Mismatch repair US Medical PG Practice Questions and MCQs
Practice US Medical PG questions for Mismatch repair. These multiple choice questions (MCQs) cover important concepts and help you prepare for your exams.
Mismatch repair US Medical PG Question 1: A 42-year-old woman presents to her primary care physician with fatigue. She reports that over the past 2 months, she has felt increasingly tired despite no changes in her diet or exercise. Her past medical history is notable for obesity, seasonal allergies, and hypertension. She takes ranitidine as needed and hydrochlorothiazide daily. Her family history is notable for colorectal cancer in her mother and maternal uncle, endometrial cancer in her maternal aunt, and ovarian cancer in her maternal grandmother. Her temperature is 98.8°F (37.1°C), blood pressure is 132/71 mmHg, pulse is 89/min, and respirations are 17/min. On exam, she has conjunctival pallor. A stool sample is hemoccult positive. A colonoscopy reveals a fungating hemorrhagic mass in the ascending colon. Which of the following processes is likely impaired in this patient?
- A. Mismatch repair (Correct Answer)
- B. Non-homologous end joining
- C. Homologous recombination
- D. Base excision repair
- E. Nucleotide excision repair
Mismatch repair Explanation: ***Mismatch repair***
- The patient's presentation with **colorectal cancer** at a relatively young age (42), combined with a strong family history of various cancers (colorectal, endometrial, ovarian) suggesting a **hereditary cancer syndrome**, points towards a defect in **mismatch repair (MMR)**.
- Defective MMR leads to an accumulation of **mutations** during DNA replication, particularly in microsatellites, and is characteristic of **Lynch syndrome** (Hereditary Nonpolyposis Colorectal Cancer, HNPCC).
*Non-homologous end joining*
- This pathway is crucial for repairing **double-strand breaks** in DNA but is often error-prone.
- Defects in non-homologous end joining are associated with conditions like **severe combined immunodeficiency** due to impaired V(D)J recombination, not typically Lynch syndrome.
*Homologous recombination*
- Involved in high-fidelity repair of **double-strand breaks** using a homologous DNA template.
- Impairments are linked to increased risk of certain cancers, such as **BRCA1/2 mutations** causing breast and ovarian cancer, but are not the primary defect in Lynch syndrome.
*Base excision repair*
- This pathway is responsible for repairing **small, non-helix-distorting base lesions** caused by oxidation, alkylation, or deamination.
- Defects can lead to increased mutagenicity but are not the primary mechanism underlying the cancer spectrum seen in Lynch syndrome.
*Nucleotide excision repair*
- This system repairs **bulky DNA adducts** and helix-distorting lesions, such as those caused by UV radiation (e.g., **pyrimidine dimers**).
- Defects are associated with conditions like **xeroderma pigmentosum**, characterized by extreme sun sensitivity and skin cancers, which is not consistent with this patient's presentation.
Mismatch repair US Medical PG Question 2: A 47-year-old man presents to his primary care physician for fatigue. Over the past 3 months, his tiredness has impacted his ability to work as a corporate lawyer. He denies any changes to his diet, exercise regimen, bowel movements, or urinary frequency. His past medical history is notable for obesity, type II diabetes mellitus, and hypertension. He takes metformin and enalapril. His family history is notable for colorectal cancer in his father and paternal grandfather and endometrial cancer in his paternal aunt. He has a 20-pack-year smoking history and drinks one 6-pack of beer a week. His temperature is 98.8°F (37.1°C), blood pressure is 129/71 mmHg, pulse is 82/min, and respirations are 17/min. On exam, he has conjunctival pallor. A stool sample is positive for occult blood. A colonoscopy reveals a small hemorrhagic mass at the junction of the ascending and transverse colon. Which of the following processes is likely impaired in this patient?
- A. Mismatch repair (Correct Answer)
- B. Homologous recombination
- C. Non-homologous end joining
- D. Nucleotide excision repair
- E. Base excision repair
Mismatch repair Explanation: ***Mismatch repair***
- The patient's presentation with **colorectal cancer** at a relatively young age and a strong family history of various cancers (colorectal, endometrial) in **first-degree and second-degree relatives** suggests Lynch syndrome (Hereditary Nonpolyposis Colorectal Cancer).
- **Lynch syndrome** is caused by inherited mutations in genes responsible for **DNA mismatch repair**, leading to an accumulation of errors and increased cancer risk.
*Homologous recombination*
- This repair mechanism is crucial for fixing **double-strand DNA breaks** using a homologous DNA template, important for genetic stability and primarily associated with genes like BRCA1/2.
- While defects in homologous recombination can lead to cancer (e.g., **breast and ovarian cancers**), it is not the primary mechanism implicated in Lynch syndrome or the patient's specific presentation of colorectal and endometrial cancer families.
*Non-homologous end joining*
- This is another major pathway for repairing **double-strand DNA breaks**, but it does so by directly ligating the broken ends, often with some loss of genetic information, and does not rely on a homologous template.
- Defects in non-homologous end joining are not typically linked to the specific spectrum of cancers seen in **Lynch syndrome**.
*Nucleotide excision repair*
- This pathway is responsible for removing bulky DNA lesions, such as those caused by **UV light (e.g., pyrimidine dimers)** or certain chemical mutagens, and its defects are associated with conditions like xeroderma pigmentosum.
- The clinical picture and family history are not characteristic of disorders related to impaired **nucleotide excision repair**.
*Base excision repair*
- This repair pathway primarily corrects small, non-bulky DNA lesions, such as **oxidized, alkylated, or deaminated bases**, that do not distort the DNA helix.
- While important for maintaining genomic integrity, defects in base excision repair are typically associated with different cancer susceptibilities and not the specific features of **Lynch syndrome**.
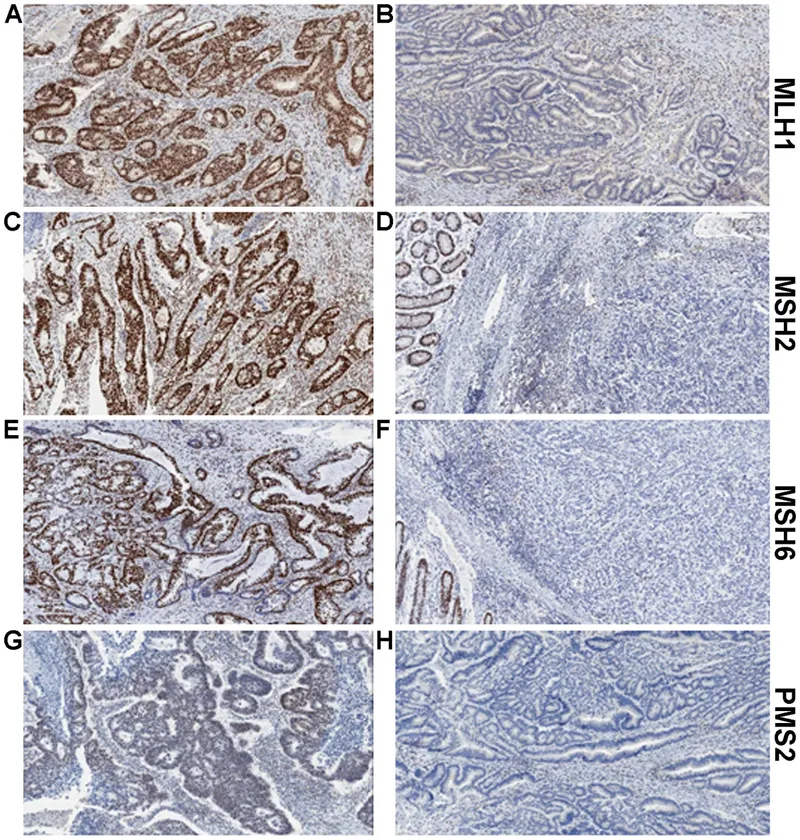
Mismatch repair US Medical PG Question 3: A 35-year-old woman, gravida 2, para 2, comes to the physician with intermenstrual bleeding and heavy menses for the past 4 months. She does not take any medications. Her father died of colon cancer at the age of 42 years. A curettage sample shows dysplastic tall, columnar, cells in the endometrium without intervening stroma. Germline sequencing shows a mutation in the MLH1 gene. Which of the following is the most likely underlying cause of neoplasia in this patient?
- A. Inability to excise bulky DNA adducts
- B. Defective checkpoint control transitions
- C. Impaired repair of deaminated DNA bases
- D. Accumulation of double-stranded DNA breaks
- E. Instability of short tandem DNA repeats (Correct Answer)
Mismatch repair Explanation: ***Instability of short tandem DNA repeats***
- The presence of a **germline mutation in the MLH1 gene**, combined with a family history of early-onset colon cancer (father died at 42), is highly indicative of **Lynch syndrome (hereditary nonpolyposis colorectal cancer or HNPCC)**.
- Lynch syndrome is caused by defects in **DNA mismatch repair (MMR) genes**, such as MLH1, which leads to microsatellite instability (MSI) where **short tandem DNA repeats** accumulate mutations due to inefficient repair.
*Inability to excise bulky DNA adducts*
- This mechanism is characteristic of defects in **nucleotide excision repair (NER)**, often seen in conditions like **xeroderma pigmentosum**, which is typically associated with skin cancers due to UV-induced DNA damage.
- The patient's presentation and MLH1 mutation point specifically to microsatellite instability, not bulky adduct repair.
*Defective checkpoint control transitions*
- Defects in cell cycle checkpoint control lead to uncontrolled cell proliferation and genomic instability, but this is a general mechanism in many cancers and not directly linked to MLH1 or microsatellite instability in the specific way that mismatch repair defects are.
- Mutations in genes like **p53** or **Rb** are classic examples of defective checkpoint controls, which is not the primary defect described by an MLH1 mutation.
*Impaired repair of deaminated DNA bases*
- This refers to defects in **base excision repair (BER)**, which is responsible for correcting small lesions like deaminated bases.
- While critical for DNA integrity, BER defects are not the primary mechanism behind Lynch syndrome and MLH1 mutations, which are specifically involved in correcting errors during DNA replication.
*Accumulation of double-stranded DNA breaks*
- This is typically associated with defects in **homologous recombination** or **non-homologous end-joining pathways**, often seen in conditions like **BRCA1/2 mutations** leading to breast and ovarian cancers.
- MLH1 mutations primarily affect mismatch repair and do not directly lead to an accumulation of double-stranded breaks.
Mismatch repair US Medical PG Question 4: A 44-year-old man comes to the physician because of fatigue and increased straining during defecation for 3 months. During this time, he has lost 5 kg (12 lb) despite no change in appetite. He has a family history of colon cancer in his maternal uncle and maternal grandfather. His mother died of ovarian cancer at the age of 46. Physical examination shows conjunctival pallor. His hemoglobin concentration is 11.2 g/dL, hematocrit is 34%, and mean corpuscular volume is 76 μm3. Colonoscopy shows an exophytic mass in the ascending colon. Pathologic examination of the resected mass shows a poorly differentiated adenocarcinoma. Genetic analysis shows a mutation in the MSH2 gene. Which of the following is the most likely diagnosis?
- A. Familial adenomatous polyposis
- B. Peutz-Jeghers syndrome
- C. Turcot syndrome
- D. Lynch syndrome (Correct Answer)
- E. Gardner syndrome
Mismatch repair Explanation: ***Lynch syndrome***
- The patient's **poorly differentiated adenocarcinoma** in the ascending colon, coupled with the **family history of colon and ovarian cancer** (early onset, diverse cancer types), and the **MSH2 gene mutation**, strongly indicates Lynch syndrome (hereditary nonpolyposis colorectal cancer).
- **Lynch syndrome** is caused by germline mutations in **DNA mismatch repair (MMR) genes** (e.g., MSH2, MLH1, MSH6, PMS2), leading to an increased risk of colorectal, ovarian, endometrial, and other cancers, often at a younger age and predominantly in the **right colon**.
*Familial adenomatous polyposis*
- This syndrome is characterized by the development of **hundreds to thousands of colorectal adenomatous polyps** during adolescence or early adulthood, a feature not mentioned in the patient's presentation.
- It is caused by a germline mutation in the **APC gene**, not MSH2, leading to an almost 100% lifetime risk of colorectal cancer if untreated.
*Peutz-Jeghers syndrome*
- This syndrome is characterized by **hamartomatous polyps** throughout the gastrointestinal tract and **mucocutaneous melanin pigmentation** (dark spots on lips, buccal mucosa, fingers/toes).
- It is associated with mutations in the **STK11 gene** and an increased risk of various cancers, but the clinical presentation and genetic mutation do not match.
*Turcot syndrome*
- Turcot syndrome is a rare condition characterized by the coexistence of **colorectal polyposis** (either FAP-like or Lynch-like) and **central nervous system tumors** (e.g., medulloblastoma, glioblastoma).
- While it can involve MMR gene mutations in some cases, the prominent feature of CNS tumors is absent in this patient's history.
*Gardner syndrome*
- This is a subtype of FAP, characterized by colorectal polyps along with **extra-intestinal manifestations** such as **osteomas** (especially in the mandible or skull), **epidermoid cysts**, and **desmoid tumors**.
- Like FAP, it is caused by mutations in the **APC gene**, and the characteristic extra-intestinal features are not described in the patient.
Mismatch repair US Medical PG Question 5: A 32-year-old woman visits her primary care provider with the results of a recent colonoscopy, which was ordered after 3 episodes of rectal bleeding in the last month. Her grandmother, mother, and sister all have been diagnosed with nonpolyposis colorectal cancer, at ages 65, 50, and 40 years, respectively. Colonoscopy for this patient revealed a large, flat, right-sided adenoma. Histopathological examination of the lesion showed villous histology and high-grade dysplasia. Which of the following helps explain the condition of this patient?
- A. Chromosomal instability
- B. Chemical carcinogenicity
- C. DNA hypermethylation
- D. Environmental carcinogenicity
- E. Microsatellite instability (Correct Answer)
Mismatch repair Explanation: ***Microsatellite instability***
- The patient's **family history** of early-onset, **nonpolyposis colorectal cancer** in three first-degree relatives across three generations strongly suggests a **hereditary syndrome**, specifically **Lynch syndrome (HNPCC)**, which is characterized by **microsatellite instability**.
- **Lynch syndrome** results from germline mutations in **DNA mismatch repair genes** (e.g., MLH1, MSH2, MSH6, PMS2), leading to accumulation of mutations in microsatellite regions and increased cancer risk. The patient's large, flat, right-sided adenoma with villous histology and high-grade dysplasia is also typical of Lynch syndrome-associated lesions.
*Chromosomal instability*
- **Chromosomal instability** (CIN) is characteristic of the **adenoma-carcinoma sequence** seen in sporadic colorectal cancer, involving large-scale chromosomal alterations (aneuploidy, translocations, deletions).
- While CIN is common in sporadic colorectal cancers, the strong family history and early onset in this patient point away from the typical CIN pathway as the primary cause.
*Chemical carcinogenicity*
- **Chemical carcinogenicity** refers to cancer development due to exposure to specific chemicals, which is generally associated with sporadic cancers and lacks the clear hereditary pattern seen here.
- While environmental factors can influence cancer risk, the striking familial presentation in this case makes a solely chemical cause unlikely.
*DNA hypermethylation*
- **DNA hypermethylation** of promoter regions leading to gene silencing, particularly the **CpG island methylator phenotype (CIMP)**, is found in both sporadic colorectal cancers and some Lynch syndrome cases (especially those with MLH1 promoter methylation).
- However, **microsatellite instability** is the direct consequence of germline mutations in mismatch repair genes, which is the fundamental defect in Lynch syndrome suggested by the patient's family history.
*Environmental carcinogenicity*
- **Environmental carcinogenicity** broadly refers to cancer caused by external factors such as diet, smoking, or radiation.
- While environmental factors can play a role in all cancers, the strong autosomal dominant inheritance pattern and early onset of nonpolyposis colorectal cancer in this patient's family point towards a specific genetic predisposition rather than solely environmental causes.
Mismatch repair US Medical PG Question 6: A 58-year-old obese male has noticed the gradual development of a soft bulge on his right groin that has been present over the past year and occasionally becomes very tender. He notices that it comes out when he coughs and strains during bowel movements. He is able to push the bulge back in without issue. After examination, you realize that he has an inguinal hernia and recommend open repair with mesh placement. After surgery, the patient returns to clinic and complains of numbness and tingling in the upper part of the scrotum and base of the penis. What nerve was most likely injured during the procedure?
- A. Ilioinguinal nerve (Correct Answer)
- B. Iliohypogastric nerve
- C. Lateral femoral cutaneous nerve
- D. Obturator nerve
- E. Genitofemoral nerve
Mismatch repair Explanation: **Ilioinguinal nerve**
- The **ilioinguinal nerve** supplies sensory innervation to the skin of the **scrotum** (or labia majora in females), the medial thigh, and the base of the penis.
- Injury to this nerve during an open inguinal hernia repair can cause **numbness and tingling** in these specific areas, consistent with the patient's symptoms.
*Iliohypogastric nerve*
- The **iliohypogastric nerve** primarily provides sensation to the skin over the **suprapubic region** and a small part of the buttock.
- Damage to this nerve would not typically result in numbness of the scrotum or base of the penis.
*Lateral femoral cutaneous nerve*
- This nerve is responsible for sensory innervation of the **lateral aspect of the thigh**.
- Its injury would lead to symptoms of numbness or pain on the lateral thigh (**meralgia paresthetica**), not the scrotum or penis.
*Obturator nerve*
- The **obturator nerve** is a motor nerve that innervates the **adductor muscles of the thigh** and provides sensory innervation to a small area of the medial thigh.
- Damage to this nerve would result in **adductor weakness** and sensory loss in the medial thigh, which does not match the patient's complaints.
*Genitofemoral nerve*
- The **genitofemoral nerve** has two branches: the genital branch (supplies the cremaster muscle and scrotal skin) and the femoral branch (supplies skin of the anterior thigh).
- While the genital branch does innervate the scrotum, injury to this nerve more commonly causes **cremasteric reflex loss** or pain radiating to the anterior thigh, and the described symptoms (base of penis) are more characteristic of ilioinguinal nerve involvement.
Mismatch repair US Medical PG Question 7: A 34-year-old woman comes to the physician for evaluation of a breast lump she noticed 2 days ago while showering. She has no history of major illness. Her mother died of ovarian cancer at age 38, and her sister was diagnosed with breast cancer at age 33. Examination shows a 1.5-cm, nontender, mobile mass in the upper outer quadrant of the left breast. Mammography shows pleomorphic calcifications. Biopsy of the mass shows invasive ductal carcinoma. The underlying cause of this patient's condition is most likely a mutation of a gene involved in which of the following cellular events?
- A. Repair of double-stranded DNA breaks (Correct Answer)
- B. Inhibition of programmed cell death
- C. Regulation of intercellular adhesion
- D. Activity of cytoplasmic tyrosine kinase
- E. Arrest of cell cycle in G1 phase
Mismatch repair Explanation: ***Repair of double-stranded DNA breaks***
- The patient's **family history** (mother with ovarian cancer at 38, sister with breast cancer at 33) and early onset of **invasive ductal carcinoma** strongly suggest an inherited cancer syndrome.
- **BRCA1 and BRCA2 genes** are tumor suppressor genes responsible for repairing **double-stranded DNA breaks**, and mutations in these genes significantly increase the risk of breast and ovarian cancers.
*Inhibition of programmed cell death*
- Mutations leading to the **inhibition of programmed cell death (apoptosis)**, such as those affecting the **Bcl-2 gene**, can contribute to cancer by allowing damaged cells to survive and proliferate.
- While relevant to cancer pathogenesis, it is not the primary mechanism associated with the specific familial breast/ovarian cancer pattern seen here, which points more directly to DNA repair defects.
*Regulation of intercellular adhesion*
- Defects in **intercellular adhesion**, often involving **E-cadherin** (CDH1 gene) mutations, are associated with cancers like **lobular breast carcinoma** and **hereditary diffuse gastric cancer**.
- This patient has **invasive ductal carcinoma**, and the specific familial pattern is less characteristic of intercellular adhesion defects.
*Activity of cytoplasmic tyrosine kinase*
- Abnormal **cytoplasmic tyrosine kinase activity** is implicated in various cancers (e.g., **HER2/neu** amplification in breast cancer, **BCR-ABL** fusion in CML).
- While HER2/neu overexpression is common in breast cancer, it is typically a somatic mutation or amplification, and not the underlying germline defect explaining the strong family history of early-onset breast and ovarian cancer.
*Arrest of cell cycle in G1 phase*
- The **arrest of the cell cycle at the G1 phase** is mainly regulated by **p53** and **Rb tumor suppressor genes**, which prevent uncontrolled cell division.
- While mutations in these genes are crucial in many cancers, the specific familial pattern (breast and ovarian cancer) points more strongly to defects in homologous recombination via BRCA1/2, a different DNA repair pathway.
Mismatch repair US Medical PG Question 8: A software engineer presents to the OPD with 'complaints of easy fatigability. He reports sitting in front of a computer for 12-14 hours a day consuming junk food, and eating few fruits and vegetables. CBC results show hemoglobin (Hb) concentration of $7 \mathrm{gm} \%$ and MCV of 120 fL . What is the most likely cause of anemia?
- A. Cyanocobalamin deficiency
- B. Acute blood loss
- C. Sideroblastic anemia
- D. Folate deficiency (Correct Answer)
- E. Iron deficiency anemia
Mismatch repair Explanation: ***Folate deficiency***
- A **macrocytic anemia** with an **MCV of 120 fL** is characteristic of folate deficiency, as folate is vital for **DNA synthesis** in red blood cell production.
- The patient's diet of **junk food** and few fruits/vegetables suggests poor nutritional intake, as folate is abundant in leafy greens and fresh produce.
*Cyanocobalamin deficiency*
- While also causing **macrocytic anemia** with high MCV, cyanocobalamin (Vitamin B12) deficiency often presents with **neurological symptoms** (e.g., neuropathy, cognitive changes) which are not mentioned.
- Dietary sources of B12 are primarily **animal products**, and while junk food is poor, a strict vegetarian/vegan diet is a stronger indicator of B12 deficiency.
*Acute blood loss*
- Acute blood loss typically causes **normocytic, normochromic anemia**, characterized by a normal MCV in the initial stages.
- While severe blood loss can lead to fatigue, the **elevated MCV** of 120 fL makes this diagnosis unlikely unless there's a pre-existing macrocytic condition.
*Sideroblastic anemia*
- Sideroblastic anemia can be **microcytic, normocytic, or macrocytic**, but it is primarily characterized by the presence of **ring sideroblasts** in the bone marrow and iron overload.
- It's often associated with **alcoholism, lead poisoning, or myelodysplastic syndromes**, and the typical features of the patient's diet and MCV do not point towards this condition.
*Iron deficiency anemia*
- Iron deficiency anemia presents with **microcytic, hypochromic anemia** with a **low MCV** (typically <80 fL), not macrocytic anemia.
- While iron deficiency is the most common cause of anemia worldwide and can result from poor diet, the **elevated MCV of 120 fL** clearly excludes this diagnosis.
Mismatch repair US Medical PG Question 9: An investigator isolates bacteria from a patient who presented with dysuria and urinary frequency. These bacteria grow rapidly in pink colonies on MacConkey agar. During replication of these bacteria, the DNA strands are unwound at the origin of replication, forming two Y-shaped replication forks that open in opposite directions. At each replication fork, daughter strands are synthesized from the template strands in a 5′ to 3′ direction. On one strand, the DNA is synthesized continuously; on the other strand, the DNA is synthesized in short segments. The investigator finds that three enzymes are directly involved in elongating the DNA of the lagging strand in these bacteria. One of these enzymes has an additional function that the others do not possess. Which of the following steps in DNA replication is unique to this enzyme?
- A. Elongation of lagging strand in 5'→3' direction
- B. Excision of nucleotides with 5'→3' exonuclease activity (Correct Answer)
- C. Prevention of reannealing of the leading strand and the lagging strand
- D. Creation of ribonucleotide primers
- E. Proofreading for mismatched nucleotides
Mismatch repair Explanation: ***Excision of nucleotides with 5'→3' exonuclease activity***
- **DNA polymerase I** possesses unique **5'→3' exonuclease activity** that allows it to remove RNA primers synthesized by primase.
- After primer removal, DNA polymerase I synthesizes DNA in the 5'→3' direction to fill the gap.
*Elongation of lagging strand in 5'→3' direction*
- While **DNA polymerase I** elongates the lagging strand, this 5'→3' synthesis function is also shared by **DNA polymerase III**, which is the primary enzyme for DNA synthesis.
- Therefore, this specific function is not unique to the enzyme in question (DNA polymerase I) as DNA polymerase III also performs 5'→3' elongation.
*Prevention of reannealing of the leading strand and the lagging strand*
- This function is carried out by **single-strand binding proteins (SSBs)**, which bind to the separated DNA strands to prevent them from reannealing and protect them from degradation.
- This is not a function of any DNA polymerase.
*Creation of ribonucleotide primers*
- The synthesis of short RNA primers required for initiation of DNA synthesis is performed by **primase**, an RNA polymerase.
- DNA polymerases do not create primers but rather extend them.
*Proofreading for mismatched nucleotides*
- **DNA polymerase I** and **DNA polymerase III** both possess **3'→5' exonuclease activity** for proofreading, allowing them to remove incorrectly incorporated nucleotides.
- Since this function is shared by DNA polymerase III, it is not unique to DNA polymerase I.
Mismatch repair US Medical PG Question 10: An investigator is studying the replication of bacterial DNA with modified nucleotides. After unwinding, the double-stranded DNA forms a Y-shaped replication fork that separates into two strands. At each of these strands, daughter strands are synthesized. One strand is continuously extended from the template strands in a 5′ to 3′ direction. Which of the following is exclusively associated with the strand being synthesized away from the replication fork?
- A. Reverse transcriptase activity
- B. Repeated activity of ligase (Correct Answer)
- C. Elongation in the 3'→5' direction
- D. Synthesis of short RNA sequences
- E. 5' → 3' exonuclease activity
Mismatch repair Explanation: ***Repeated activity of ligase***
- The lagging strand, synthesized away from the replication fork, is made in fragments (**Okazaki fragments**) due to the 5' to 3' synthesis direction of DNA polymerase.
- **DNA ligase** repeatedly joins these Okazaki fragments together, forming a continuous strand.
*Reverse transcriptase activity*
- **Reverse transcriptase** synthesizes DNA from an RNA template, which is not involved in normal bacterial DNA replication.
- This enzyme is characteristic of **retroviruses** and certain eukaryotic telomere maintenance.
*Elongation in the 3'→5' direction*
- DNA polymerases only synthesize new DNA strands in the **5' to 3' direction**.
- While reading the template strand in the 3' to 5' direction, the daughter strand is always built from 5' to 3'.
*Synthesis of short RNA sequences*
- **RNA primers** are synthesized by **primase** on both the leading and lagging strands to initiate DNA synthesis.
- This process is not exclusive to the strand synthesized away from the replication fork; the leading strand also requires an initial RNA primer.
*5' → 3' exonuclease activity*
- The **5' to 3' exonuclease activity** of DNA polymerase I in bacteria is responsible for removing RNA primers.
- This activity occurs on both the leading and lagging strands, as both require primer removal.
More Mismatch repair US Medical PG questions available in the OnCourse app. Practice MCQs, flashcards, and get detailed explanations.
 identifying and correcting a base pair mismatch in a newly synthesized DNA strand)
identifying and correcting a base pair mismatch in a newly synthesized DNA strand)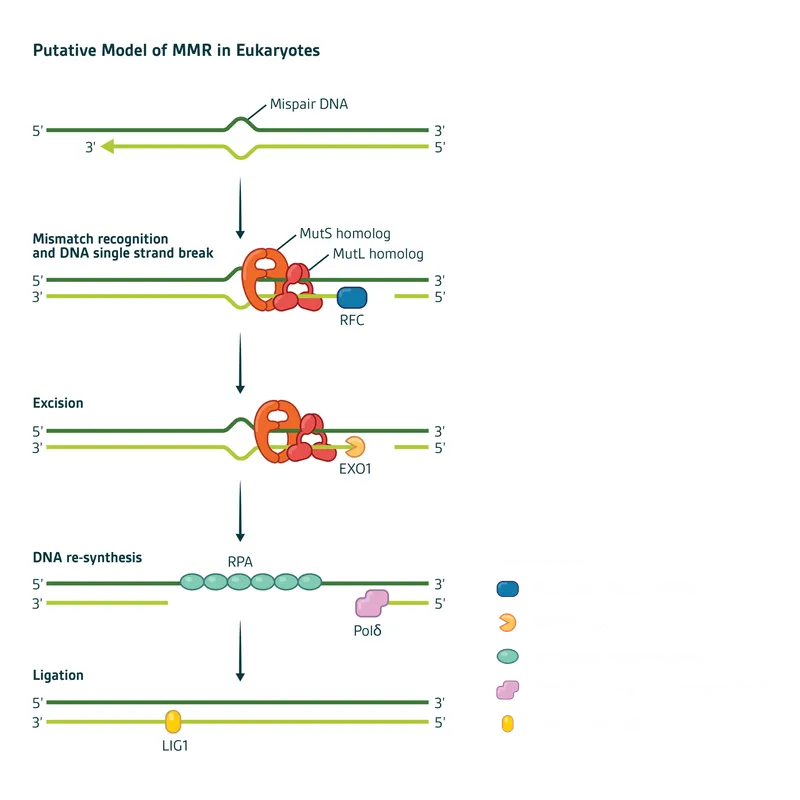 pathway diagram with MutS and MutL homologs)
pathway diagram with MutS and MutL homologs)