Nucleotide excision repair US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Nucleotide excision repair. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Nucleotide excision repair US Medical PG Question 1: A 7-month-old boy presents to the family physician with extensive scaliness and pigmentation of sun-exposed skin areas. His mother says that these symptoms were absent until mid-spring and then became significantly worse after their trip to California in the summer. The child was born in December to a consanguineous couple after an uncomplicated pregnancy. He is breastfed and receives mashed potatoes, bananas, and carrots as complementary foods. His weight is 8.5 kg (18.7 lb) and length is 70 cm (2 ft 96 in). The patient’s vital signs are within normal limits for his age. On physical examination, there is freckling, scaling, and erythema on the sunlight-exposed areas of the face, trunk, and upper and lower extremities. No blistering, scarring, hypertrichosis, or alopecia is noted. The rest of the exam is unremarkable. Which process is most likely disrupted in this patient?
- A. Nucleotide-excision DNA repair (Correct Answer)
- B. Base-excision DNA repair
- C. Hydroxylation of proline and lysine in the procollagen molecule
- D. Conversion of uroporphyrinogen III to coproporphyrinogen III
- E. NAD production
Nucleotide excision repair Explanation: ***Nucleotide-excision DNA repair***
- The patient's symptoms (extensive scaliness, pigmentation, freckling, scaling, and erythema on sun-exposed areas) that worsen with sun exposure are characteristic of **xeroderma pigmentosum**.
- This condition is caused by a defect in **nucleotide-excision repair (NER)**, which is essential for repairing DNA damage, particularly from UV radiation.
*Base-excision DNA repair*
- **Base-excision repair (BER)** primarily addresses single-base damage, like oxidized or alkylated bases, not the bulky adducts formed by UV light.
- Defects in BER are associated with conditions like colorectal cancer, but not the specific photosensitivity seen here.
*Hydroxylation of proline and lysine in the procollagen molecule*
- This process is essential for proper collagen synthesis; defects lead to disorders like **Ehlers-Danlos syndrome** or **scurvy**.
- These conditions manifest with skin fragility, bruising, and joint hypermobility, not the prominent photosensitivity observed.
*Conversion of uroporphyrinogen III to coproporphyrinogen III*
- This step is involved in **heme synthesis**; defects can lead to **porphyrias**, which often cause photosensitivity and blistering.
- However, the patient's presentation of scaling, freckling, and erythema without blistering or scarring is less typical for porphyria.
*NAD production*
- **NAD (nicotinamide adenine dinucleotide)** is a crucial coenzyme in many metabolic pathways; its deficiency can lead to pellagra-like symptoms (dermatitis, diarrhea, dementia).
- While pellagra can involve sun-exposed skin, it typically involves a more diffuse, symmetrically inflamed rash with hyperpigmentation and thickening, rather than the discrete freckling and scaling described.
Nucleotide excision repair US Medical PG Question 2: A mother from rural Louisiana brings her 4-year-old son to a pediatrician. Her son is intellectually disabled, and she hopes that genetic testing will help determine the cause of her son's condition. She had previously been opposed to allowing physicians to treat her son, but his impulsive behavior and learning disabilities are making it difficult to manage his care on her own. On exam, the child has a long, thin face with a large jaw, protruding ears, and macroorchidism. The physician also hears a high-pitched holosystolic murmur at the apex of the heart that radiates to the axilla. Which of the following trinucleotide repeats is most likely affected in this individual?
- A. GAA on chromosome 9
- B. CGG on the sex chromosome X (Correct Answer)
- C. CTG on chromosome 19
- D. CTG on chromosome 8
- E. CAG on chromosome 4
Nucleotide excision repair Explanation: ***CGG on the sex chromosome X***
- The constellation of **intellectual disability**, a **long, thin face with a large jaw**, **protruding ears**, and **macroorchidism** are classic features of **Fragile X syndrome**.
- Fragile X syndrome is caused by an expansion of the **CGG trinucleotide repeat** in the **FMR1 gene** on the **X chromosome**. The **high-pitched holosystolic murmur at the apex radiating to the axilla** suggests **mitral valve prolapse**, which is also frequently associated with Fragile X.
*GAA on chromosome 9*
- This describes the **GAA trinucleotide repeat expansion** associated with **Friedreich's ataxia**, affecting the **FXN gene** on **chromosome 9**.
- Friedreich's ataxia is characterized by **progressive ataxia**, **dysarthria**, and **loss of vibratory/proprioceptive sensation**, not macroorchidism or the specific facial features seen here.
*CTG on chromosome 19*
- This describes the **CTG trinucleotide repeat expansion** associated with **myotonic dystrophy type 1**, affecting the **DMPK gene** on **chromosome 19**.
- Myotonic dystrophy is characterized by **myotonia** (delayed muscle relaxation), **muscle weakness**, and **cataracts**, which are not consistently present in this case.
*CTG on chromosome 8*
- While **CTG repeats** are involved in some genetic conditions, the specific association with **chromosome 8** as a cause for the described symptoms (intellectual disability, specific facial features, macroorchidism, and mitral valve prolapse) is not a common trinucleotide repeat disorder.
- This option does not correspond to a recognized trinucleotide repeat disorder that presents with the given clinical picture.
*CAG on chromosome 4*
- This describes the **CAG trinucleotide repeat expansion** associated with **Huntington's disease**, affecting the **HTT gene** on **chromosome 4**.
- Huntington's disease typically presents with **chorea**, **psychiatric symptoms**, and **dementia** later in life, not with the childhood onset intellectual disability and physical features described.
Nucleotide excision repair US Medical PG Question 3: While performing a Western blot, a graduate student spilled a small amount of the radiolabeled antibody on her left forearm. Although very little harm was done to the skin, the radiation did cause minor damage to the DNA of the exposed skin by severing covalent bonds between the nitrogenous bases and the deoxyribose sugar, leaving several apurinic/apyrimidinic sites. Damaged cells would most likely repair these sites by which of the following mechanisms?
- A. Nucleotide excision repair
- B. Nonhomologous end joining repair
- C. Homologous recombination
- D. Mismatch repair
- E. Base excision repair (Correct Answer)
Nucleotide excision repair Explanation: **Base excision repair**
- This mechanism is specifically involved in correcting **single-base DNA damage** or **modified bases**, such as **apurinic/apyrimidinic (AP) sites**.
- It involves removing the damaged base by a **DNA glycosylase**, creating an AP site, which is then processed by an **AP endonuclease** to cleave the phosphodiester backbone, followed by DNA polymerase and ligase.
*Nucleotide excision repair*
- Primarily repairs **bulky DNA lesions**, such as **thymine dimers** caused by UV radiation, or damage from chemical adducts that distort the DNA helix.
- It involves excising a larger oligonucleotide containing the damage, not just a single base.
*Nonhomologous end joining repair*
- This pathway is used to repair **double-strand DNA breaks**, where both strands of the DNA molecule are broken.
- It is a "quick-and-dirty" repair mechanism that ligates the broken ends together, often leading to small insertions or deletions.
*Homologous recombination*
- A repair mechanism for **double-strand DNA breaks** that uses a homologous DNA template (e.g., sister chromatid) to accurately repair the break.
- This process is highly accurate but occurs only when a homologous template is available, typically during the S and G2 phases of the cell cycle.
*Mismatch repair*
- Corrects **base-pair mismatches** and **small insertions/deletions** that occur during DNA replication, which were not corrected by DNA polymerase proofreading.
- It targets newly synthesized DNA strands based on methylation patterns in the parental strand.
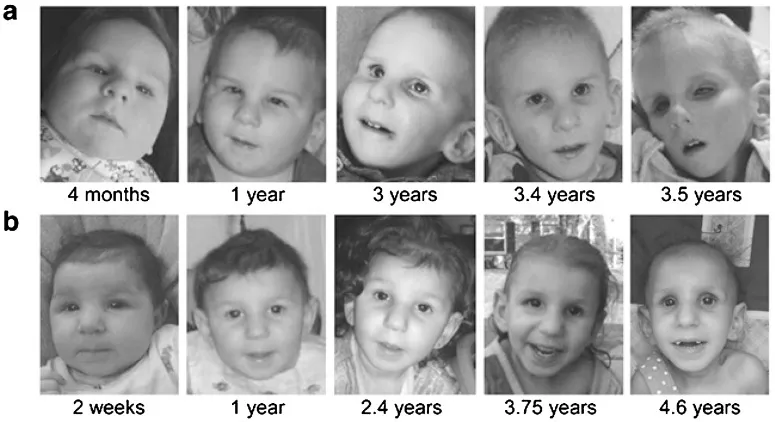
Nucleotide excision repair US Medical PG Question 4: A 10-year-old boy is brought into your clinic by his mother for sunburns that have not been healing. The mother states that he easily gets sunburned. The mother admits she gave birth to him at home and has never taken him to see a doctor. The patient walks with a wide stance gait and appears unstable on his feet. He has an extensive erythematous, scaling, hyperkeratotic rash on his face, neck, arms and legs. After extensive workup, the patient is found to have a genetic disorder that results in defective absorption of an important vitamin. Which of the following is likely to be low if measured?
- A. Vitamin K
- B. Niacin (Correct Answer)
- C. Folate
- D. Vitamin A
- E. Vitamin B12
Nucleotide excision repair Explanation: ***Niacin***
- The constellation of **sunburns that don't heal**, a **wide-stanced unstable gait**, and an **erythematous, scaling, hyperkeratotic rash** (consistent with dermatitis) strongly suggests **pellagra**.
- Pellagra is caused by a deficiency of **niacin (Vitamin B3)**, which is characterized by the "3 Ds": **dermatitis**, **diarrhea**, and **dementia (or neurological symptoms like ataxia)**.
*Vitamin K*
- Deficiency typically leads to **bleeding disorders** due to impaired coagulation, which is not indicated by the patient's symptoms.
- While newborns often receive a **vitamin K shot**, his current symptoms are unrelated to its deficiency.
*Folate*
- Folate deficiency primarily causes **megaloblastic anemia** and can lead to **neural tube defects** in developing fetuses.
- It does not explain the characteristic dermatological and neurological symptoms described.
*Vitamin A*
- Vitamin A deficiency is known to cause **night blindness** and **xerophthalmia** (dry eyes), and impaired immune function.
- While it plays a role in skin health, the specific rash and gait abnormalities point away from primary vitamin A deficiency.
*Vitamin B12*
- Deficiency leads to **megaloblastic anemia** with **neurological symptoms** such as peripheral neuropathy, but the dermatological manifestations (scaling, hyperkeratotic rash) and unhealing sunburn are not typical.
- The gait could be linked to neurological symptoms, but the overall presentation is better explained by niacin deficiency.
Nucleotide excision repair US Medical PG Question 5: A 54-year-old woman with breast cancer comes to the physician because of redness and pain in the right breast. She has been undergoing ionizing radiation therapy daily for the past 2 weeks as adjuvant treatment for her breast cancer. Physical examination shows erythema, edema, and superficial desquamation of the skin along the right breast at the site of radiation. Sensation to light touch is intact. Which of the following is the primary mechanism of DNA repair responsible for preventing radiation-induced damage to neighboring neurons?
- A. Homology-directed repair
- B. Base excision repair
- C. Nonhomologous end joining repair (Correct Answer)
- D. DNA mismatch repair
- E. Nucleotide excision repair
Nucleotide excision repair Explanation: ***Nonhomologous end joining repair***
- This pathway is crucial for repairing **double-strand DNA breaks**, which are a major form of damage caused by **ionizing radiation**.
- It directly ligates the broken DNA ends without requiring a homologous template, making it an efficient but potentially error-prone repair mechanism.
*Homology-directed repair*
- This pathway is also used to repair **double-strand DNA breaks** but requires a **homologous DNA template** (usually a sister chromatid) for accurate repair.
- While highly accurate, it is typically active during the S and G2 phases of the cell cycle and is generally slower and less dominant than NHEJ for immediate radiation-induced damage in non-dividing cells like neurons.
*Base excision repair*
- This mechanism primarily corrects damage to individual DNA bases, such as **oxidative damage**, alkylation, or deamination.
- It is not the primary mechanism for repairing the **double-strand breaks** induced by ionizing radiation.
*DNA mismatch repair*
- This pathway corrects errors that arise during **DNA replication**, specifically mismatched base pairs or small insertions/deletions.
- It is not involved in repairing radiation-induced DNA damage like **double-strand breaks**.
*Nucleotide excision repair*
- This pathway repairs bulky DNA lesions, such as those caused by **UV radiation** (e.g., pyrimidine dimers) or chemical mutagens.
- It removes a segment of DNA containing the damage but is not the primary repair mechanism for **double-strand breaks** caused by ionizing radiation.
Nucleotide excision repair US Medical PG Question 6: A 5-year-old girl is brought to the physician by her mother because of a 1-month history of a painful ulcer on her face. She has developed painful sunburns in the past with minimal UV exposure. Examination of the skin shows a 2-cm ulcerated nodule on the left cheek. There are scaly, hyperpigmented papules and plaques over the skin of the entire body. Ophthalmologic examination shows decreased visual acuity, clouded corneas, and limbal injection. Examination of a biopsy specimen from the facial lesion shows poorly-differentiated squamous cell carcinoma. Impairment of which of the following proteins is the most likely cause of this patient's condition?
- A. Rb nuclear protein
- B. Base-specific glycosylase
- C. Excision endonuclease (Correct Answer)
- D. ATM serine/threonine kinase
- E. DNA helicase
Nucleotide excision repair Explanation: ***Excision endonuclease***
- This patient's presentation with **painful sunburns**, **early-onset squamous cell carcinoma** on the face, and **ocular abnormalities (clouded corneas, decreased visual acuity)** is highly suggestive of **xeroderma pigmentosum (XP)**.
- XP is an autosomal recessive disorder caused by a defect in **nucleotide excision repair (NER)**, which is responsible for removing DNA damage primarily induced by **UV radiation**. **Excision endonucleases** are key enzymes in the initiation phase of NER, recognizing and excising the damaged DNA segment.
*Rb nuclear protein*
- The **Rb nuclear protein** is a tumor suppressor involved in cell cycle regulation (G1/S checkpoint).
- Impairment of Rb is associated with **retinoblastoma** and several other cancers, but not typically with this specific constellation of light sensitivity, skin cancer, and ocular damage seen in XP.
*Base-specific glycosylase*
- **Base-specific glycosylases** are involved in **base excision repair (BER)**, which primarily corrects small, non-helix-distorting base lesions (e.g., deaminated or alkylated bases).
- While important for DNA repair, defects in BER would not explain the extreme UV sensitivity and subsequent skin cancers characteristic of xeroderma pigmentosum, as these are primarily linked to UV-induced pyrimidine dimers.
*ATM serine/threonine kinase*
- **ATM (ataxia-telangiectasia mutated) kinase** is a critical protein involved in initiating the cellular response to **DNA double-strand breaks**.
- Defects in ATM cause **ataxia-telangiectasia**, characterized by cerebellar ataxia, immunodeficiency, and a predisposition to lymphoid malignancies, but not the specific skin and eye findings of XP.
*DNA helicase*
- **DNA helicases** are enzymes that unwind DNA and are involved in various DNA processes, including replication, recombination, and repair.
- While critical for many functions, a general defect in **DNA helicase** would lead to a broader range of severe developmental and cellular defects, and is not specifically linked to the clinical phenotype of xeroderma pigmentosum which results from specific NER pathway defects.
Nucleotide excision repair US Medical PG Question 7: A 5-month-old male infant from a consanguineous marriage presents with severe sunburns and freckling in sun exposed areas. The mother explains that the infant experiences these sunburns every time the infant goes outside despite applying copious amounts of sunscreen. Which of the following DNA repair mechanisms is defective in this child?
- A. Non-homologous end joining
- B. Homologous recombination
- C. Base excision repair
- D. Mismatch repair
- E. Nucleotide excision repair (Correct Answer)
Nucleotide excision repair Explanation: ***Nucleotide excision repair***
- The symptoms of **severe sunburns** and **freckling in sun-exposed areas** are classic manifestations of **Xeroderma Pigmentosum (XP)**.
- XP is caused by a defect in **nucleotide excision repair (NER)**, which is crucial for removing **UV-induced DNA damage**, such as **pyrimidine dimers**.
*Non-homologous end joining*
- This mechanism repairs **double-strand DNA breaks** by directly ligating the broken ends, often with some loss of genetic information.
- Defects in non-homologous end joining are associated with conditions like **immunodeficiency** and increased cancer risk, but not with UV sensitivity like XP.
*Homologous recombination*
- This high-fidelity repair pathway uses a **homologous DNA template** to accurately repair **double-strand breaks** and interstrand crosslinks.
- Impaired homologous recombination is linked to conditions like **Fanconi anemia** and increased risk of certain cancers, but not primarily to UV hypersensitivity.
*Base excision repair*
- **Base excision repair (BER)** is responsible for removing **damaged or modified bases** from DNA, such as oxidized or alkylated bases.
- Defects in BER can lead to increased spontaneous mutagenesis and cancer, but do not explain the specific sensitivity to UV light seen in this infant.
*Mismatch repair*
- **Mismatch repair (MMR)** corrects errors that occur during DNA replication, such as **base mismatches** or small insertions/deletions.
- Defective MMR is strongly associated with **hereditary nonpolyposis colorectal cancer (Lynch syndrome)**, but not with severe reactions to sun exposure.
Nucleotide excision repair US Medical PG Question 8: A 3-year-old male child is found to have a disease involving DNA repair. Specifically, he is found to have a defect in the endonucleases involved in the nucleotide excision repair of pyrimidine dimers. Which of the following is a unique late-stage complication of this child's disease?
- A. Telangiectasia
- B. Colorectal cancer
- C. Malignant melanoma (Correct Answer)
- D. Lymphomas
- E. Endometrial cancer
Nucleotide excision repair Explanation: **Malignant melanoma**
- The described condition is **xeroderma pigmentosum**, an autosomal recessive disorder characterized by a defect in **nucleotide excision repair (NER)**, specifically the inability to remove **pyrimidine dimers** caused by **UV radiation**.
- This severely impaired DNA repair leads to an extreme predisposition to **UV-induced skin cancers**, including basal cell carcinomas, squamous cell carcinomas, and, most aggressively, **malignant melanoma**, which is a unique and life-threatening late-stage complication.
*Telangiectasia*
- **Telangiectasias** are dilated small blood vessels that appear on the skin or mucous membranes and can be associated with various conditions.
- While skin abnormalities are prevalent in xeroderma pigmentosum due to sun damage, **melanoma** is a more specific and severe late-stage complication directly resulting from the DNA repair defect.
*Colorectal cancer*
- **Colorectal cancer** is typically associated with other DNA repair defects, such as those in the **mismatch repair system**, as seen in conditions like **Lynch syndrome**.
- It is not a primary or most significant late-stage complication of xeroderma pigmentosum, which is primarily characterized by skin cancers.
*Lymphomas*
- **Lymphomas** are cancers of the lymphatic system, often linked to immune deficiencies or specific genetic translocations.
- While individuals with genetic syndromes can have increased cancer risks, **lymphoma** is not the hallmark late-stage complication of xeroderma pigmentosum; skin cancers are the predominant concern.
*Endometrial cancer*
- **Endometrial cancer** is a gynecological cancer often associated with hormonal factors or genetic predispositions like Lynch syndrome, which involves mismatch repair defects.
- This type of cancer is not a characteristic or unique late-stage complication of xeroderma pigmentosum, whose pathology is centered on **UV-induced DNA damage** and subsequent skin malignancies.
Nucleotide excision repair US Medical PG Question 9: An investigator studying DNA mutation mechanisms isolates single-stranded DNA from a recombinant bacteriophage and sequences it. The investigator then mixes it with a buffer solution and incubates the resulting mixture at 70°C for 16 hours. Subsequent DNA resequencing shows that 3.7 per 1,000 cytosine residues have mutated to uracil. Which of the following best describes the role of the enzyme that is responsible for the initial step in repairing these types of mutations in living cells?
- A. Connecting the phosphodiester backbone
- B. Cleavage of the phosphodiester bond 3' of damaged site
- C. Creation of abasic site (Correct Answer)
- D. Release of the damaged nucleotide
- E. Addition of free nucleotides to 3' end
Nucleotide excision repair Explanation: ***Creation of abasic site***
- The mutation of **cytosine to uracil** is an example of **deamination**, which is repaired by the **base excision repair (BER)** pathway.
- The initial step in BER involves **DNA glycosylase**, which *removes* the damaged base (uracil) from the sugar-phosphate backbone by hydrolyzing the **N-glycosidic bond**, creating an **abasic site**.
*Connecting the phosphodiester backbone*
- This is the function of **DNA ligase**, which acts at the *final step* of DNA repair pathways to seal the nicks in the backbone.
- It does not initiate the repair process for deaminated bases.
*Cleavage of the phosphodiester bond 3' of damaged site*
- This is typically performed by an **AP endonuclease (APE1)** after the abasic site has been created.
- It is a *subsequent step* in BER, not the initial one for removing the damaged base itself.
*Release of the damaged nucleotide*
- While the damaged base is eventually *released*, the initial enzyme (DNA glycosylase) specifically removes the **base**, leaving the sugar and phosphate intact.
- The entire nucleotide (base, sugar, and phosphate) is typically removed later by an **AP lyase** or APE1, after the initial glycosylase action.
*Addition of free nucleotides to 3' end*
- This is the function of **DNA polymerase**, which fills in the gap after the damaged nucleotide and surrounding region have been excised.
- This occurs *after* the initial recognition and removal of the damaged base, not as the primary repair step.
Nucleotide excision repair US Medical PG Question 10: Replication in eukaryotic cells is a highly organized and accurate process. The process involves a number of enzymes such as primase, DNA polymerase, topoisomerase II, and DNA ligase. In which of the following directions is DNA newly synthesized?
- A. 3' --> 5'
- B. N terminus --> C terminus
- C. C terminus --> N terminus
- D. 3' --> 5' & 5' --> 3'
- E. 5' --> 3' (Correct Answer)
Nucleotide excision repair Explanation: ***5' --> 3'***
- DNA polymerase can only add **nucleotides** to the 3' end of a growing strand, meaning synthesis always proceeds in a **5' to 3' direction**.
- This is true for both the **leading strand** (synthesized continuously) and the **lagging strand** (synthesized discontinuously via Okazaki fragments).
*3' --> 5'*
- While the parental template strand is read in the 3' to 5' direction, the *newly synthesized* DNA strand is always built in the **opposite, antiparallel 5' to 3' direction**.
- DNA polymerase lacks the ability to add new nucleotides to the **5' phosphate group** of the growing strand.
*N terminus --> C terminus*
- This directional notation refers to the synthesis of **proteins**, where amino acids are added to the C (carboxyl) terminus of the growing polypeptide chain.
- It does not apply to the synthesis direction of **nucleic acids (DNA or RNA)**.
*C terminus --> N terminus*
- This directional notation is incorrectly applied; protein synthesis always proceeds from the **N (amino) terminus to the C (carboxyl) terminus**.
- This has no relevance to the synthesis direction of **DNA**.
*3' --> 5' & 5' --> 3'*
- Although DNA replication involves two strands, one is synthesized continuously in the **5' → 3' direction (leading strand)** and the other discontinuously, but still *each fragment* is synthesized in the **5' → 3' direction (lagging strand)**.
- No new DNA strand is synthesized in the **3' → 5' direction**.
More Nucleotide excision repair US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.