSDs (Types I, III, IV, VI, IX)
- Primary manifestation: Hepatomegaly with hypoglycemia
- Pathophysiology: Impaired hepatic glucose release
- Onset timing: Early infancy (3-6 months)
- Fasting tolerance: <4 hours before hypoglycemia
- Type I: 2-3 hours (most severe)
- Type III: 4-6 hours (moderate)
- Type VI: 6-8 hours (mildest)
-
Myopathic GSDs (Types II, V, VII)
- Primary manifestation: Exercise intolerance, muscle weakness
- Pathophysiology: Impaired muscle energy metabolism
- Onset timing: Variable (infancy to adulthood)
- Exercise response: Abnormal lactate and CK elevation
- Type II: Progressive weakness from infancy
- Type V: Exercise cramping in young adults
- Type VII: Exercise intolerance with hemolysis
| GSD Type | Enzyme Defect | Primary Organs | Key Clinical Features | Diagnostic Marker |
|---|---|---|---|---|
| Type I | Glucose-6-phosphatase | Liver, kidney | Severe hypoglycemia, nephromegaly | Lactate >4 mmol/L |
| Type II | Acid α-glucosidase | Heart, muscle | Cardiomegaly, hypotonia | CK >500 U/L |
| Type III | Debranching enzyme | Liver, muscle | Hepatomegaly + myopathy | Normal lactate |
| Type IV | Branching enzyme | Liver, muscle | Cirrhosis, failure to thrive | Abnormal glycogen structure |
| Type V | Muscle phosphorylase | Skeletal muscle | Exercise intolerance only | Flat lactate curve |
💡 Master This: Hepatic GSDs present with fasting hypoglycemia and hepatomegaly by 6 months of age, while myopathic GSDs present with exercise intolerance and normal glucose but elevated CK (>300 U/L baseline, >1000 U/L post-exercise).
The tissue-specific expression of glycogen enzymes explains why Type I affects liver and kidney (G6Pase expression), Type V affects only skeletal muscle (muscle-specific phosphorylase), and Type II affects heart and muscle (lysosomal enzyme distribution). Connect this classification mastery through diagnostic precision to understand how laboratory patterns confirm specific enzyme defects.
⚡ The Clinical Spectrum: GSD Classification Mastery
🔬 Diagnostic Precision: Laboratory Pattern Recognition
📌 Remember: GLUCOSE LACTATE CK - Glucose Low Under Conditions Of Stress Especially; Lactate Always Checked To Assess Type Enzyme; CK Key for muscle involvement detection.
Fasting Laboratory Patterns
Fasting studies reveal enzyme-specific metabolic signatures:
-
Type I (von Gierke) Pattern
- Glucose: <50 mg/dL after 2-3 hours fasting
- Lactate: >4 mmol/L (normal <2 mmol/L)
- Uric acid: >8 mg/dL (normal <6 mg/dL)
- Triglycerides: >500 mg/dL (normal <150 mg/dL)
- Mechanism: G6P accumulation → lactate + lipogenesis
- Ketones: Absent (impaired gluconeogenesis)
-
Type III (Cori) Pattern
- Glucose: <60 mg/dL after 4-6 hours fasting
- Lactate: Normal (<2 mmol/L)
- CK: Elevated (200-800 U/L, normal <200 U/L)
- ALT/AST: Mildly elevated (2-3x normal)
- Mechanism: Partial glycogenolysis possible
- Ketones: Present (normal gluconeogenesis)
| GSD Type | Fasting Glucose | Lactate | CK | Uric Acid | Key Distinguisher |
|---|---|---|---|---|---|
| Type I | <50 mg/dL (2-3h) | >4 mmol/L | Normal | >8 mg/dL | Severe lactic acidosis |
| Type III | <60 mg/dL (4-6h) | Normal | 200-800 U/L | Normal | CK elevation + normal lactate |
| Type V | Normal | Flat curve* | >1000 U/L* | Normal | Exercise-induced only |
| Type VI | <70 mg/dL (6-8h) | Normal | Normal | Normal | Mild, late-onset |
⭐ Clinical Pearl: Glucagon stimulation test shows no glucose rise but marked lactate increase in Type I GSD, while Type III shows partial glucose response and no lactate elevation. This single test differentiates the two most common hepatic GSDs.
Exercise Testing Protocols
Exercise testing unmasks myopathic GSDs through metabolic stress responses:
-
Standard Protocol
- Baseline: CK, lactate, glucose measurement
- Exercise: 5 minutes forearm exercise at 70% maximum
- Post-exercise: Serial measurements at 1, 3, 5, 10 minutes
- Normal response: 3-5x lactate increase, CK <300 U/L
-
Type V (McArdle) Response
- Lactate: Flat curve (no increase from baseline)
- CK: >1000 U/L within 24 hours
- Glucose: Normal throughout
- Clinical: Muscle cramping within 2-3 minutes
💡 Master This: Type I GSDs cannot fast >3 hours without severe hypoglycemia (<40 mg/dL) and lactic acidosis (pH <7.3), while Type V GSDs have normal glucose but develop muscle necrosis (CK >5000 U/L) with intense exercise.
Confirmatory testing requires enzyme analysis in appropriate tissues: liver biopsy for hepatic GSDs, muscle biopsy for myopathic GSDs, and skin fibroblasts for Type II. Genetic testing increasingly replaces tissue diagnosis, with >95% detection rates for common mutations. Connect this diagnostic precision through treatment algorithms to understand how laboratory patterns guide therapeutic interventions.
🔬 Diagnostic Precision: Laboratory Pattern Recognition
⚖️ Treatment Algorithms: Evidence-Based Management Strategies
📌 Remember: CORNSTARCH SAVES - Continuous Oral Raw Natural Starch Taken According Regimen Controls Hypoglycemia; Supplementation And Vitamins Essential Support. Raw cornstarch provides slow glucose release over 4-6 hours.
Dietary Management Protocols
Nutritional therapy forms the cornerstone of GSD management with type-specific approaches:
-
Type I (von Gierke) Protocol
- Cornstarch dosing: 1.6-2.5 g/kg every 4 hours (including overnight)
- Protein target: 10-15% total calories (normal kidney function)
- Carbohydrate: 65-70% total calories as complex carbohydrates
- Fructose/sucrose: Strictly avoided (worsens lactic acidosis)
- Monitoring: Glucose >70 mg/dL, lactate <4 mmol/L
- Growth: Normal linear growth achieved in >90% patients
-
Type III (Cori) Protocol
- High-protein diet: 25-30% total calories as protein
- Cornstarch: 1.0-1.5 g/kg every 6 hours
- Exercise: Moderate aerobic exercise encouraged
- Liver monitoring: Annual ultrasound for fibrosis assessment
- Outcome: Hepatomegaly resolution in 70% by adolescence
| GSD Type | Cornstarch Dose | Frequency | Protein % | Key Restrictions | Success Metric |
|---|---|---|---|---|---|
| Type I | 1.6-2.5 g/kg | Every 4h | 10-15% | No fructose/sucrose | Glucose >70 mg/dL |
| Type III | 1.0-1.5 g/kg | Every 6h | 25-30% | None specific | Normal growth |
| Type V | Not indicated | N/A | 20-25% | Avoid intense exercise | CK <500 U/L |
| Type VI | 0.5-1.0 g/kg | Every 8h | 15-20% | None specific | Normal development |
Pharmacological Interventions
Medical therapy addresses specific complications and metabolic abnormalities:
-
Type I Complications Management
- Allopurinol: 100-300 mg daily for hyperuricemia (target <6 mg/dL)
- ACE inhibitors: For proteinuria (>300 mg/day)
- Statins: For triglycerides >500 mg/dL
- Citrate supplementation: 2-3 mEq/kg/day for nephrolithiasis prevention
- Renal monitoring: Annual creatinine, proteinuria assessment
- Success rate: >85% maintain normal renal function with treatment
-
Type II Enzyme Replacement Therapy
- Alglucosidase alfa: 20 mg/kg IV every 2 weeks
- Avalglucosidase alfa: 20 mg/kg IV every 2 weeks (newer formulation)
- Monitoring: 6-minute walk test, pulmonary function, CK levels
- Outcomes: Improved survival (>90% at 5 years vs <10% untreated)
💡 Master This: Type I GSD patients require lifelong cornstarch every 4 hours including overnight feeds to prevent hypoglycemic seizures, while Type II patients show measurable cardiac improvement within 6 months of enzyme replacement therapy with ejection fraction increases of 10-15%.
Surgical interventions include liver transplantation for Type IV GSD (100% mortality without transplant) and gastrostomy tube placement for severe Type I cases requiring continuous overnight feeding. Gene therapy trials show promising results for Type I with >50% reduction in cornstarch requirements. Connect this treatment mastery through multi-system integration to understand how therapeutic interventions prevent long-term complications across organ systems.
⚖️ Treatment Algorithms: Evidence-Based Management Strategies
🔗 Multi-System Integration: The Metabolic Network
📌 Remember: ORGANS TALK - Organ Responses Generate Adaptive Networks Systematically; Tissue Alterations Lead Kinetic changes across metabolic pathways. Each organ's glycogen needs affect whole-body glucose homeostasis.
Hepato-Renal-Cardiac Integration
Type I GSD demonstrates complex multi-organ interactions:
-
Hepatic Consequences
- Massive hepatomegaly: 5-8x normal size by age 2
- Hepatic adenomas: >75% develop by adulthood
- Malignant transformation: 10-15% of adenomas become hepatocellular carcinoma
- Portal hypertension: Rare but life-threatening when present
-
Renal Manifestations
- Nephromegaly: 2-3x normal kidney size
- Glomerular hyperfiltration: GFR >150 mL/min/1.73m²
- Progressive proteinuria: >300 mg/day by adolescence
- Chronic kidney disease: 30-40% develop CKD stage 3+ by age 30
- Mechanism: Glucose-6-phosphate accumulation → renal tubular dysfunction
-
Cardiovascular Adaptations
- Increased cardiac output: 20-30% above normal
- Pulmonary hypertension: Secondary to hepatomegaly
- Systemic hypertension: Related to renal dysfunction
- Cardiomyopathy: Rare but reported in severe cases
| Organ System | Primary Effect | Timeline | Monitoring Frequency | Intervention Threshold |
|---|---|---|---|---|
| Liver | Hepatomegaly, adenomas | 6 months - 20 years | Every 6 months (MRI) | Adenoma >5 cm |
| Kidney | Nephromegaly, proteinuria | 5-15 years | Every 6 months | Proteinuria >300 mg/day |
| Heart | Increased output | Immediate | Annually (echo) | EF <55% |
| Growth | Short stature | 2-10 years | Every 3 months | <5th percentile |
Muscle-Cardiac-Respiratory Integration
Type II GSD (Pompe disease) shows progressive multi-system involvement:
-
Cardiac Manifestations
- Hypertrophic cardiomyopathy: 100% of infantile cases
- Left ventricular outflow obstruction: >50% develop
- Conduction abnormalities: Short PR interval (<120 ms)
- Heart failure: Primary cause of death in untreated infants
-
Respiratory Consequences
- Diaphragmatic weakness: Progressive respiratory failure
- Sleep apnea: >80% of late-onset cases
- Ventilator dependence: Inevitable without treatment
- Aspiration risk: Bulbar muscle weakness
-
Skeletal Muscle Effects
- Proximal weakness: Legs before arms
- CK elevation: 5-20x normal (500-4000 U/L)
- Exercise intolerance: Progressive limitation
- Wheelchair dependence: Average age 40 (untreated late-onset)
💡 Master This: Type II GSD creates a "glycogen traffic jam" in lysosomes, causing cellular dysfunction across all muscle types. Enzyme replacement therapy reverses cardiac hypertrophy in >90% of patients within 12 months but has limited effect on established skeletal muscle damage.
Cutting-edge research reveals autophagy dysfunction as a secondary mechanism in Type II GSD, leading to clinical trials combining enzyme replacement with autophagy enhancers. Substrate reduction therapy using pharmacological chaperones shows promise for enhancing enzyme stability and improving outcomes. Connect this multi-system understanding through rapid mastery tools to develop comprehensive clinical assessment and management frameworks.
🔗 Multi-System Integration: The Metabolic Network
🎯 Clinical Mastery Arsenal: Rapid Assessment Tools
📌 Remember: RAPID GSD - Recognize Age Pattern Identify Distribution; Glucose Status Determines urgency. Age + organ pattern + glucose level = immediate GSD type suspicion.
The 3-Minute GSD Assessment Protocol
Systematic approach for emergency and outpatient settings:
-
Step 1: Age-Pattern Recognition (30 seconds)
- <6 months + hepatomegaly = Type I or III (urgent glucose check)
- <2 years + cardiomegaly = Type II (urgent cardiac assessment)
- Young adult + exercise intolerance = Type V or VII (CK check)
- Any age + failure to thrive = Consider any hepatic GSD
-
Step 2: Organ Distribution (60 seconds)
- Liver only: Types I, VI, IX (check fasting tolerance)
- Liver + muscle: Types III, IV (check CK + glucose)
- Muscle only: Types II, V, VII (check exercise history)
- Heart prominent: Type II (urgent echocardiogram)
-
Step 3: Metabolic Status (90 seconds)
- Current glucose: <70 mg/dL = immediate intervention
- Fasting tolerance: <4 hours = severe GSD (likely Type I)
- Exercise response: Cramping + normal glucose = myopathic GSD
- Growth pattern: Poor growth = inadequate management
| Clinical Scenario | Age | Key Features | Immediate Action | Likely GSD |
|---|---|---|---|---|
| Hypoglycemic seizure | 3 months | Hepatomegaly, lactic acidosis | IV glucose, frequent feeds | Type I |
| Heart failure | 6 months | Cardiomegaly, hypotonia | Echo, ERT evaluation | Type II |
| Exercise cramping | 20 years | Normal glucose, high CK | Exercise restriction | Type V |
| Chronic hepatomegaly | 5 years | Normal glucose, mild CK elevation | Liver MRI, genetic testing | Type III |
Essential Clinical Arsenal
High-yield numbers for immediate clinical decision-making:
-
Critical Glucose Thresholds
- <40 mg/dL: Immediate IV glucose required
- <50 mg/dL: High suspicion for Type I GSD
- <60 mg/dL after 4h fast: Possible Type III
- <70 mg/dL after 6h fast: Consider Type VI
-
CK Interpretation
- >1000 U/L post-exercise: Type V or VII
- 200-800 U/L baseline: Type III (liver + muscle)
- >5000 U/L: Muscle necrosis risk
- Normal: Excludes myopathic GSDs
-
Lactate Patterns
- >4 mmol/L fasting: Type I (pathognomonic)
- Normal fasting: Type III, V, VI, VII
- Flat exercise curve: Type V (McArdle)
- >8 mmol/L post-exercise: Normal response
💡 Master This: GSD emergency management follows ABC priorities: Always check glucose first, Be prepared for IV access, Continuous monitoring until stable. Type I patients can develop hypoglycemic coma within 2-3 hours of missed feeding, requiring immediate glucose and long-term cornstarch protocol.
Advanced integration includes genetic counseling for family planning (25% recurrence risk for autosomal recessive GSDs), transition planning for adult care (specialized metabolic clinics), and emergency action plans for school and travel. These rapid assessment tools enable life-saving interventions while comprehensive evaluation proceeds systematically.
🎯 Clinical Mastery Arsenal: Rapid Assessment Tools
Practice Questions: Glycogen storage diseases
Test your understanding with these related questions
A 15-year-old boy is sent from gym class with a chief complaint of severe muscle aches. In class today he was competing with his friends and therefore engaged in weightlifting for the first time. A few hours later he was extremely sore and found that his urine was red when he went to urinate. This concerned him and he was sent to the emergency department for evaluation. Upon further questioning, you learn that since childhood he has always had muscle cramps with exercise. Physical exam was unremarkable. Upon testing, his creatine kinase level was elevated and his urinalysis was negative for blood and positive for myoglobin. Thinking back to biochemistry you suspect that he may be suffering from a hereditary glycogen disorder. Given this suspicion, what would you expect to find upon examination of his cells?












